Background.
The coronavirus disease 2019 (COVID-19) pandemic has had a variable course across the United States. Understanding its evolving impact on heart and lung transplantation (HT and LT) will help with planning for next phases of this pandemic as well as future ones.
Methods.
We used Scientific Registry of Transplant Recipients data from before the pandemic to predict the number of waitlist registrations and transplants expected to occur between March 15, 2020, and December 31, 2020 (if no pandemic had occurred), and compared these expectations to observed rates. The observed era was divided into wave 1 (March 15–May 31), wave 2 (June 1–September 30), and wave 3 (October 1–December 31). We used multilevel Poisson regression to account for center- and state-level COVID-19 incidence.
Results.
During wave 1, rates of heart registrations and transplants were 28% (incidence rate ratio [IRR]: 0.72 [95% confidence interval (CI), 0.67-0.77]) and 13% (IRR: 0.87 [95% CI, 0.80-0.93]) lower than expected; lung registrations and transplants were 40% (IRR: 0.60 [95% CI, 0.54-0.66]) and 28% (IRR: 0.72 [95% CI, 0.66-0.79]) lower. Decreases were greatest in states with the highest incidence where registrations were 53% (IRR: 0.47 [95% CI, 0.36-0.62]) and 59% (IRR: 0.41 [95% CI, 0.29-0.58]) and transplants were 57% (IRR: 0.43 [95% CI, 0.31-0.60]) and 58% (IRR: 0.42 [95% CI, 0.29-0.62]) lower than expected. Whereas HT largely recovered during waves 2 and 3, LT continued to fall short of expectations through the end of the year.
Conclusions.
The COVID-19 pandemic in the US substantially reduced thoracic transplant access. Ongoing evaluation of the risks and benefits of this dramatic practice change is critical to inform clinical decision-making moving forward.
INTRODUCTION
The emergence of a novel coronavirus led to a global pandemic unprecedented in modern history.1-3 The United States has seen 3 major waves of the pandemic, in addition to wide geographic variation, with some states observing an early and rapid escalation of cases, whereas others successfully limited the impact of the disease with policies supporting social distancing. At some times and in some places, healthcare resources were stretched thin, with the number of cases outstripping the number of hospital beds and intensive care resources. Since organ transplantation is a resource-intensive endeavor, challenges in clinical decision-making and hospital policy-making have involved a complex balance between risk to patients on the waitlist (including community acquisition of coronavirus disease 2019 [COVID-19]), the risk of transplantation (including nosocomial acquisition and potentially increased severity of disease while immunosuppressed), and utilization of hospital resources (including prioritizing hospital beds, intensive care unit beds, and ventilators for COVID-19 patients).
In kidney transplantation (KT), new listings dropped on average by 18%, deceased-donor transplants by 24%, and living-donor transplants by 87% from March 15 to April 30, with even higher declines of 41% new listings, 61% deceased-donor KT, and nearly 100% living-donor KT in states with the highest COVID-19 burden.4 This dramatic decrease is understandable given the relatively low-risk treatment alternative of dialysis. However, the risk/benefit calculus is quite different with heart and lung transplantation (HT and LT). For example, 25% of LT recipients require hospitalization, and 81% of HT recipients require life support before surgery.5,6 Waitlist deaths are much higher with end-stage heart and lung disease (ESHD and ESLD), ranging from 8.7 to 30.1 deaths per 100 waitlist-y for HT and 6.6–121.8 for LT, compared with 1.7–9.5 for KT.5-7 And, not surprisingly, outcomes of COVID-19 infection in HT and LT recipients are quite poor.8-27 However, the evolving impact of the pandemic on the practice of HT and LT has not been quantified.
Given the acuity of disease among patients awaiting HT and LT, any decrease in transplant access must be understood to inform decision-making in future phases of this pandemic as well as future pandemics. To quantify the impact of the pandemic on HT and LT in the United States, we used Scientific Registry of Transplant Recipients (SRTR) data from before the pandemic to predict the number of waitlist registrations and transplants expected to occur between March 15 and December 31, 2020 (if no pandemic had occurred), and compared these expectations with observed rates.
MATERIALS AND METHODS
Data Source
This study used data from the SRTR data system. The SRTR data system includes data on all donors, waitlisted candidates, and transplant recipients in the United States submitted by members of the Organ Procurement and Transplantation Network (OPTN) and has been described elsewhere.28 The Health Resources and Services Administration of the US Department of Health and Human Services provides oversight of the activities of OPTN and SRTR contractors.
The project design was approved by the Johns Hopkins Medicine Institutional Review Board before data acquisition and deemed exempt from ethics board review.
