PURPOSE
Adjuvant chemotherapy after D2 gastrectomy is standard for resectable locally advanced gastric cancer (LAGC) in Asia. Based on positive findings for perioperative chemotherapy in European phase III studies, the phase III PRODIGY study (ClinicalTrials.gov identifier: NCT01515748) investigated whether neoadjuvant docetaxel, oxaliplatin, and S-1 (DOS) followed by surgery and adjuvant S-1 could improve outcomes versus standard treatment in Korean patients with resectable LAGC.
PATIENTS AND METHODS
Patients 20-75 years of age, with Eastern Cooperative Oncology Group performance status 0-1, and with histologically confirmed primary gastric or gastroesophageal junction adenocarcinoma (clinical TNM staging: T2-3N+ or T4Nany) were randomly assigned to D2 surgery followed by adjuvant S-1 (40-60 mg orally twice a day, days 1-28 every 6 weeks for eight cycles; SC group) or neoadjuvant DOS (docetaxel 50 mg/m2, oxaliplatin 100 mg/m2 intravenously day 1, S-1 40 mg/m2 orally twice a day, days 1-14 every 3 weeks for three cycles) before D2 surgery, followed by adjuvant S-1 (CSC group). The primary objective was progression-free survival (PFS) with CSC versus SC. Two sensitivity analyses were performed: intent-to-treat and landmark PFS analysis.
RESULTS
Between January 18, 2012, and January 2, 2017, 266 patients were randomly assigned to CSC and 264 to SC at 18 Korean study sites; 238 and 246 patients, respectively, were treated (full analysis set). Follow-up was ongoing in 176 patients at data cutoff (January 21, 2019; median follow-up 38.6 months [interquartile range, 23.5-62.1]). CSC improved PFS versus SC (adjusted hazard ratio, 0.70; 95% CI, 0.52 to 0.95; stratified log-rank P = .023). Sensitivity analyses confirmed these findings. Treatments were well tolerated. Two grade 5 adverse events (febrile neutropenia and dyspnea) occurred during neoadjuvant treatment.
CONCLUSION
PRODIGY showed that neoadjuvant DOS chemotherapy, as part of perioperative chemotherapy, is effective and tolerable in Korean patients with LAGC.
INTRODUCTION
Adjuvant treatment for locally advanced gastric cancer (LAGC) has evolved over two decades. After much debate, the efficacy of this approach was established in four pivotal trials1-4; however, standard adjuvant treatment differs regionally. Standard of care is postoperative chemoradiation in North America, based on the US intergroup study,3 perioperative chemotherapy (epirubicin plus cisplatin plus fluorouracil) in Europe, based on the MAGIC trial,2 and postoperative S-1 or capecitabine plus oxaliplatin (CAPOX) in Eastern Asia, based on the ACTS-GC4 and CLASSIC trials.1 Considerable effort has been invested in improving adjuvant treatment outcomes in each region, primarily by intensifying chemotherapy.5-8 Alternative approaches involve modifying adjuvant strategies used elsewhere, for example, adding radiation to adjuvant chemotherapy in Asia9 and Europe.10 Three studies have shown chemotherapy intensification to be beneficial.11-13 FLOT4 demonstrated that perioperative docetaxel, oxaliplatin, and fluorouracil (FLOT) was superior to epirubicin plus cisplatin plus fluorouracil,11 whereas JACCRO GC-0713 and ARTIST 212 showed that intensified postoperative adjuvant chemotherapy regimens were superior to standard regimens in patients with advanced disease.
CONTEXT
Key Objective
There is no global standard adjuvant strategy for patients with locally advanced gastric cancer (LAGC). Perioperative chemotherapy, widely used in the United States and Europe, is not standard of care in Asia. We designed the phase III PRODIGY study to investigate whether neoadjuvant docetaxel, oxaliplatin, and S-1 followed by surgery and adjuvant S-1 (CSC) could improve outcomes versus standard surgery followed by adjuvant S-1 (SC) in Korean patients with LAGC.
Knowledge Generated
Adding neoadjuvant chemotherapy to standard D2 surgery plus adjuvant chemotherapy was beneficial in this setting: progression-free survival was statistically significantly improved for CSC- versus SC-treated patients and hazard ratios favored CSC in most subgroups. Statistically significant downstaging was observed in the CSC arm. Neoadjuvant treatment was well tolerated, treatment-related hospitalizations were few, and mortality was low.
Relevance
This trial establishes perioperative chemotherapy as an appropriate new standard-of-care option for patients with LAGC in Asia, analogous to the therapeutic approach commonly used in Western countries.
Unlike the United States and Europe, neoadjuvant chemotherapy is not currently standard for LAGC in Korea. Our earlier phase II study showed that neoadjuvant docetaxel, oxaliplatin, and S-1 (DOS) is feasible in terms of tolerability and resection rate in Korean patients with potentially resectable LAGC.14 We designed the PRODIGY study to investigate whether neoadjuvant chemotherapy with DOS followed by surgery and adjuvant S-1 chemotherapy (CSC) could improve outcomes in Korean patients with resectable LAGC versus up-front surgery followed by adjuvant S-1 (SC).
PATIENTS AND METHODS
Study Design and Participants
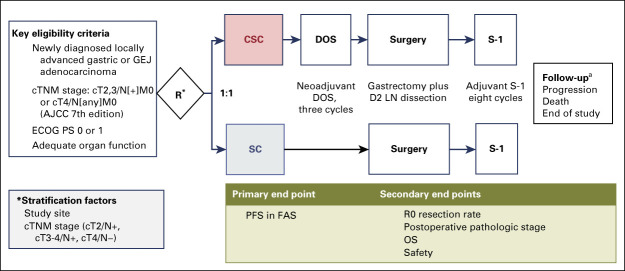
PRODIGY was a phase III, open-label, randomized study of neoadjuvant DOS followed by surgery plus adjuvant S-1 versus surgery followed by adjuvant S-1 in patients with resectable advanced gastric cancer (Appendix Fig A1, online only).
Eligible patients were 20-75 years of age, with Eastern Cooperative Oncology Group performance status 0-1, and a new histologically confirmed primary gastric or gastroesophageal junction adenocarcinoma that was locally advanced but amenable to curative resection, that is, clinical TNM staging cT2-3N+ or cT4Nany stage (American Joint Committee on Cancer [AJCC] 7th Edition).
The study was approved by ethics committees or institutional review boards at participating institutions. All patients provided written informed consent.
Patients and investigators were not blinded to the treatment received; the Independent Data Monitoring Committee (IDMC) monitored safety data and evaluated effectiveness at the interim analysis.
