Abstract
Almost all patients with autoimmune polyendocrine syndrome type 1 (APS-1) have neutralizing antibodies against type 1 interferons (IFN), important mediators of antiviral defense. Recently, neutralizing anti-IFN antibodies were shown to be a risk factor of severe COVID-19. Here we show in a cohort of 44 patients with APS-1 that higher titers of neutralizing anti-IFNα4 antibodies are associated with a higher and earlier incidence of VZV reactivation (herpes zoster). The patients also present with uncommonly severe clinical sequelae of herpetic infections. APS-1 patients had decreased humoral immune responses to varicella zoster virus, but cellular responses were comparable to healthy controls. These results suggest that blocking the type I interferon pathway in patients with APS-1 patients leads to a clinically significant immune deficiency, and susceptibility to herpesviruses should be taken into account when treating patients with APS-1.
Keywords: AIRE, Herpes simplex, Varicella zoster, Herpes zoster, Autoantibody, Immunodeficiency, Type I interferon
Abbreviations: APS-1, autoimmune polyendocrine syndrome type 1; IFN, interferon; HSV, herpes simplex virus; VZV, varicella zoster virus
1. Introduction
Almost all patients with the rare inherited autoimmune polyendocrine syndrome type 1 (APS-1), caused by bi-allelic mutations in the AIRE gene, develop high levels of neutralizing antibodies against type I interferons (IFN), often before any clinical symptoms of autoimmunity [1]. The antibodies have been shown to decrease in vitro the expression of interferon-regulated genes [2]. Recently the anti-IFN antibodies in patients with APS-1 were linked to a markedly increased risk of severe COVID-19 pneumonia [3]. In a child with APS-1 severe COVID-19 disease was successfully treated with plasmapheresis, removing the neutralizing anti-IFN from circulation [4]. It has also been suggested that patients with anti-IFN-omega antibodies may have a reduced risk of diabetes [5]. Another family of autoantibodies common in patients with APS-1, neutralizing anti-interleukin-17 and -22 antibodies, has been linked to susceptibility to C. albicans infections, one of the hallmarks of APS-1 [7,8].
Type I interferons are important early mediators of antiviral immunity and have also been shown to contribute to the control of latent herpesvirus infections, especially those caused by alphaherpesvirinae herpes simplex virus (HSV) and varicella zoster virus (VZV). Inborn errors in type-I IFN signaling lead to susceptibility to viral infections including fatal HSV encephalitis in a child with IFNAR1 deficiency [[9], [10], [11]]. IFNα has also been used to treat herpes infections [12]. Moreover, in clinical trials of sifalimumab, a monoclonal anti-IFNα antibody, and of anifrolumab, a monoclonal anti-IFNα receptor antibody, 5.9% and 7.4%, respectively, of systemic lupus erythematosus patients receiving the treatment experienced herpes zoster due to VZV reactivation [13,14]. In APS-1, only two case reports of severe herpes infections have been published [15,16].
We show that patients with APS-1 are susceptible to a severe clinical course of herpesvirus infections. The patients in our cohort had VZV reactivation already in childhood and adolescence and this was especially common in patients with high titers of anti-IFN antibodies.
2. Materials and methods
2.1. Subjects
Patients were identified from the large Finnish cohort of over 90 patients with APS-1 [15]. All living patients (n = 65) with a confirmed diagnosis of APS-1 were invited to participate in the study, 44 (68%) of them consented. The APS-1 diagnosis had been confirmed genetically, by identification of biallelic mutations in AIRE, in all participating patients. The recruitment of the patients has been previously described [17]. The mean age of the 44 patients was 38 years (11–70) and 27 (61%) were women. Blood samples were obtained from 40 patients. The control group for laboratory tests consisted of 46 healthy volunteers with a mean age of 38 years (21–69); 29 (63%) of them were women. The incidence and severity of VZV infections was compared with 80 age- and gender-matched healthy control subjects who were recruited for a study on bone health in patients with APS-1 [17]. Their mean age was 40 (7–73) and 46 (58%) were females.
An ethical approval was obtained from the Research Ethics Committee of the Hospital District of Helsinki and Uusimaa. All study participants or their guardians (for subjects aged <18 years) gave an informed written consent. The study was performed according to the principles of Declaration of Helsinki.
2.2. Clinical data and blood samples
Clinical details were collected from hospital records, through patient interviews, and with a questionnaire including questions about medical history, infections, medications, and other relevant parameters. All patients were clinically examined by a medical doctor (S.L.). Fasting blood samples were collected in the morning between 7 and 10 am after an 8–12-h fast and before morning medications. Sera were isolated with standard protocol and stored at −80 °C until analyses.