Time Periods
We identified all transplant candidates and recipients from February 1, 2020, to December 31, 2020. We categorized candidates by date of listing and recipients by date of transplant into waves (Transition: February 1–March 14, wave 1: March 15–May 31, wave 2: June 1–September 30, and wave 3: October 1–December 31). These time periods were selected to best reflect the varying incidence in COVID-19 over time, according to the national reported incidence of COVID-19 in the United States per the New York Times GitHub.
State-level Cumulative Incidence of COVID-19 Infection
We used data from http://covidtracking.com/ to calculate the state-level (including the District of Columbia and Puerto Rico) cumulative incidence of reported COVID-19 cases per 100 000 people per day in each month; the last week of March, June, and November, respectively, were used as they reflected the highest incidence of COVID-19 within each wave. We then divided states according to their COVID-19 burden (per 100 000 people per d) into the following categories: low (<4 cases), medium (4–8 cases), high (8–12 cases), and very high (>12 cases) for waves 1 and 2, and low (<35 cases), medium (35–50 cases), high (50–75 cases), and very high (>75 cases) for wave 3. These categories were based on prior studies and according to thresholds that placed a sufficient number of states in each category.4,29
National Trends in Waitlisting and Transplantation
To describe the impact of COVID-19 on national trends in waitlisting and transplants, we generated Locally Weighted Scatterplot Smoothing graphs using SRTR data from February 1, 2020, to December 31, 2020. Briefly, this method allows us to visualize trends as it generates a line of best fit using robust locally weighted regression on a scatterplot of our exposure over time.30
Center-level Impact of COVID-19 on Waitlisting and Transplantation
To determine the center-level impact of COVID-19 on waitlists events (registrations and removal for transplant) for comparison to observed counts, we used data from each center by month from: (i) November 2018 to February 2020 for heart (in light of changes to heart allocation policy in October 2018) and (ii) January 2016 to February 2020 for lung. Using hierarchical Poisson regression with a center-level random intercept, we modeled the number of waitlist events per center per month after accounting for candidate characteristics. For heart, we adjusted for age, sex, race/ethnicity, etiology of ESHD, pulmonary hypertension, OPTN waitlist status, and type of insurance. For lung, we adjusted for age, sex, race/ethnicity, etiology of ESLD, smoking status, and type of insurance. Lung allocation score (LAS) was not included in the final model since it did not impact the model’s predictive ability. We then used these models to predict the number of events expected to occur across centers within each wave (if no pandemic had occurred).
To compare the observed and expected counts among centers, we used Poisson regression adjusting for state-level COVID-19 burden (low, medium, high, and very high). Given that Poisson regression by definition models the log of the observed counts as the outcome, we used an offset (the log of expected counts) to obtain incidence rate ratios (IRRs) that directly compare the ratio of observed to expected events. For analyses that compared between-state burden, we used “low rates” as our reference given that most centers fell into this category.
Statistical Analysis
To compare recipient characteristics, we used Pearson’s chi-squared tests for categorical variables, and the Kruskal-Wallis test for nonnormally distributed continuous variables. All analyses were performed using Stata 16.0/MP for Linux (College Station, TX).
RESULTS
Study Population
Heart Transplant Recipients
A total of 461 (326/mo) HT were performed during the Transition, 673 (266/mo) in wave 1, 1324 (333/mo) in wave 2, and 912 (305/mo) in wave 3 (Table 1). The composition of HT recipients between waves did not vary by age, sex, race, insurance type, or etiology of ESHD. The distribution of OPTN status varied significantly with time, primarily because of an increase in status 2 from 34.3% to 39.9% and a decline in status 4 from 25.3% to 18.5% between waves 1 and 3 (P = 0.004). The average time to transplantation decreased significantly from a median of 1.6 (interquartile range [IQR]: 0.4–7.2) to 1.0 (IQR: 0.3–3.9) mo between wave 1 and 3 (P < 0.001).
TABLE 1.