Procedures
Patients were randomly assigned (1:1) to CSC or SC by interactive web-response system according to computer-generated random assignment list. Random assignment was stratified by site and cTNM staging (cT2/N+, cT3-4/N+, cT4/N−), performed using computed tomography (CT) alone; positron-emission tomography and laparoscopy were used if needed to ensure no distant metastases. Baseline CT scans were uploaded to a website and reviewed by a central reviewer (J.S.L.) to assign clinical TNM stage before random assignment and determine eligibility and stratification.
CSC patients began neoadjuvant treatment within 7 days of random assignment. CSC treatment was docetaxel (Aventis Pharma, Dagenham, UK) 50 mg/m2 and oxaliplatin (Aventis Pharma, Dagenham, UK) 100 mg/m2 intravenously on day 1, with S-1 (Taiho Pharmaceutical Co Ltd, Japan) 40 mg/m2 orally twice a day on days 1-14 every 3 weeks for three cycles. Cycles were delayed and doses modified as described in Appendix Table A1 (online only), based on CBCs performed at the start of each cycle and toxicities reported during the previous cycle. Standard surgery was D2 gastrectomy 1-3 weeks after neoadjuvant chemotherapy (CSC group) or within 2 weeks of random assignment (SC group). Both groups received adjuvant S-1 40-60 mg orally twice a day depending on body surface area (BSA) on days 1-28 every 6 weeks for eight cycles. Adjuvant therapy continued unless patients met treatment discontinuation criteria (Appendix Table A1). Patients were followed for safety for ≥ 30 days after the last investigational product dose.
For CSC patients, tumor response was evaluated by additional preoperative abdominal-pelvic CT before cycle 2 and after cycle 3. If tumor progression was demonstrated during the neoadjuvant period, treatment was discontinued; surgery or another anticancer treatment could be initiated at the investigator's discretion.
The goal of surgery was R0 resection, defined as curative resection of gastric primary lesions and regional lymph nodes without evidence of distant metastasis or residual tumor cells grossly and at resection margin. Postoperative disease stage and R0 resection rate were confirmed using AJCC cancer staging criteria (7th Edition). Tumor assessment was conducted as follows: physical examination every 3 months for the first year and every 6 months thereafter; abdominal-pelvic CT every 6 months; and esophagogastroduodenoscopy every 12 months. If progressive disease (PD) was suspected, additional evaluation could be performed irrespective of the relevant period. Follow-up continued as described above until death or study closing date, whichever was earlier.
Outcomes
The primary objective was to compare progression-free survival (PFS) for CSC versus SC. Secondary objectives were to compare overall survival (OS), postoperative pathologic stage, R0 resection rate, and safety in the two groups.
PFS was defined as PD or death, with PD defined as follows: (1) in the CSC arm only, RECIST PD during neoadjuvant chemotherapy, and (2) in both the CSC and SC arms, (a) finding of distant metastasis or reporting of distant metastasis from pathology irrespective of intraoperative curative resection; (b) persistence of visually observed cancer cells at resection margin (R2) or microscopic cancer cells at resection margin from postoperative histology (R1) that could not be further removed; or (c) recurrence, either local or at distant sites, during follow-up after R0 resection (Appendix 2, online only).
Adverse events (AEs), hematologic toxicities, clinical examination (physical examination, blood pressure, BSA, body weight, and Eastern Cooperative Oncology Group performance status), special tests (chest x-ray and ECG), and laboratory data were collected. AEs were recorded using National Cancer Institute Common Toxicity Criteria for Adverse Events (version 4.03); toxicity data were collected at postbaseline visits.
Statistical Analysis
The intent-to-treat analysis included all randomly assigned patients. The full analysis set (FAS) included all randomly assigned patients satisfying inclusion or exclusion criteria. CSC patients who started neoadjuvant chemotherapy but could not have tumor evaluation (eg, because of toxic death) were included in the FAS to avoid bias. The safety analysis set included patients with ≥ 1 dose of neoadjuvant DOS (for the safety set of neoadjuvant chemotherapy in the CSC arm), all patients who underwent surgery (for the safety set of surgery in both the CSC and SC arms), and adjuvant S-1 chemotherapy (for the safety set of adjuvant S-1 in both the CSC and SC arms). Medication compliance or administration and all clinical safety data were summarized using the safety analysis set.
Based on assumption of 3-year PFS of 70% in the CSC arm and 60% in the SC arm (ie, hazard ratio [HR] = 0.698), 244 events and ≥ 238 patients per group were required for comparison of PFS with 80% power and an alpha of .05. Given an estimated 10% dropout rate, 530 patients were required. One interim efficacy analysis was planned after 135 events and a final efficacy analysis after median follow-up of > 3 years and 244 PFS events. The statistical analysis plan is described in Appendix 2.
Two sensitivity analyses were planned: analysis on the intent-to-treat population to assess whether excluding patients with no treatment after random assignment affected study findings; and a landmark PFS analysis in which death and progression before the landmark time (6 months after random assignment) were defined as events at the landmark time.
The IDMC periodically monitored the safety of patients exposed to investigational product and assessed efficacy at the interim analysis.
Statistical analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC).
This trial is registered with ClinicalTrials.gov (identifier NCT01515748).
RESULTS
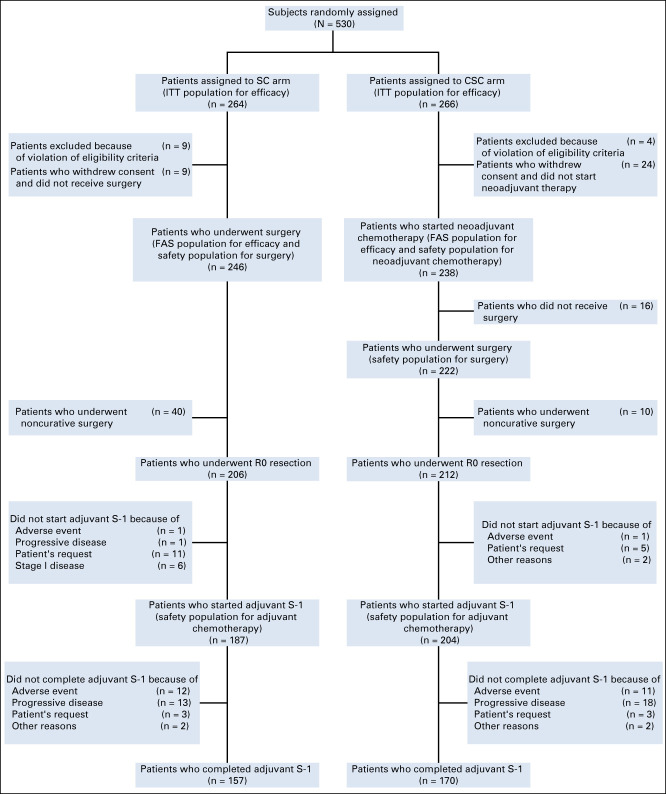
A total of 693 patients were recruited at 18 Korean hospitals between January 18, 2012, and January 2, 2017, 163 of whom were screening failures; the intent-to-treat population comprised 530 patients. Of these, 266 were randomly assigned to CSC and 264 to SC. Forty-six patients were excluded from the FAS: 33 withdrew consent after random assignment, nine in the SC arm and 24 in the CSC arm. Thirteen patients did not satisfy eligibility criteria, mainly because of inadequate organ function for chemotherapy (SC, n = 9; CSC, n = 4). The FAS comprised 484 patients, 238 in the CSC group and 246 in the SC group. The trial profile is shown in Figure 1.