2.3. Quantification of anti-VZV antibodies
To analyze anti-VZV-IgG-antibodies, polysorp microtiter wells (Nunc A/S) were coated with 0.25 μg VZV lysate antigen per well. The antigen was prepared from VZV strain Ellen -infected human fibroblast cell cultures. To minimize non-specific binding, wells were first blocked with PBS containing 1% BSA for 1 h at 37 °C. Sera were diluted 1:100 in PBS containing 0.05% Tween 20 and 1% BSA (dilution buffer), added to the wells in duplicate, and incubated for 2 h at 37 °C. After incubation, wells were washed three times with PBS containing 0.1% Tween 20 (PBS-T). Horseradish peroxidase (HRP)-conjugated anti-human-IgG (Dako) was then added at a dilution of 1:6000 in dilution buffer and wells were incubated for 1 h at 37 °C. After washing three times with PBS-T, 3,3′,5,5′-tetramethylbenzidine (TMB, Sigma-Aldrich) substrate was added and color reaction was stopped after 10 min with 0.5 M H2SO4. OD values were measured at 450 nm with Hidex Sense (Hidex). Results were calculated by dividing the OD value of a serum sample by the mean OD value of the positive control, multiplied by 100, and expressed as enzyme immunoassay units (EIU).
2.4. Quantification of T cell response to VZV
PBMC were isolated from 10 APECED patients (Table I ) and 10 healthy age and sex matched controls using Ficoll gradient centrifugation (GE Health Sciences). The cells were frozen using the CTL freezing kit and thawn before analysis according to the manufacturer's instructions (Cellular Technology Ltd). Gamma-interferon Elispot assay was performed using a commercially available kit from Mabtech, as previously described [18]. Briefly, 200,000 cells/well were stimulated overnight with inactivated VZV (Jena Biosciences) at 2 mg/well. Interferon-γ-producing cells were identified by staining the wells with biotinylated anti-IFN-γ antibody, followed by streptavidin-HRP and TMB substrate. The spots were counted by Elispot reader (Elispot Reader System ELRIFL04, AID), and the data shown as spots per well (200,000 cells) after subtracting the background observed in nonstimulated wells.
Table I.
Clinical characteristics of APS-1 patients.
| Patient | Anti-IFNα4 (U) | VZV-IgG (EIU) | VZV | VZV reactivation | HSV | Unusual HSVa | Unusual primary VZVb | Hospitalizedc | Elispot | AIRE genotype | Age groupd |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 294 | 110 | + | + | + | HMZ | 50–59 | ||||
| 2 | 195 | 69 | + | + | + | HMZ | 50–59 | ||||
| 3 | 143 | 80 | + | + | + | + | HMZ | 30–39 | |||
| 4 | 141 | 64 | + | + | + | HMZ | 30–39 | ||||
| 5 | 129 | 51 | + | + | + | HTZ | 20–29 | ||||
| 6 | 122 | 37 | + | + | HMZ | 30–39 | |||||
| 7 | 113 | 86 | + | + | HTZ | 30–39 | |||||
| 8 | 112 | 33 | + | + | HMZ | 30–39 | |||||
| 9 | 109 | 71 | + | + | HMZ | >60 | |||||
| 10 | 107 | 100 | + | HTZ | 10–19 | ||||||
| 11 | 103 | 68 | + | HMZ | 20–29 | ||||||
| 12 | 95 | 69 | + | + | + | + | + | HMZ | 30–39 | ||
| 13 | 95 | 80 | + | + | HMZ | 30–39 | |||||
| 14 | 94 | 72 | + | + | + | HMZ | 40–49 | ||||
| 15 | 94 | 51 | + | HMZ | <10 | ||||||
| 16 | 94 | 72 | + | + | HMZ | 20–29 | |||||
| 17 | 93 | 45 | + | + | + | HMZ | 40–49 | ||||
| 18 | 92 | 77 | + | + | HTZ | 10–19 | |||||
| 19 | 91 | 51 | + | + | + | + | + | + | HMZ | 40–49 | |
| 20 | 91 | 85 | + | + | + | + | HMZ | 40–49 | |||
| 21 | 91 | 47 | + | + | HMZ | >60 | |||||
| 22 | 88 | 78 | + | HMZ | >60 | ||||||
| 23 | 88 | 82 | + | + | + | + | HTZ | 20–29 | |||
| 24 | 88 | 70 | + | + | HMZ | 40–49 | |||||
| 25 | 87 | 69 | + | + | + | HMZ | 40–49 | ||||
| 26 | 87 | 84 | + | HMZ | 20–29 | ||||||
| 27 | 87 | 71 | + | + | HMZ | 40–49 | |||||