Characteristics of heart transplant recipients throughout the COVID-19 pandemic
| Characteristic | Wave 1 | Wave 2 | Wave 3 | Overall P |
|---|---|---|---|---|
| March–May (N = 673) | June–September (N = 1324) | October–December (N = 912) | ||
| Age (y), % | 0.1 | |||
| 0–17 | 11.1 | 14.4 | 11.8 | |
| 18–34 | 9.1 | 8.9 | 8.7 | |
| 35–49 | 17.4 | 19.6 | 16.3 | |
| 50–64 | 44.1 | 40.1 | 42.9 | |
| ≥65 | 18.3 | 17 | 20.3 | |
| Female sex, % | 26.9 | 28.7 | 28.7 | 0.7 |
| Race, % | 0.8 | |||
| White | 61.7 | 59.7 | 58.9 | |
| Black | 23.6 | 25.3 | 24.3 | |
| Hispanic/Latino | 10 | 10.4 | 11.3 | |
| Other | 4.8 | 4.5 | 5.5 | |
| Insurance, % | 0.3 | |||
| Private | 45.9 | 45.8 | 45.3 | |
| Public | 53.3 | 53.5 | 54.6 | |
| Other | 0.7 | 0.8 | 0.1 | |
| Primary ESHD diagnosis, % | 0.2 | |||
| Ischemic cardiomyopathy | 21.2 | 19.8 | 24.9 | |
| Nonischemic cardiomyopathy | 62.6 | 62.9 | 58.3 | |
| Transplant | 2.7 | 3.3 | 3.7 | |
| Congenital | 8.8 | 9.6 | 9 | |
| Other | 4.8 | 4.4 | 4.1 | |
| OPTN urgency, % | 0.004 | |||
| Status 1A | 8.9 | 10.7 | 10 | |
| Status 1B/2 | 2.4 | 4 | 2.1 | |
| Adult status 1 | 6.8 | 7.8 | 7 | |
| Adult status 2 | 34.3 | 38.4 | 39.9 | |
| Adult status 3 | 17.4 | 15 | 16.3 | |
| Adult status 4 | 25.3 | 18 | 18.5 | |
| Adult status 5–6 | 4.9 | 6.1 | 6.1 | |
| Pulmonary hypertension, % | 17.9 | 18.5 | 16.3 | 0.5 |
| Time to transplant (mo), median (IQR) | 1.6 (0.4, 7.2) | 0.9 (0.3, 5.4) | 1.0 (0.3, 3.9) | <0.001 |
COVID-19, coronavirus disease 2019; ESHD, end-stage heart disease; IQR, interquartile range; OPTN, organ procurement and transplantation network.
Lung Transplant Recipients
A total of 363 (257/mo) LT were performed during the Transition, 431 (170/mo) in wave 1, 871 (219/mo) in wave 2, and 638 (213/mo) in wave 3 (Table 2). The composition of LT recipients between waves did not vary by age, sex, race, insurance type, history of tobacco use, etiology of ESLD or LAS. The number of single-lung transplants increased from 20.6% to 25.1% between waves 1 and 3 (P = 0.03). The average time to transplantation decreased significantly from 1.5 mo (IQR: 0.4–4.6) to 0.9 mo (IQR: 0.3–.8) between waves 1 and 3 (P = 0.007).
TABLE 2.
Characteristics of lung transplant recipients throughout the COVID-19 pandemic
| Characteristic | Wave 1 | Wave 2 | Wave 3 | Overall P |
|---|---|---|---|---|
| March–May (N = 431) | June–September (N = 871) | October–December (N = 638) | ||
| Age (y), % | 0.3 | |||
| 0–17 | 0.7 | 1.5 | 1.4 | |
| 18–34 | 7.9 | 5.3 | 4.2 | |
| 35–49 | 10 | 10.3 | 9.9 | |
| 50–64 | 45 | 46.2 | 45.1 | |
| ≥65 | 36.4 | 36.7 | 39.3 | |
| Female sex, % | 43.2 | 42.6 | 40.3 | 0.6 |
| Race, % | 0.6 | |||
| White | 75.9 | 74.7 | 75.1 | |
| Black | 10.2 | 11 | 9.2 | |
| Hispanic/Latino | 11.1 | 9.8 | 11.6 | |
| Other | 2.8 | 4.5 | 4.1 | |
| History of cigarette use, % | 54.5 | 58.4 | 57.2 | 0.4 |
| Insurance, % | 0.4 | |||
| Private | 42.2 | 38.7 | 40.9 | |
| Public | 57.1 | 60.8 | 58 | |
| Other | 0.7 | 0.5 | 1.2 | |
| Primary ESLD diagnosis, % | 0.07 | |||
| A: Obstructive lung disease | 27.6 | 26.5 | 23 | |
| B: Pulmonary vascular disease | 7 | 6.3 | 6.6 | |
| C: Cystic fibrosis | 4.6 | 2.5 | 2 | |
| D: Restrictive lung diseases | 60.8 | 64.6 | 68.3 | |
| LAS, % | 0.4 | |||
| 0–33 | 17.2 | 19.4 | 17 | |
| 34–37 | 22.5 | 21.2 | 23.7 | |
| 38–45 | 22.3 | 22.8 | 26.5 | |
| 46–100 | 38.1 | 36.5 | 32.9 | |
| Time to transplant (mo), (median) IQR | 1.5 (0.4, 4.6) | 1.2 (0.3, 4.7) | 0.9 (0.3, 2.8) | 0.007 |
| Single-lung transplant, % | 20.6 | 19.6 | 25.1 | 0.03 |
COVID-19, coronavirus disease 2019; ESLD, end-stage lung disease; IQR, interquartile range; LAS, lung allocation score.