FIG 1.
CONSORT diagram showing the study disposition. CSC, neoadjuvant chemotherapy plus surgery plus adjuvant chemotherapy; FAS, full analysis set; ITT, intent-to-treat; SC, surgery plus adjuvant chemotherapy.
Demographic and clinical characteristics of FAS patients are shown in Table 1. The CSC and SC groups were generally comparable. Gastroesophageal junction primary tumors were uncommon. Also notable was clinical stage: cT4 was the most common T stage and cN0 was rare; most patients were clinical stage III and relatively few were clinical stage II.
TABLE 1.
Baseline Demographics and Disease Characteristics for the Full Analysis Set

Neoadjuvant Chemotherapy
Overall, 214 (89.9%) of 238 CSC patients received all three cycles of DOS. The mean (±standard deviation [SD]) relative dose intensities were 95.1% (±8.5%) for docetaxel, 95.2% (±8.5%) for oxaliplatin, and 89.6% (±15.0%) for S-1. Reasons for not completing neoadjuvant therapy were AEs (n = 13; 5.5%), PD (n = 2; 0.8%), patient request (n = 3; 1.3%), and other reasons (n = 6; 2.5%). Five (2.1%) of 238 patients had PD as a response to neoadjuvant therapy.
AEs occurring during neoadjuvant chemotherapy are shown in Appendix Table A2 (online only). Grade ≥ 3 treatment-emergent AEs included neutropenia (30 of 238 patients; 12.6%), febrile neutropenia (n = 22; 9.2%), and diarrhea (n = 12; 5.0%). Two grade 5 AEs (febrile neutropenia and dyspnea) occurred during neoadjuvant treatment.
Surgery
The details of surgical procedures are summarized in Table 2. Sixteen patients who started neoadjuvant chemotherapy did not undergo surgery for the following reasons: consent withdrawal (n = 6), death (n = 4), PD during neoadjuvant therapy (n = 3), lost to follow-up (n = 2), or AE before surgery (n = 1). The median time to surgery from study entry was 1.9 weeks in the SC group and 11.6 weeks in the CSC group; the median time to surgery from completion of neoadjuvant chemotherapy was 1.71 weeks. Among patients who had surgery, the R0 resection rate was 95% with CSC (212 of 222 patients) versus 84% with SC (206 of 246 patients); in the FAS, the R0 resection rate was 89% with CSC (212 of 238 patients) versus 84% with SC (206 of 246 patients). D2 lymph node dissection rates were similar in both arms. Clinically significant (grade ≥ 3) surgery-related complications were uncommon, and there were no differences between the two groups in complications and hospital stays. One surgery-related death (pulmonary embolism) and one death not related to surgery occurred in the CSC arm.
TABLE 2.
Surgery Undertaken in the Full Analysis Set
Postoperative pathology findings for the 468 patients who underwent surgery are shown in Table 3. Patients receiving CSC had a pathologic complete response rate of 10.4% (23 of 222 patients), with significantly more tumor downstaging versus SC (P < .0001).
TABLE 3.
Postoperative Pathology Findings (patients who underwent surgery)

Adjuvant Chemotherapy
Overall, 391 of the 418 patients with an R0 resection (SC, n = 206; CSC, n = 212) received adjuvant chemotherapy. Reasons for not starting adjuvant chemotherapy in the SC arm were AE (n = 1 [0.5%]; surgery-related GI anastomotic leak), PD (n = 1; 0.5%), patient's request (n = 11; 5.3%), and other reasons (n = 6; 2.9%). In the CSC arm, reasons were AE (n = 1 [0.5%]; pulmonary embolism, unknown association with surgery), patient's request (n = 5; 2.3%), and other reasons (n = 2; 0.9%).
Adjuvant chemotherapy was delayed by > 6 weeks because of vomiting in one CSC patient. More SC than CSC patients received no adjuvant chemotherapy as this is not standard of care for SC patients with pathologic stage I disease.
A total of 157 (84.0%) of 187 SC patients and 170 (83.3%) of 204 CSC patients who started adjuvant chemotherapy completed all eight cycles. Reasons for not completing adjuvant chemotherapy were PD or death (13 [7.0%] of 187 SC patients; 18 [8.8%] of 204 CSC patients), AEs (12 SC patients [6.4%]; 11 CSC patients [5.4%]), patient request (three SC patients [1.6%]; three CSC patients [1.5%]), and other reasons (two SC patients [1.1%]; two CSC patients [1.0%]). The mean (±SD) relative S-1 dose intensity delivered was 86.0% (± 9.5%) in the SC group and 84.0% (± 11.1%) in the CSC group.
AEs occurring during adjuvant therapy are summarized in Appendix Table A3 (online only). The most common grade ≥ 3 AE was neutropenia, which occurred in 10 of 187 patients (5.3%) in the SC safety population and 13 of 204 patients (6.4%) in the CSC safety population; febrile neutropenia occurred in one (0.5%) of 187 SC patients and was not observed in the CSC group. There was no adjuvant chemotherapy-related mortality.
Treatment Outcomes
At the interim analysis (May 31, 2016), the between-group difference did not reach the prespecified significance threshold (.0031) and the study continued. Fewer PFS events than expected were observed, and the IDMC recommended protocol revision to allow final analysis after the median follow-up of 3 years was reached. In the final analysis, the adjusted alpha was .049 after 183 PFS events.