| 28 | 85 | + | HMZ | 10–19 | |||||||
| 29 | 84 | 95 | + | + | + | + | + | HMZ | 50–59 | ||
| 30 | 84 | 72 | + | + | HMZ | 40–49 | |||||
| 31 | 83 | 15 | # | HTZ | 10–19 | ||||||
| 32 | 83 | 64 | + | + | HMZ | 50–59 | |||||
| 33 | 81 | + | + | HTZ | 50–59 | ||||||
| 34 | 75 | 58 | + | HMZ | 40–49 | ||||||
| 35 | 73 | 99 | + | + | HMZ | 30–39 | |||||
| 36 | 66 | 86 | + | + | HMZ | >60 | |||||
| 37 | 61 | 99 | + | + | HTZ | 50–59 | |||||
| 38 | 53 | 72 | + | + | HTZ | 50–59 | |||||
| 39 | 50 | 81 | + | + | HMZ | 50–59 | |||||
| 40 | 47 | + | + | HMZ | 10–19 | ||||||
| 41 | 44 | 56 | + | + | + | HMZ | 30–39 | ||||
| 42 | 41 | 105 | + | HMZ | 30–39 | ||||||
| 43 | 35 | + | + | HTZ | 10–19 | ||||||
| 44 | 6 | 79 | + | HMZ | 10–19 |
IFN, interferon, VZV, varicella zoster virus, HSV, herpes simplex virus, HMZ, homozygous for the variant c.769C > T in AIRE, HMZ, compound heterozygous for the variant c.769C > T in AIRE.
# Vaccinated.
HSV infection with prolonged or atypical symptoms or non-mucosal location.
VZV primary infection with hospitalization or patient reported severe blistering or high fever.
Hospitalized either due to VZV primary infection or reactivation or HSV infection.
Age is given as age group to preserve anonymity.
2.5. Interleukin-22 and IFN-α4 radioligand binding assay
Serum IL-22 and IFN-α4 autoantibodies were measured by immunoprecipitation using radiolabeled IL-22 and IFN-α4 proteins. Human cDNA (IL-22 RC209995, IFN-a4 SC304903, OriGene) was cloned into pTNT vector (L5610, Promega). The vectors were used for in vitro transcription and translation with a Sp6 TNT coupled reticulocyte-lysate system (Promega) in the presence of 35S methionine. Immunoprecipitation was conducted in 96-well plates (Thermo Scientific). Two positive control sera from patients with APS-1 with anti-IL-22 and anti-IFN-α4 antibodies were included in each plate. 4% BSA was used as a negative control. All serum samples were analyzed in duplicate. Radiolabeled protein (30.000 cpm) and 2.5 ml of serum sample was added to each well, and samples incubated overnight. Serum antibodies were then immobilized to protein A Sepharose (nProtein A Sepharose 4 Fast Flow, GE Healthcare) in 96-well filtration plates (Millipore) during 45 min of incubation. The plates were washed multiple times and then dried. Measurement of the radioactivity was performed in a microbeta counter (1450 Microbeta TriLux, Wallac) after adding scintillation fluid (PerkinElmer). Index values were calculated according to the following equation: ((cpm sample-cpm negative standard)/(cpm positive standard-cpm negative standard)) x100.
2.6. Statistical analysis
Statistical analyses were performed using SPSS version 23 (IBM). Nonparametric Mann–Whitney U test was used to study differences between groups. A p-value of <0.05 was considered significant.
3. Results
3.1. VZV reactivations are prevalent in patients with APS-1 and they have more severe clinical sequelae of herpesvirus infections
Our study included 44 patients with genetically confirmed APS-1, accounting for approximately two-thirds of the currently diagnosed surviving patients in Finland (Table I). Except for one vaccinated patient, all others had had a wild-type primary infection by VZV, requiring hospitalization of three (6.8%) of them (at the age of 1, 3 and 4 years). More subjectively, an additional 6 patients (13.6%) described their primary infection as unusually severe, with severe blistering and high fever. None of the 80 age and gender-matched healthy controls reported hospitalization or an exceptionally severe primary infection.