Trends in Waitlist Events
Heart Transplant Candidates
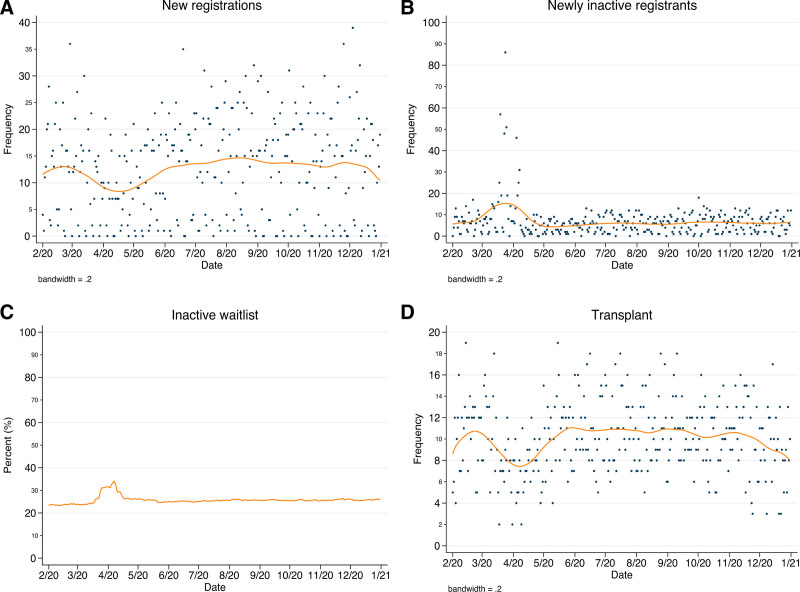
Waitlist registrations declined from an average of 13 per day during the Transition to 9 in wave 1 before rebounding to 14 early in wave 2 and remaining stable thereafter (Figure 1A). The number of newly inactive registrants increased from an average of 7–21 per day between the Transition and beginning of wave 1, declined to 8 through wave 1, and remained stable at an average of 6 through waves 2 and 3 (Figure 1B). The proportion of total inactive waitlist registrants increased from 24.3% at the beginning of wave 1 to 34.1% 3 wks later before returning to an average of 25.5% through waves 2 and 3 (Figure 1C). Transplants per day declined from 11 to 7 at the beginning of wave 1, rebounded to prepandemic volume by the beginning of wave 2, and then declined to an average of 9 through wave 3 (Figure 1D).
FIGURE 1.
Heart transplant waitlist events before and after onset of the pandemic. Counts of new DDHT waitlist registrations (A) and patients moved to inactive status (B) per d, with LOWESS smooth; (C) proportion of prevalent inactive waitlist per d; (D) counts of DDHT per d, with LOWESS smooth. DDHT, deceased-donor heart transplant; LOWESS, Locally Weighted Scatterplot Smoothing.
Lung Transplant Candidates
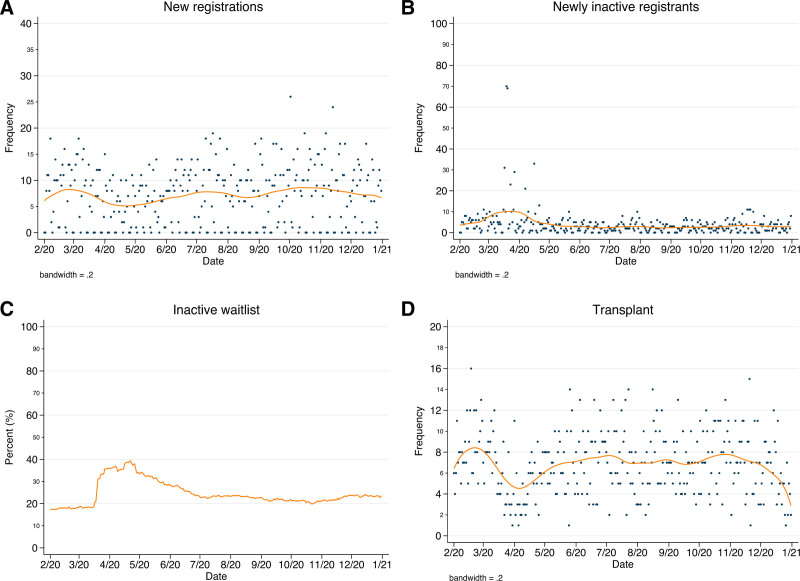
Waitlist registrations declined from an average of 8 per day during the Transition to 5 in wave 1 before slowly rebounding to prepandemic volume by the beginning of wave 3 and remaining stable thereafter (Figure 2A). The number of newly inactive registrants increased from 5 to 18 per day between the Transition and beginning of wave 1, decreased to 3 through wave 1 and remained stable thereafter (Figure 2B). The proportion of total inactive waitlist registrants increased from 18.3% at the beginning of wave 1 to 39.3% 1 mo later before decreasing gradually to 19.8% by the beginning of wave 3; this began to rise once again to a high of 24.2% by year’s end (Figure 2C). Transplants per day declined from 8 to 4 at the beginning of wave 1 before rebounding to 7 during wave 2 then decreasing slightly to 6 by the end of the year (Figure 2D).