After median follow-up of 38.6 (interquartile range, 23.5-62.1) months and 183 PFS events, PFS was significantly superior in the CSC arm (HR for PFS adjusted for stratification factors, 0.70; 95% CI, 0.52 to 0.95; stratified log-rank P =.023; Fig 2A). Three-year PFS rates were 66.3% (95% CI, 59.6 to 72.1) with CSC and 60.2% (95% CI, 53.6 to 66.3) with SC. Sensitivity analyses confirmed these findings in the intent-to-treat population PFS analysis (HR, 0.69; 95% CI, 0.51 to 0.93; P = .016; Appendix Fig A2A, online only) and the 6-month landmark PFS analysis (HR, 0.74; 95% CI, 0.54 to 1.00; P = .043; Appendix Fig A2B). Similar results were observed in most subgroups (Fig 3).
FIG 2.
Kaplan-Meier survival estimates in the full analysis set: (A) progression-free survival and (B) preliminary overall survival. CSC, neoadjuvant chemotherapy plus surgery plus adjuvant chemotherapy; HR, hazard ratio; SC, surgery plus adjuvant chemotherapy.
FIG 3.
Progression-free survival analyses for subgroups in the full analysis set. CSC, neoadjuvant chemotherapy plus surgery plus adjuvant chemotherapy; GEJ, gastroesophageal junction; HR, hazard ratio; SC, surgery plus adjuvant chemotherapy.
OS was not statistically significantly better in CSC versus SC patients (HR, 0.84; 95% CI, 0.60 to 1.19; P = .338; Fig 2B). Three-year OS was 74.2% (95% CI, 67.7 to 79.6) with CSC and 73.4% (95% CI, 67.0 to 78.7) with SC. OS results were generally consistent across patient subgroups (Appendix Fig A3, online only).
DISCUSSION
The phase III PRODIGY study has shown the benefit of adding neoadjuvant chemotherapy to standard D2 surgery plus adjuvant chemotherapy in Asian patients with resectable LAGC. The study met its primary end point: PFS was statistically significantly improved in CSC- versus SC-treated patients and HRs favored CSC in most subgroups. Notably, neoadjuvant therapy benefit appeared greatest in patients with more advanced disease. Statistically significant downstaging was observed in all categories in the CSC arm versus SC (all P < .0001). Neoadjuvant treatment-related hospitalizations were few and mortality was low in PRODIGY. The toxicity of DOS in PRODIGY was lower than in the previous phase II trial, likely because of the protocol specifying CBCs at the start of every 3-week cycle to ensure adequate recovery of bone marrow function before the next cycle, in contrast to weekly monitoring in the phase II study to capture nadir absolute neutrophil and platelet counts.14
The neoadjuvant treatment given to the CSC group—dose-intensive three-drug DOS regimen—is likely responsible for the activity of the regimen. Three-drug FLOT has become a new standard perioperative regimen in Europe based on results from FLOT4.11,15 In the phase II part of that study, FLOT gave a pathologic complete response rate of 16%, comparable with the 14.6% reported by Park et al14 for their phase II study of preoperative DOS. Although these data indicate that docetaxel-containing triplet chemotherapy is a promising neoadjuvant regimen, care is needed to ensure tolerability in Asian patients, who are more vulnerable than White patients to myelosuppression caused by docetaxel.16 Asian patients may not find the higher docetaxel dose intensity in FLOT as tolerable as the DOS regimen used in this study. Although comparison of results across studies performed under different conditions, using different schedules, and in different patient populations is made with caveats, apparently conflicting data have been reported for neoadjuvant oral fluoropyrimidine plus platinum doublets. Addition of neoadjuvant S-1 plus cisplatin to standard D2 surgery plus adjuvant S-1 failed to show a benefit in the Japanese phase III JCOG0501 trial, in which most patients had type 4 gastric cancer.17 By contrast, however, patients treated with perioperative S-1 plus oxaliplatin (SOX) in RESOLVE had significantly improved 3-year disease-free survival versus postoperative CAPOX (62% v 55%; HR, 0.79; 95% CI, 0.62 to 0.99; P = .045).18 This difference might be attributed to JCOG0501 using two cycles of S-1 (40-60 mg orally twice a day on days 1-21) plus cisplatin (60 mg/m2 on day 8) every 4 weeks, whereas the neoadjuvant regimen in RESOLVE comprised three cycles of SOX (S-1 40-60 mg orally twice daily on days 1-14 plus oxaliplatin 130 mg/m2 intravenously on day 1, every 3 weeks), thereby delivering more cycles and higher platinum dose intensity than JCOG0501. These findings further support the use of neoadjuvant therapy in Asian patients with LAGC.
It should be noted that PRODIGY was not powered to observe a statistically significant difference in OS as this was not the primary end point. Based on the current number of OS events, the observed power is only 17%. Furthermore, we could not achieve the planned number of PFS events because inclusion of patients with early-stage disease and a better prognosis than expected, owing to inaccurate clinical staging, reduced the power of the study. Clinical-stage overestimation has been reported by others: Fukagawa et al19 conducted a prospective study of preoperative diagnostic criteria in the JCOG1302A study to evaluate the accuracy of clinical staging. They concluded that specification of cT3-4 and cN1-3 disease rather than cT3/T4 tumors would maximize inclusion of patients with stage III disease and minimize inclusion of those with stage I disease, an approach used in the JCOG1509 study.20 Efforts should be made, including clinical staging with CT scans, to rigorously enroll patients with more advanced clinical stage disease and avoid recruiting patients with early-stage disease who are better treated with surgery alone avoiding the toxicity of neoadjuvant chemotherapy.
Defining PD in neoadjuvant LAGC studies with PFS as the primary end point is challenging due, in part at least, to the fact that peritoneal seeding is not easily visualized using CT scans. Identification at surgery of distant metastasis missed in earlier CT scans precluded curative gastrectomy and only allowed for palliative surgery (bypass) or open and closure, necessitating a change in subsequent therapy for the patient that was not consistent with the planned treatment. The definition of PD in PRODIGY, although differing from other settings, is not without precedent as others have included incomplete resection as PD events in similar studies.21 Enhancing complete resection is one of the markers of efficacy associated with neoadjuvant chemotherapy and incomplete resection because of missed distant metastases as a PD event was more common in the SC arm, resulting in early separation of the PFS curves, an observation that was not changed in the 6-month landmark analysis.
