Normothermic machine perfusion has emerged as a method of assessing livers before implantation and permits livers to be split ex vivo while perfused under normothermic conditions. This combines the advantages of both ex vivo (convenience) and in situ techniques (shorter cold ischemic time).1,2 Liver splitting during normothermic perfusion with continuous perfusion and survival of both grafts beyond 6 h has never been described.3,4 We report an ex vivo split performed during normothermic machine perfusion followed by continuous perfusion of both grafts until they became clearly nonviable at 6 d.
A 65-y-old liver weighing 2200 g was procured by the donation after circulatory death pathway following an out-of-hospital cardiac arrest. The liver was declined for transplantation according to donation after circulatory death acceptance criteria at our center and consented for research. This was approved by the Sydney Local Health District Ethics Review Committee (HREC/18/RPAH/748/X18-0523). After a cold ischemic time of 315 min, the liver was connected to the Liver Assist perfusion machine (Organ Assist, Groningen, the Netherlands), which we modified by adding a dialysis membrane (Prismaflex, Baxter, Illinois) and long-term oxygenators (Quadrox-iD Pediatric, Macquet, Getinge Group, Rastatt, Germany). Nutritional support included infusions of taurocholic acid (7.7 mg/h), methylprednisolone (50 mg/24 h), parenteral nutrition, insulin, and glucagon.
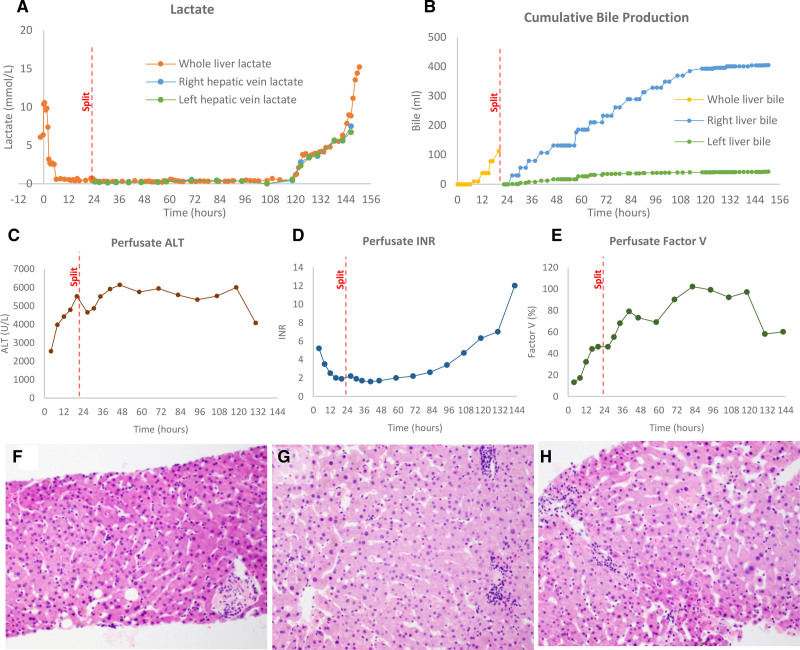
After 20 h of normothermic perfusion (36°C) using a red-cell–based perfusate anticoagulated with heparin, the lactate was 0.68, arterial pH 7.30, glucose 9 mmol/L, bile pH 7.25, and the cumulative bile volume 40 mL. With these results, the liver was assessed as suitable for splitting. Our predetermined criteria were lactate <2.5 mmol/L and ≥2 of the following: bile production, arterial pH >7.3, evidence of glucose metabolism, homogeneous perfusion, and bile pH >7.4.5 The split was performed by dissection and isolation of the left hepatic artery, left portal vein, and left hepatic vein with the liver in the prone position (caudate lobe and hilum facing upwards). Parenchymal transection was performed 1 cm to the right side of the falciform ligament using a harmonic scalpel (Ethicon, Cincinnati, Ohio). A Y-connector was added to the hepatic artery and portal vein to facilitate perfusion of both lobes in the same machine. Perfusion was reestablished by cannulation of the left hepatic artery and left portal vein after arterial and portovenous ischemic times of 5 min and 4 min, respectively. The total surgical time was 140 min. At experiment completion, the left lateral segment graft weighed 333 g, and the extended right graft weighed 1452 g. Organ viability was demonstrated before and after the split by stable liver biochemistry, production of bile, and coagulation factors (Figure 1A–E). Histological assessment of biopsies demonstrated viable hepatocytes in all sections without evidence of perivenular necrosis or loss of hepatocyte cohesion (Figure 1F–H).