FIGURE 2.
Lung transplant waitlist events before and after onset of the pandemic. Counts of new DDLT waitlist registrations (A) and patients moved to inactive status (B) per d, with LOWESS smooth; (C) proportion of prevalent inactive waitlist per d; (D) counts of DDLT per d, with LOWESS smooth. DDLT, deceased-donor lung transplant; LOWESS, Locally Weighted Scatterplot Smoothing.
State-level COVID-19 Incidence
Heart
In wave 1, there were 28 states with low incidence, 5 states with medium incidence, 3 states with high incidence, and 3 states with very high incidence (MI, NJ, and NY) of COVID-19. In wave 2, there were 7 states with low incidence, 13 states with medium incidence, 6 states with high incidence, and 13 states with very high incidence (AL, AR, AZ, CA, FL, GA, LA, MS, NC, SC, TN, TX, and UT). In wave 3, there were 11 states with low incidence, 8 states with medium incidence, 14 states with high incidence, and 6 states with very high incidence (CO, IN, KS, MN, NE, and WI).
Lung
In wave 1, there were 21 states with low incidence, 4 states with medium incidence, 2 states with high incidence, and 3 states with very high incidence (MI, NJ, and NY) of COVID-19. In wave 2, there were 4 states with low incidence, 11 states with medium incidence, 4 states with high incidence, and 11 states with very high incidence (AL, AZ, CA, FL, GA, LA, NC, SC, TN, TX, and UT). In wave 3, there were 8 states with low incidence, 6 states with medium incidence, 11 states with high incidence, and 5 states with very high incidence (CO, IN, MN, NE, and WI).
Center-level Variation in Observed Versus Expected Events
Heart Transplant Candidates
In wave 1, there were 28% fewer listings than expected (IRR: 0.72 [95% confidence interval (CI), 0.67-0.77]) (Table 3). Decreases were seen across all categories of geographic COVID-19 incidence with the greatest decrease among centers in states with very high incidence (IRR: 0.30 [95% CI, 0.17-0.52]). Similarly, there were 13% fewer transplants than expected (IRR: 0.87 [95% CI, 0.80-0.93]), with the greatest decrease among centers in states with very high incidence (IRR: 0.43 [95% CI, 0.31-0.60]).
TABLE 3.
Observed center-level heart waitlist events as a proportion of expected events, March 15–December 31, 2020
| New listings | DDHT | |
|---|---|---|
| COVID-19 incidence | IRR (CI) | IRR (CI) |
| Wave 1: March–May | ||
| Overall | 0.72 (0.67-0.77) | 0.87 (0.80-0.93) |
| Low | 0.79 (0.73-0.86) | 0.97 (0.89-1.06) |
| Medium | 0.56 (0.45-0.69)a | 0.75 (0.61-0.92)a |
| High | 0.74 (0.55-1.01) | 0.80 (0.58-1.10) |
| Very high | 0.47 (0.36-0.62)a | 0.43 (0.31-0.60)a |
| Wave 2: June–September | ||
| Overall | 1.05 (1.00-1.11) | 1.09 (1.03-1.15) |
| Low | 1.17 (1.04-1.31) | 1.16 (1.02-1.33) |
| Medium | 1.00 (0.92-1.09)a | 1.00 (0.91-1.11) |
| High | 1.35 (1.13-1.62) | 1.27 (1.03-1.56) |
| Very high | 1.02 (0.95-1.09)a | 1.10 (1.02-1.20) |
| Wave 3: October–December | ||
| Overall | 1.02 (0.97-1.08) | 0.99 (0.93-1.06) |
| Low | 1.05 (0.93-1.17) | 0.98 (0.85-1.12) |
| Medium | 1.11 (1.01-1.21) | 1.12 (1.01-1.24) |
| High | 0.98 (0.88-1.09) | 0.93 (0.83-1.06) |
| Very high | 0.81 (0.66-0.98)a | 0.77 (0.62-0.95) |
Bold denotes statistically significant IRRs
aIRRs that are statistically significantly different from the IRR in states with the lowest per-capita reported COVID-19 cases.
CI, confidence interval; DDHT, deceased-donor heart transplant; IRR, incidence rate ratio.