The current standard adjuvant chemotherapy regimen in Asia is 1 year of adjuvant S-1 or 6 months of CAPOX.22 We used 1 year of S-1 as adjuvant chemotherapy as there was no evidence that CAPOX was better than S-1 and we believed adjuvant S-1 would be better tolerated than adjuvant CAPOX, especially following neoadjuvant chemotherapy and gastrectomy. Indeed, the tolerability of adjuvant chemotherapy was excellent and no new safety signals were observed. Notably, 84% of patients starting adjuvant S-1 completed eight cycles, similar to the completion rate in the phase II study14 and better than the ACTS-GC trial, in which only 66% of patients finished the planned 1 year of S-1 treatment.4 Moreover, in the FLOT4 study, 71% of patients starting adjuvant therapy in the epirubicin, cisplatin, plus capecitabine comparator group, and 76% of those in the FLOT group received all allocated cycles.11 The high completion of 1 year of S-1 in PRODIGY is primarily because of the tolerability of DOS and S-1 versus the FLOT regimen, and patients being better able to tolerate intensive chemotherapy regimens in the neoadjuvant setting than after gastrectomy.23 Well-tolerated neoadjuvant chemotherapy, therefore, need not negatively affect delivery of appropriate adjuvant chemotherapy. The recent positive results of the JACCRO GC-07 and ARTIST 2 studies,12,13 which showed that doublet regimens (docetaxel plus S-1 or SOX) are better than S-1 alone as adjuvant chemotherapy in patients with more advanced disease, suggest that the optimal adjuvant regimen after neoadjuvant DOS requires further investigation.
Some study limitations should be considered. Many patients with early-stage disease were included in PRODIGY although clinical-stage inclusion criteria used were similar to if not stricter than those used in MAGIC or FLOT4. Comparison of pathologic disease stage of patients enrolled in FLOT4 and those in PRODIGY is not possible as FLOT4 did not include an arm in which patients underwent surgery first. However, a difference is apparent in relapse-free survival results for the two studies, with PRODIGY having a better PFS rate at 3 years than FLOT4. Another limitation is that the HRs for PFS and absolute PFS benefit are small and OS results are immature. Finally, many adjuvant chemotherapy options remain to be explored, including those used in the recent JACCRO GC-07 and ARTIST 2 studies.
In conclusion, addition of neoadjuvant DOS to D2 gastrectomy and adjuvant S-1 led to significant tumor downstaging and improved PFS with acceptable safety in the PRODIGY study. These results suggest that this strategy should be considered a standard treatment for patients in Asia with resectable advanced gastric or gastroesophageal cancer. Importantly, the results of PRODIGY support one common treatment strategy—perioperative chemotherapy with surgery—for patients with LAGC in East Asia as well as in the West.
ACKNOWLEDGMENT
The authors would like to acknowledge the contribution of all the patients and their families, and study personnel who contributed to the PRODIGY study. They thank the members of the IDMC for overseeing the study conduct and Hana Cho, MD (Sanofi Korea), for her contribution to the study and development of the manuscript. Statistical analysis was conducted by Song Hee Han (LSK Global PS). Medical writing assistance was provided by Lee Miller and Deirdre Carman of Miller Medical Communications Ltd. A list of study centers in addition to those represented in the author list is provided in Appendix 1 (online only).
APPENDIX 1
Participating Study Centers and Principal Investigators
Kyung Hee University Hospital: Chi Hoon Maeng
Keimyung University Dongsan Medical Center: Jin Young Kim
Korea University Guro Hospital: Sang Cheul Oh
Kosin University Gospel Hospital: Sang Ho Lee
National Cancer Center: Young-Woo Kim
Dong-A University Hospital: Min Chan Kim
The Catholic University of Korea, Seoul St Mary's Hospital: In-Ho Kim
Asan Medical Center, University of Ulsan: Yoon-Koo Kang
Soon Chun Hyang University Hospital Seoul: Namsu Lee
Korea Institute of Radiological & Medical Sciences: Hang-Jong Yu
Severance Hospital, Yonsei University Health System: Jae-Ho Cheong
Ajou University Hospital: Jin-Hyuk Choi
Chungnam National University Hospital: Ji Young Sul
Kyungpook National University Chilgok Hospital: Jong Gwang Kim
Hallym University Sacred Heart Hospital: Dae Young Zang
Chonnam National University Hwasun Hospital: Young-Kyu Park
Hallym University Dongtan Sacred Heart Hospital: Dong Woo Shin
Kangbuk Samsung Hospital: Chang-Hak Yoo
APPENDIX 2
Supplemental Methods
Inclusion Criteria.
Patients had to have adequate organ function. Patients were considered lymph node-positive (N+) if, irrespective of the lymph node shape, the short axis was ≥ 8 mm or shortest diameter was ≥ 5 mm with central necrosis, round shape, perinodal infiltration, or prominent enhancement.
Exclusion Criteria.
Patients satisfying any of the following criteria could not be randomly assigned in this study:
Patients younger than 20 years or older than 76 years of age (inclusive).
Patients with the Eastern Cooperative Oncology Group performance status ≥ 2.
-
Patients with the medical history of gastric cancer (gastroesophageal junction included), including all of the following cases:
a. Patients who had surgery for gastric cancer (gastroesophageal junction included)
b. Patients who received adjuvant chemotherapy, or preoperative chemotherapy and/or radiotherapy and/or immunotherapy for treatment of gastric cancer (gastroesophageal junction included).
-
Patients with the medical history of other malignancy. However, patients with the following could be included in this study:
a. Adequately treated basal cell or squamous cell carcinoma and in situ cervical carcinoma
b. Other cancer that exceeded 5 years after completion of chemotherapy and remained disease-free for 5 years or more.
Patients with a distant metastasis (M1) including a distant lymph node (retro-pancreatic, para-aortic, periportal, retroperitoneal, and mesenteric lymph nodes) of gastric or gastroesophageal junction adenocarcinoma.
-
Patients who cannot undergo curative resection at the discretion of a surgeon.
a. Patients with T4b with completely resectable involvement of the surrounding organ with no distant metastasis could be enrolled.
Patients who participated in another study or administered another investigational product within 30 days before signing the Informed Consent Form.
Patients who had any of the following within 6 months before signing the Informed Consent Form: myocardial infarction, severe or unstable angina, coronary or peripheral artery bypass surgery, New York Heart Association Class III or IV congestive heart failure, stroke, or transient ischemic attack.
Patients who had deep vein thrombosis within 4 weeks before signing the Informed Consent Form.
Patients with a previous medical history of uncontrolled seizure, CNS, or psychologic disorder that is so clinically significant that it is impossible to obtain the Informed Consent Form or the severity may interfere with oral administration of medication.
Patients with uncontrolled active infection or sepsis, previously known acquired immune deficiency syndrome, HIV infection, or previously known active hepatitis B or C.