FIGURE 1.
Perfusate and tissue markers of liver function before and after liver splitting during normothermic ex vivo machine perfusion of human livers. A, Lactate from packed red cells was rapidly cleared at the start of perfusion and maintained at a low level until 120 h of ex vivo perfusion. This was followed by a rapid decline parallel to death of both grafts. The lactate from individual grafts was sampled directly from respective hepatic veins. B, Bile was produced at a constant rate by the whole liver until splitting and then by each lobe individually. Bile flow slowed and ceased first in the left lobe and then the right lobe as the graft reached 120 h. C, Alanine aminotransferase (ALT) remained high throughout ex vivo perfusion. D and E, Indirect (international normalized ratio [INR]) and direct markers (factor V) of liver synthetic function demonstrated functional liver during perfusion before ceasing from 120 h of perfusion. F–H, Hematoxylin and eosin staining of core biopsies taken presplitting (F), and postsplitting from the right graft (G) and the left graft (H) (original objective ×20). In all sections, there are occasional apoptotic hepatocytes but no evidence of perivenular necrosis or loss of hepatocyte cohesion consistent with liver viability before and after splitting.
This is the first liver split performed during ex vivo normothermic perfusion to demonstrate prolonged viability (>6 d) and continuous perfusion of both grafts. This technique not only provides a potential model for comparing therapeutics and regeneration but also provides the opportunity to perform viability testing on each graft. Current methods of assessing graft viability ex vivo remain imperfect, and at present, the only definite way to confirm graft viability is through clinical transplantation. Future research should focus on identifying novel methods of ex vivo viability assessment and understanding why these grafts became nonviable after 120 h. A study to assess individual graft function using prolonged ex vivo perfusion on separate machines after splitting is currently underway to investigate these questions.
Footnotes
Published online 7 September, 2021.
The authors declare no funding or conflicts of interest.
N.-S.L. participated in research design, wrote the article, performed the research, and analyzed the data. M.L. participated in research design and performed the research. A.J. participated in research design. K.E., N.M., A.A., and N.K. performed the research. J.K. participated in the research design and analyzed the data. K.L., G.M., and M.C. participated in research design and reviewed the article. C.P. participated in research design, analyzed the data, and reviewed the article.
REFERENCES
- 1.Stephenson BTF, Bonney GK, Laing RW, et al. Proof of concept: liver splitting during normothermic machine perfusion. J Surg Case Rep. 2018;3:rjx218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brockmann JG, Vogel T, Coussios C, et al. Liver splitting during normothermic organ preservation. Liver Transpl. 2017;23:701–706. [DOI] [PubMed] [Google Scholar]
- 3.Attard JA, Osei-Bordom D-C, Boteon Y, et al. Ex situ normothermic split liver machine perfusion: protocol for robust comparative controls in liver function assessment suitable for evaluation of novel therapeutic interventions in the pre-clinical setting. Front Surg. 2021;8:627332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang V, Karimian N, Detelich D, et al. Split-liver ex situ machine perfusion: a novel technique for studying organ preservation and therapeutic interventions. J Clin Med. 2020;9:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mergental H, Laing RW, Kirkham AJ, et al. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat Commun. 2020;11:2939. [DOI] [PMC free article] [PubMed] [Google Scholar]