These dramatic decreases resolved by wave 2, with even slightly more new listings than expected (IRR: 1.05 [95% CI, 1.00-1.11]). Resolution of wave 1 decreases were seen across all categories of geographic incidence, with the greatest increase among centers in states with high incidence (IRR: 1.35 [95% CI, 1.13-1.62]). Similarly, decreases in transplants during wave 1 resolved by wave 2, with even slightly more transplants than expected (IRR: 1.09 [95% CI, 1.03-1.15]); this was also seen across all categories of incidence, with the greatest increase among centers in states with high incidence (IRR: 1.27 [95% CI, 1.03-1.56]).
The stability of wave 2 remained in wave 3, other than in very high incidence states, which had 19% fewer new listings (IRR: 0.81 [95% CI, 0.66-0.98]) and 23% fewer transplants (IRR: 0.77 [95% CI, 0.62-0.95]) than expected.
Lung Transplant Candidates
In wave 1, there were 40% fewer listings than expected (IRR: 0.60 [95% CI, 0.54-0.66]) (Table 4). Decreases were seen across all categories of geographic COVID-19 incidence with the greatest decrease among centers in states with very high incidence (IRR: 0.41 [95% CI, 0.29-0.58]). Similarly, there were 28% fewer transplants than expected (IRR: 0.72 [95% CI, 0.66-0.79]); this was observed across all categories of incidence with the greatest decrease among centers in states with very high incidence (IRR: 0.42 [95% CI, 0.29-0.62]).
TABLE 4.
Observed center-level lung waitlist events as a proportion of expected events, March 15–December 31, 2020
| New listings | DDLT | |
|---|---|---|
| COVID-19 incidence | IRR (CI) | IRR (CI) |
| Wave 1: March–May | ||
| Overall | 0.60 (0.54-0.66) | 0.72 (0.66-0.79) |
| Low | 0.63 (0.57-0.71) | 0.80 (0.72-0.90) |
| Medium | 0.56 (0.45-0.70) | 0.66 (0.52-0.83) |
| High | 0.68 (0.45-1.03) | 0.48 (0.26-0.86) |
| Very high | 0.41 (0.29-0.58)a | 0.42 (0.29-0.62)a |
| Wave 2: June–September | ||
| Overall | 0.81 (0.76-0.86) | 0.93 (0.87-1.00) |
| Low | 0.90 (0.76-1.06) | 0.93 (0.78-1.11) |
| Medium | 0.71 (0.64-0.79)a | 0.99 (0.89-1.10) |
| High | 0.77 (0.54-1.09) | 0.77 (0.53-1.12) |
| Very high | 0.88 (0.79-0.96) | 0.90 (0.81-1.00) |
| Wave 3: October–December | ||
| Overall | 0.89 (0.82-0.95) | 0.91 (0.84-0.98) |
| Low | 0.87 (0.73-1.02) | 0.98 (0.83-1.15) |
| Medium | 1.00 (0.89-1.11) | 0.82 (0.72-0.94) |
| High | 0.81 (0.72-0.92) | 0.96 (0.84-1.09) |
| Very high | 0.73 (0.55-0.98) | 0.92 (0.68-1.26) |
Bold denotes statistically significant IRRs.
aIRRs that are statistically significantly different from the IRR in states with the lowest per-capita reported COVID-19 cases.
CI, confidence interval; DDLT, deceased-donor lung transplant; IRR, incidence rate ratio.
Wave 2 was characterized by partially attenuated but persistent decreases in transplant volume. There were 19% fewer listings than expected (IRR: 0.81 [95% CI, 0.76-0.86]). This was observed across all categories of incidence with the greatest decrease among centers in states with medium incidence (IRR: 0.71 [95% CI, 0.64-0.79]). There were 7% fewer LT performed than expected (IRR: 0.93 [95% CI, 0.87-1.00]) driven predominantly by centers in states with very high incidence (IRR: 0.90 [95% CI, 0.81-1.00]).
Decreases in volume continued into wave 3 with 11% fewer listings than expected (IRR: 0.89 [95% CI, 0.82-0.95]). This was observed across the majority of categories of incidence with the greatest decrease among centers in states with very high incidence (IRR: 0.73 [95% CI, 0.55-0.98]). There were 9% fewer LT performed than expected (IRR: 0.91 [95% CI, 0.84-0.98]) driven predominantly by centers in states with medium incidence (IRR: 0.82 [95% CI, 0.72-0.94]).