Patients with severe acute or chronic disease that may limit the ability to participate in the study or make it difficult to interpret the results of the study.
Patients who have not fully recovered from another procedure.
-
Patients who may experience a problem with absorption after oral administration of the investigational product, as follows:
a. Patients with intolerability with oral administration, malabsorption, or absorption disorder
b. Patients who have not recovered from the lack of physical completeness of the upper GI tract
c. Ileus
d. Chronic inflammatory bowel disease
e. Extensive small bowel resection and other diseases that limit drug absorption (eg, gastric dumping syndrome, rapid intestinal transit, and malabsorption after bowel surgery).
In cases of female patients of childbearing potential or male patients with a female partner of childbearing potential, patients who do not consent to use of generally accepted effective contraception during the investigational product administration period or for at least 6 months after completion of the investigational product administration.
Breastfeeding or pregnant women. Women of childbearing potential with a positive pregnancy test.
-
Inadequate bone marrow and organ function before administration of the investigational product:
a. Absolute neutrophil count < 1.5 × 109/L
b. Platelet count < 100 × 109/L
c. Hemoglobin ≤ 9 g/dL
d. AST > 2.5 × upper limit of normal (ULN); ALT > 2.5 × ULN
e. Alkaline phosphatase > 2.5 × ULN
f. Total bilirubin > 1.5 × ULN
g. Serum creatinine > 1.5 × ULN. (Creatinine clearance is calculated by using the Cockcroft-Gault formula with 24-hour urine collection; patients with creatinine clearance < 60 mL/min will be excluded.)
Peripheral neuropathy with grade ≥ 2 (National Cancer Institute Common Toxicity Criteria for Adverse Events [NCI CTCAE] version 4.03) clinical symptoms.
Grade ≥ 2 (NCI CTCAE version 4.03) hearing loss.
Grade ≥ 2 (NCI CTCAE version 4.03) severe tumor bleeding.
Medical history of hypersensitivity reaction to the investigational product (docetaxel, oxaliplatin, and S-1 [tegafur, gimeracil, and oteracil]).
Patients using immunosuppressants and prohibited concomitant medication.
Assessments
Assessments included chest x-ray, abdominal-pelvic computed tomography (CT) for clinical staging of advanced gastric cancer, and other tests to rule out M1 disease, conducted within 14 days before random assignment. Preoperative abdominal-pelvic CT was performed ≤ 5 days before cycle 2 and after completion of cycle 3 to follow the target lesion confirmed at baseline and identify signs of progressive disease (PD). Additional tumor assessments could be conducted at any time if disease progression was clinically suspected. Abdominal-pelvic CT after neoadjuvant chemotherapy was read by the central reviewer. Laparoscopy was undertaken if peritoneal seeding was suspected based on CT or physical examination. Clinical laboratory tests, conducted before each cycle, included hematology (hemoglobin, CBC, absolute neutrophil count, and platelet count) and blood chemistry (sodium, potassium, calcium, blood urea nitrogen, creatinine, creatinine clearance, total protein, albumin, ALT, AST, total bilirubin, alkaline phosphatase, and glucose).
Before the start of each 6-week cycle, patients underwent clinical examination, chest x-ray, and clinical laboratory safety examination.
Outcomes
PD was defined as follows according to RECIST (version 1.1). In the neoadjuvant chemotherapy plus surgery plus adjuvant chemotherapy (CSC) arm, PD was determined according to RECIST (version 1.1) during the neoadjuvant chemotherapy period. In the event of PD determination based on the sum of diameters of lesions, the date of such determination of PD was defined as the last tumor assessment date of the lesion. Beyond the neoadjuvant chemotherapy period, the same definition of PD applied to both groups. Irrespective of curative resection, if an intraoperative distant metastasis was observed, or a distant metastasis was reported from pathology, it was considered PD, and the date of surgery defined as the PD demonstration date. If residual cancer cells were finally confirmed at the resection margin during postoperative histology (R1), or if residual cancer cells were visually identified at the resection margin during surgery but could not be completely resected (R2), this was considered PD and the date of surgery defined as the PD demonstration date. In the event of finding a recurrence or distant metastasis or a new lesion during follow-up after R0 complete resection, this was defined as the first tumor assessment date when it was observed. For a patient determined to have PD, administration of the investigational product was discontinued according to permanent treatment discontinuation criteria as defined. These patients subsequently received standard treatment and were followed for survival or death. In case of R1 resection, one repeat surgery was allowed to achieve R0 resection; the outcome of this surgery determined the final resection status.
The time to progression was calculated until the first date of demonstration of progression.
Downstaging was determined not by comparison of baseline clinical stage and postoperative pathologic stage in the CSC arm, but by comparison of pathologic stages of patients in the surgery plus adjuvant chemotherapy and CSC arms. As patients were randomly assigned to one of these two arms, by comparing the postoperative pathologic stages of the two arms, we recognize that downstaging was achieved by neoadjuvant chemotherapy. There were, therefore, no criteria for downstaging, rather a statistical comparison of pathologic stages of patients in the two arms.
Pretreatment Schedule
Docetaxel.
Premedication was administered before docetaxel administration and included a glucocorticosteroid-class drug. Premedication was administered according to the practice at the relevant sites. Examples included oral administration of dexamethasone 8 mg in the evening before treatment (day 0), intravenous administration 30 minutes before docetaxel infusion (day 1), oral administration on the evening of day 1, or on the morning and evening of day 2.
Oxaliplatin.
To prevent nausea and vomiting, antiemetics (eg, 5-hydroxytryptamine3 antagonists) were administered with dexamethasone or methylprednisolone. Antiemetic administration was prescribed according to the practice at the relevant site.
Discontinuation Criteria
In the following cases, the patient's investigational product administration could be discontinued. However, unless the patient withdrew consent for participation in this study, assessment and follow-up were continuously performed:
The patient could ask for discontinuation of the investigational product administration at any time, irrespective of the reason. For a patient incapable of voluntary self-expression, administration of the study drug could be discontinued at the request of their legally acceptable representative.
-
If continuous administration of the investigational product could be harmful to the patient, at the discretion of the investigator, because of:
a. Disease progression
b. Unacceptable adverse event not controlled with symptomatic treatment, dose delay, or dose adjustment that interfered with subsequent administration of the investigational product
c. Finding of a second primary cancer during the study, so that the study-specific treatment could not be continued at the discretion of the investigator, and the patient agreed with this decision.
Intercurrent disease making it impossible to administer the investigational product.
Pregnancy.
At the special request of the sponsor.