DISCUSSION
In this national study of HT and LT waitlisting and transplantation during the US COVID-19 pandemic, we found substantial reductions in new listings and transplants immediately following the declaration of a national state of emergency on March 15, even in areas that were not experiencing heavy COVID-19 burden. During wave 1, the number of heart waitlist registrations and transplants were 28% and 13% lower than expected; lung waitlist registrations and transplants were 40% and 28% lower. Decreases were greatest among centers in states with the highest COVID-19 burden where registrations were 53% and 59%, and transplants were 57% and 58%, lower than expected. These trends began to reverse in subsequent months. Heart registration and transplant volume recovered by wave 2, even exceeding expectations. Wave 3 once again brought decreases in registration and transplant volume among centers in states with very high incidence, whereas practice in less burdened states remained stable. Lung registration and transplant volume partially recovered during waves 2 and 3 but continued to fall short of expectations through the end of the year.
Our findings are consistent with national surveys in March and May 2020 in which 81% of HT and LT centers reported restricting operations with the most stringent measures being applied in regions hit hardest by the pandemic.31,32 Notably, despite 64% and 71% of HT and LT centers reporting persistent practice restrictions in May, 49% of responding centers anticipated resuming transplantation at full capacity by June. Our study extends these surveys by quantifying the impact of these restricted operations using actual waitlist and transplant data rather than center self-report and by studying this impact across multiple waves of the pandemic. The fact that declines in observed registrations and transplants were greatest in states with very high COVID-19 incidence during wave 1 is consistent with reports of national diversion of healthcare resources during this era. In the present study, the composition of OPTN class at the time of HT showed a modest increase in class 2 and decrease in class 4; the distribution of LAS at the time of LT did not vary significantly from predictions. The former is in line with survey responses indicating some centers were prioritizing sicker patients, whereas the latter appears contrary.
Although this study is built on the strength of a national mandated registry with detailed data collection on waitlist and transplant events, it must also be understood in the context of its limitations. For example, our use of national registry data precludes assessment of important and interesting clinical questions such as why a particular patient became inactive on the waitlist, why a particular patient was selected for transplantation, why centers reduced transplantation, etc. Despite this limitation, our use of national registry data facilitates broadly generalizable inferences, especially in the context of linking data to state-level COVID-19 incidence rates.
In summary, we found that access to HT and LT was substantially reduced in the early US COVID-19 era as evidenced by decreases in waitlist registrations, HT and LT through wave 1. HT access rebounded to, and in some cases exceeded, predictions through waves 2 and 3. Despite this overall recovery, states with very high COVID-19 incidence once again exhibited decreased heart registrations and transplants in wave 3. In contrast, lung registrations and transplants partially recovered but remained below predictions through waves 2 and 3 driven predominantly by states with the highest incidence of COVID-19. These findings suggest that the pandemic has had a dramatic and lasting effect on access to thoracic transplantation. Additional and ongoing data collection and monitoring as well as guidance from high performing centers are critical to inform clinical practice and mitigate the impact of the pandemic moving forward.
ACKNOWLEDGMENTS
The authors celebrate the contributions of collaborator and coauthor Alena Eftihia Frey, a heart and kidney transplant recipient, a federal- and state-level policy advocate for organ donation, and a student at Johns Hopkins Bloomberg School of Public Health who left the world too early on June 19, 2020. She will be remembered by her colleagues for her unyielding enthusiasm and selfless energy. The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. The data reported here have been supplied by the Hennepin County Research Institute as the contractor for the SRTR. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government.
Footnotes
Deceased June 19, 2020.
Published online 7 September, 2021.
Data are available by request from the Scientific Registry of Transplant Recipients (SRTR).
A.H., J.D.M., A.F., R.S.H., E.L.B., J.S., J.M.G.-W., D.L.S., and A.B.M. participated in project concept and design. A.H., J.D.M., A.F., J.M.G.-W., D.L.S., and A.B.M. participated in data acquisition, analysis, and interpretation. A.H. and J.D.M. drafted the article. A.F., R.S.H., E.L.B., J.S., J.M.G.-W., D.L.S., and A.B.M. provided critical article revisions for important intellectual content. J.M.G.-W., D.L.S., and A.B.M. provided funding and project supervision. All authors gave final approval of the article being submitted for publication. A.H. and J.D.M. have contributed equally to this article.
The authors declare no conflicts of interest.
This research was made possible with generous support of the Ben-Dov family. This work was supported by grant numbers K01DK101677 (A.B.M.), K24DK101828 (D.L.S.), and T32DK007732 (A.H.) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
REFERENCES
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. [DOI] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyarsky BJ, Werbel WA, Durand CM, et al. Early national and center-level changes to kidney transplantation in the United States during COVID-19 epidemic. Am J Transplant. 2020;20:3131–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2018 Annual Data Report: Lung. Am j Transplant. 2020;20(Suppl s1):427–508. [DOI] [PubMed] [Google Scholar]
- 6.Colvin M, Smith JM, Hadley N, et al. OPTN/SRTR 2018 Annual Data Report: Heart. Am j Transplant. 2020;20(Suppl s1):340–426. [DOI] [PubMed] [Google Scholar]
- 7.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2018 Annual Data Report: Kidney. Am J Transplant. 2020;20(s1):20–130. [DOI] [PubMed] [Google Scholar]
- 8.Li F, Cai J, Dong N. First cases of COVID-19 in heart transplantation from China. j Heart Lung Transplant. 2020;39:496–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernández-Ruiz M, Andrés A, Loinaz C, et al. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant. 2020;20:1849–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren Z-L, Hu R, Wang Z-W, et al. Epidemiological and clinical characteristics of heart transplant recipients during the 2019 coronavirus outbreak in Wuhan, China: a descriptive survey report. J Hear Lung Transplant. 2020;39:412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Latif F, Farr MA, Clerkin KJ, et al. Characteristics and outcomes of recipients of heart transplant with coronavirus disease 2019. jama Cardiol. 2020;5:1165–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holzhauser L, Lourenco L, Sarswat N, et al. Early experience of COVID-19 in 2 heart transplant recipients: case reports and review of treatment options. Am J Transplant. 2020;20:2916–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ketcham SW, Adie SK, Malliett A, et al. Coronavirus disease-2019 in heart transplant recipients in Southeastern Michigan: a case series. j Card Fail. 2020;26:457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Dai H, Xie X. Solid organ transplantation during the COVID-19 pandemic. Front Imunnol. 2020;11:1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koczulla RA, Sczepanski B, Koteczki A, et al. SARS-CoV-2 infection in two patients following recent lung transplantation. Am J Transplant. 2020;20:2928–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcault C, Fodil S, Dupont T, et al. Solid organ transplant recipients during COVID-19 pandemic. Am j Transplant. 2020;20:2960–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stachel MW, Gidea CG, Reyentovich A, et al. COVID-19 pneumonia in a dual heart-kidney recipient. j Heart Lung Transplant. 2020;39:612–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keller BC, Le A, Sobhanie M, et al. Early COVID-19 infection after lung transplantation. Am j Transplant. 2020;20:2923–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tschopp J, L’Huillier AG, Mombelli M, et al. ; Swiss Transplant Cohort Study (STCS). First experience of SARS-CoV-2 infections in solid organ transplant recipients in the Swiss Transplant Cohort Study. Am j Transplant. 2020;20:2876–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aigner C, Dittmer U, Kamler M, et al. COVID-19 in a lung transplant recipient. J Heart Lung Transplant. 2020;39:610–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myers CN, Scott JH, Criner GJ, et al. ; Temple University COVID-19 Research Group. COVID-19 in lung transplant recipients. Transpl Infect Dis. 2020;22:e13364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cozzi E, Faccioli E, Marinello S, et al. COVID-19 pneumonia in lung transplant recipients: report of 2 cases. Am J Transplant. 2020;20:2933–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morlacchi LC, Rossetti V, Gigli L, et al. COVID-19 in lung transplant recipients: a case series from Milan, Italy. Transpl Infect Dis. 2020;22:e13356. [DOI] [PubMed] [Google Scholar]
- 24.Kadosh BS, Pavone J, Wu M, et al. Collapsing glomerulopathy associated with COVID-19 infection in a heart transplant recipient. J Heart Lung Transplant. 2020;39:855–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jang K, Khatri A, Majure DT. COVID-19 leading to acute encephalopathy in a patient with heart transplant. J Heart Lung Transplant. 2020;39:853–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fishman JA, Grossi PA. Novel Coronavirus-19 (COVID-19) in the immunocompromised transplant recipient: #Flatteningthecurve. Am j Transplant. 2020;20:1765–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahluwalia M, Givertz MM, Mehra MR. A proposed strategy for management of immunosuppression in heart transplant patients with COVID-19. Clin Transplant. 2020;34:e14032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massie AB, Kucirka LM, Segev DL.Big data in organ transplantation: registries and administrative claims. Am J Transplant. 2014;14:1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strauss AT, Boyarsky BJ, Garonzik-Wang JM, et al. Liver transplantation in the United States during the COVID-19 pandemic: national and center-level responses. Am J Transplant. 2021;21:1838–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74:829–836. [Google Scholar]
- 31.Boyarsky BJ, Chiang TP-Y, Werbel WA, et al. Early impact of COVID-19 on transplant center practices and policies in the United States. Am j Transplant. 2020;20:1809–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyarsky BJ, Ruck JM, Chiang TP-Y, et al. Evolving impact of COVID-19 on transplant center practices and policies in the United States. Clin Transplant. 2020;34:e14086. [DOI] [PubMed] [Google Scholar]