If the patient was lost to follow-up.
Dose Modifications
Dose adjustment of the investigational product was allowed, based on the worst (nadir) grade of toxicity that occurred at any time during a cycle. The reduced dose was applied from the cycle with the event, and subsequent re-escalation was not allowed. Dose-adjustment criteria and reduced dose levels are shown in Appendix Table A1.
Statistical Analysis
Three safety reviews and one interim efficacy analysis were planned. The interim efficacy analysis was conducted after 135 progression-free survival (PFS) events, at which time the difference between groups did not reach the prespecified significance threshold of .0031; the Independent Data Monitoring Committee recommended study continuation. The final efficacy analysis was originally planned after a median follow-up of > 3 years and when 244 PFS events had occurred; however, fewer PFS events than expected were observed because of the inclusion of patients with early-stage disease and the Independent Data Monitoring Committee recommended protocol revision to allow the final analysis to be performed when either the specified number of PFS events had occurred or median follow-up was reached. This calculation was carried out by considering one interim analysis, using the group sequential approach with efficacy boundaries suggested by the O'Brien-Fleming alpha spending function. To compare PFS distribution between the two groups, a two-sided 5% significance level and up to 7.5 years of follow-up were assumed, including 4.5 years' enrollment period. PFS was compared between the two treatment groups using a log-rank test stratified according to site and TNM stage (cT4/N−, T2/N+, T3-4/N+; American Joint Committee on Cancer 7th Edition) specified at random assignment at the overall 5% significance level. Survival curves were estimated using the Kaplan-Meier method. Median PFS and corresponding 95% CIs, and 3-year PFS were presented by treatment group.
FIG A1.
Design of the PRODIGY study. aAbdominopelvic CT every 6 months and esophagogastroduodenoscopy every 1 year after surgery. AJCC, American Joint Committee on Cancer; CSC, neoadjuvant chemotherapy plus surgery plus adjuvant chemotherapy; CT, computed tomography; DOS, docetaxel, oxaliplatin, and S-1; ECOG PS, Eastern Cooperative Oncology Group performance status; FAS, full analysis set; GEJ, gastroesophageal junction; LN, lymph node; OS, overall survival; PFS, progression-free survival; R, random assignment; SC, surgery plus adjuvant chemotherapy.
FIG A2.
Sensitivity analyses of progression-free survival (A) for the ITT population and (B) at the 6-month landmark analysis. CSC, neoadjuvant chemotherapy plus surgery plus adjuvant chemotherapy; HR, hazard ratio; ITT, intent-to-treat; SC, surgery plus adjuvant chemotherapy.
FIG A3.
Subgroup analyses for overall survival in the full analysis set. CSC, neoadjuvant chemotherapy plus surgery plus adjuvant chemotherapy; GEJ, gastroesophageal junction; HR, hazard ratio; SC, surgery plus adjuvant chemotherapy.
TABLE A1.
Dose-Adjustment Criteria and Doses
TABLE A2.
Adverse Events Occurring in > 10% of Patients Undergoing Neoadjuvant Chemotherapy (n = 238)

TABLE A3.
Adverse Events Occurring in > 10% of Patients Undergoing Adjuvant Chemotherapy
Yoon-Koo Kang
Consulting or Advisory Role: DAEHWA Pharmaceutical, Bristol-Myers Squibb, Zymeworks, ALX Oncology, Amgen, Novartis, MacroGenics, Surface Oncology
Min-Hee Ryu
Honoraria: DAEHWA Pharmaceutical, Bristol-Myers Squibb, Lilly, Ono Pharmaceutical, MSD, Taiho Pharmaceutical, Novartis, Daiichi Sankyo, AstraZeneca
Consulting or Advisory Role: DAEHWA Pharmaceutical, Bristol-Myers Squibb, Lilly, Ono Pharmaceutical, MSD, Taiho Pharmaceutical, Novartis, Daiichi Sankyo, AstraZeneca
Sun Young Rha
Consulting or Advisory Role: MSD Oncology, Ipsen, Daiichi Sankyo, Eisai, Amgen, Indivumed
Speakers' Bureau: Lilly, Eisai
Research Funding: MSD Oncology, Bristol-Myers Squibb, Eisai, Roche/Genentech, MedPacto, ASLAN Pharmaceuticals, SillaJen, Bayer, Immunomet
Gyunji Kim
Employment: Sanofi, Novartis
Stock and Other Ownership Interests: Sanofi
YeonJu Lee
Employment: Sanofi
Stock and Other Ownership Interests: Sanofi
Jee Hyun Lee
Employment: Sanofi
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the European Society for Medical Oncology 2019 Congress, Barcelona, Spain, September 27-October 1, 2019.
SUPPORT
Supported by Sanofi-Aventis, Taiho Pharmaceutical, and Jeil Pharmaceutical Co, Ltd (investigational product supply).
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Qualified researchers can request access to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report forms, statistical analysis plan, and data set specifications. Patient-level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data-sharing criteria, eligible studies, and process for requesting access are at: https://www.clinicalstudydatarequest.com.
AUTHOR CONTRIBUTIONS
Conception and design: Yoon-Koo Kang, Jeong Hwan Yook, Young-Kyu Park, Young-Woo Kim, Sang Cheul Oh, Jong Gwang Kim, Gyunji Kim, Sung Hoon Noh
Provision of study materials or patients: Yoon-Koo Kang, Jeong Hwan Yook, Young-Kyu Park, Young-Woo Kim, Jin Young Kim, Min-Hee Ryu, Sun Young Rha, Ik Joo Chung, In-Ho Kim, Sang Cheul Oh, Taeil Son, Mi Ran Jung, Mi Hwa Heo, Hark Kyun Kim, ChoHyun Park, Chang Hak Yoo, Jin-Hyuk Choi, Dae Young Zang, You Jin Jang, Ji Young Sul, Jong Gwang Kim, Beom Su Kim, Seung-Hoon Beom, Sang Hee Cho, Seung Wan Ryu, Baek-Yeol Ryoo, Moon-Won Yoo, Nam Su Lee, Sang Ho Lee, Sung Hoon Noh
Collection and assembly of data: All authors.
Data analysis and interpretation: Yoon-Koo Kang, Jeong Hwan Yook, Young-Kyu Park, Young-Woo Kim, Sang Cheul Oh, Jong Gwang Kim, Sung Hoon Noh
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
PRODIGY: A Phase III Study of Neoadjuvant Docetaxel, Oxaliplatin, and S-1 Plus Surgery and Adjuvant S-1 Versus Surgery and Adjuvant S-1 for Resectable Advanced Gastric Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Yoon-Koo Kang
Consulting or Advisory Role: DAEHWA Pharmaceutical, Bristol-Myers Squibb, Zymeworks, ALX Oncology, Amgen, Novartis, MacroGenics, Surface Oncology
Min-Hee Ryu
Honoraria: DAEHWA Pharmaceutical, Bristol-Myers Squibb, Lilly, Ono Pharmaceutical, MSD, Taiho Pharmaceutical, Novartis, Daiichi Sankyo, AstraZeneca
Consulting or Advisory Role: DAEHWA Pharmaceutical, Bristol-Myers Squibb, Lilly, Ono Pharmaceutical, MSD, Taiho Pharmaceutical, Novartis, Daiichi Sankyo, AstraZeneca
Sun Young Rha
Consulting or Advisory Role: MSD Oncology, Ipsen, Daiichi Sankyo, Eisai, Amgen, Indivumed
Speakers' Bureau: Lilly, Eisai
Research Funding: MSD Oncology, Bristol-Myers Squibb, Eisai, Roche/Genentech, MedPacto, ASLAN Pharmaceuticals, SillaJen, Bayer, Immunomet
Gyunji Kim
Employment: Sanofi, Novartis
Stock and Other Ownership Interests: Sanofi
YeonJu Lee
Employment: Sanofi
Stock and Other Ownership Interests: Sanofi
Jee Hyun Lee
Employment: Sanofi
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bang YJ Kim YW Yang HK, et al. : Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): Phase 3 open-label, randomised controlled trial. Lancet 379:315-321, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Cunningham D Allum WH Stenning SP, et al. : Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355:11-20, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Macdonald JS Smalley SR Benedetti J, et al. : Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 345:725-730, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Sakuramoto S Sasako M Yamaguchi T, et al. : Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357:1810-1820, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Cunningham D Stenning SP Smyth EC, et al. : Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): Primary analysis results of a multicentre, open-label, randomised phase 2-3 trial. Lancet Oncol 18:357-370, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuchs CS Niedzwiecki D Mamon HJ, et al. : Adjuvant chemoradiotherapy with epirubicin, cisplatin, and fluorouracil compared with adjuvant chemoradiotherapy with fluorouracil and leucovorin after curative resection of gastric cancer: Results from CALGB 80101 (Alliance). J Clin Oncol 35:3671-3677, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang YK Chang HM Yook JH, et al. : Adjuvant chemotherapy for gastric cancer: A randomised phase 3 trial of mitomycin-C plus either short-term doxifluridine or long-term doxifluridine plus cisplatin after curative D2 gastrectomy (AMC0201). Br J Cancer 108:1245-1251, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsuburaya A Yoshida K Kobayashi M, et al. : Sequential paclitaxel followed by tegafur and uracil (UFT) or S-1 versus UFT or S-1 monotherapy as adjuvant chemotherapy for T4a/b gastric cancer (SAMIT): A phase 3 factorial randomised controlled trial. Lancet Oncol 15:886-893, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Lee J Lim DH Kim S, et al. : Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: The ARTIST trial. J Clin Oncol 30:268-273, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Cats A Jansen EPM van Grieken NCT, et al. : Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): An international, open-label, randomised phase 3 trial. Lancet Oncol 19:616-628, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Al-Batran SE Homann N Pauligk C, et al. : Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 393:1948-1957, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Park SH Lim DH Sohn TS, et al. : A randomized phase III trial comparing adjuvant single-agent S1, S-1 with oxaliplatin, and postoperative chemoradiation with S-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: The ARTIST 2 trial. Ann Oncol 32:368-374, 2021 [DOI] [PubMed] [Google Scholar]
- 13.Yoshida K Kodera Y Kochi M, et al. : Addition of docetaxel to oral fluoropyrimidine improves efficacy in patients with stage III gastric cancer: Interim analysis of JACCRO GC-07, a randomized controlled trial. J Clin Oncol 37:1296-1304, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park I Ryu MH Choi YH, et al. : A phase II study of neoadjuvant docetaxel, oxaliplatin, and S-1 (DOS) chemotherapy followed by surgery and adjuvant S-1 chemotherapy in potentially resectable gastric or gastroesophageal junction adenocarcinoma. Cancer Chemother Pharmacol 72:815-823, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Al-Batran SE Hofheinz RD Pauligk C, et al. : Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): Results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol 17:1697-1708, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Kenmotsu H, Tanigawara Y: Pharmacokinetics, dynamics and toxicity of docetaxel: Why the Japanese dose differs from the western dose. Cancer Sci 106:497-504, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwasaki Y Terashima M Mizusawa J, et al. : Gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer (JCOG0501): An open-label, phase 3, randomized controlled trial. Gastric Cancer 24:492-502, 2021 [DOI] [PubMed] [Google Scholar]
- 18.Ji J Shen L Li Z, et al. : Perioperative chemotherapy of oxaliplatin combined with S-1 (SOX) versus postoperative chemotherapy of SOX or oxaliplatin with capecitabine (XELOX) in locally advanced gastric adenocarcinoma with D2 gastrectomy: A randomized phase III trial (RESOLVE trial). Ann Oncol 30, 2019. (suppl 5; abstr LBA42) [Google Scholar]
- 19.Fukagawa T Katai H Mizusawa J, et al. : A prospective multi-institutional validity study to evaluate the accuracy of clinical diagnosis of pathological stage III gastric cancer (JCOG1302A). Gastric Cancer 21:68-73, 2018 [DOI] [PubMed] [Google Scholar]
- 20.Tokunaga M Mizusawa J Machida N, et al. : Phase III trial to evaluate the efficacy of neoadjuvant chemotherapy with S-1 plus oxaliplatin followed by D2 gastrectomy with adjuvant S-1 in locally advanced gastric cancer: Japan Clinical Oncology Group study JCOG1509 (NAGISA trial). J Clin Oncol 35:TPS4134, 2017. (suppl 15; abstr TPS4134) [Google Scholar]
- 21.Ychou M Boige V Pignon JP, et al. : Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 29:1715-1721, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Guideline Committee of the Korean Gastric Cancer Association (KGCA) DWGRP : Korean practice guideline for gastric cancer 2018: An evidence-based, multi-disciplinary approach. J Gastric Cancer 19:1-48, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smyth EC Verheij M Allum W, et al. : Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 27:v38-v49, 2016. (suppl 5) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers can request access to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report forms, statistical analysis plan, and data set specifications. Patient-level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data-sharing criteria, eligible studies, and process for requesting access are at: https://www.clinicalstudydatarequest.com.