Reactivation of VZV as herpes zoster had been experienced by 9 patients with APS-1 (9/44; 20.5%) and by 8 out of 80 control subjects (10.0%). In 3 patients with APS-1 the reactivation had occurred in childhood or adolescence, and in APS-1 patients the median age at VZV reactivation was 30 years (range 1.9–58.0, Fig. 1 ). One APS-1 patient required hospitalization (Suppl Table I). In the healthy controls, the median age at VZV reactivation was clearly higher at 45 years (range, 27–63, Fig. 1), and none of them had required hospital care.
Fig. 1.
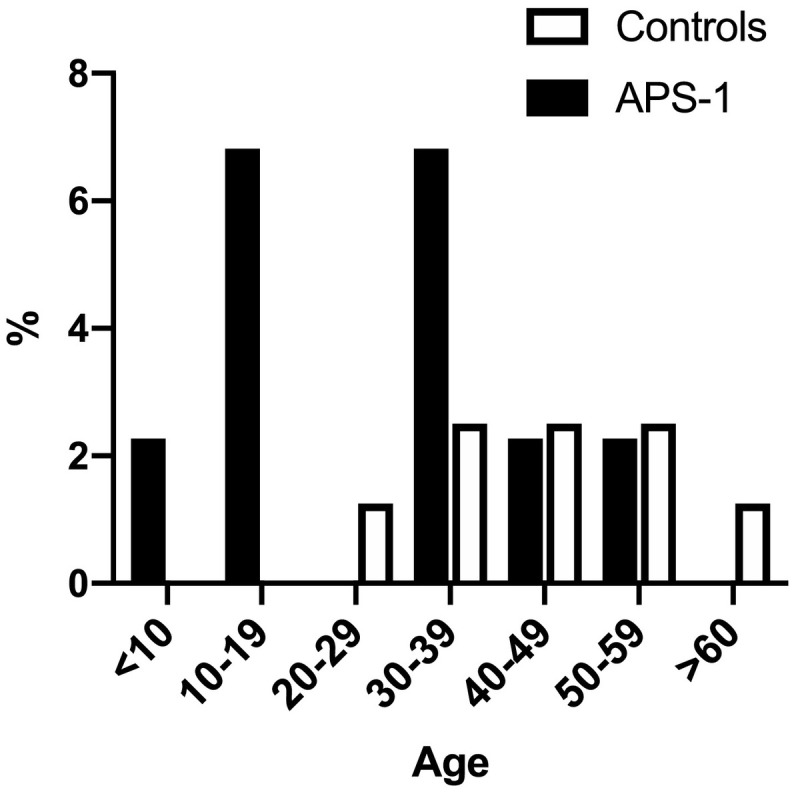
The incidence of VZV reactivation in different age groups in patients with APS-1 (n = 44) and in age- and gender-matched healthy controls (n = 80).
In the APS-1 group, a clinical HSV infection was reported by 18 patients (40.9%). Seven patients (15.9%) presented with atypical symptoms, nonmucosal manifestations, or prolonged duration. These included one meningitis and one erythema multiforme. Three patients required hospitalization one of whom had also been hospitalized due to primary VZV infection. Among the 80 controls, one (1.3%) had needed prophylactic medication due to recurrent genital HSV infections, and one had a nonmucosal manifestation. None of the controls had required hospitalization due to HSV infection.
3.2. Patients with VZV reactivation or severe herpesvirus infection exhibit high levels of anti-IFNα4 antibodies
We used a radioligand binding assay to measure anti-interferon alpha (anti-IFNα) antibodies in our patients. Anti-IFNα4 was selected as the indicator antibody, since in previous studies it has been the subtype inducing a particularly strong autoantibody formation, and the anti-IFNα4 antibodies also show a good correlation with other anti-IFNα antibodies [19]. At the same time, anti-IFNα4 antibodies show low cross-reactivity with other families of type 1 interferons. Thirty-six of the 40 patients (90%) had detectable levels of serum anti-IFNα4 antibodies, including all 9 patients with a history of VZV reactivation. The patients with a previous VZV reactivation had significantly higher levels of anti-IFNα4 antibodies than the patients who had not experienced a reactivation (Fig. 2 ). Of the patients with VZV reactivation, eight exhibited anti-IFNα4 levels above the median and one at the median for the whole APS-1 cohort. Also five out of the six patients hospitalized because of VZV or HSV disease had anti-IFNα4 levels above the median and four out of seven patients with atypical manifestations of HSV had higher and one at the median level of anti-IFNα4 antibodies (Table I).
Fig. 2.
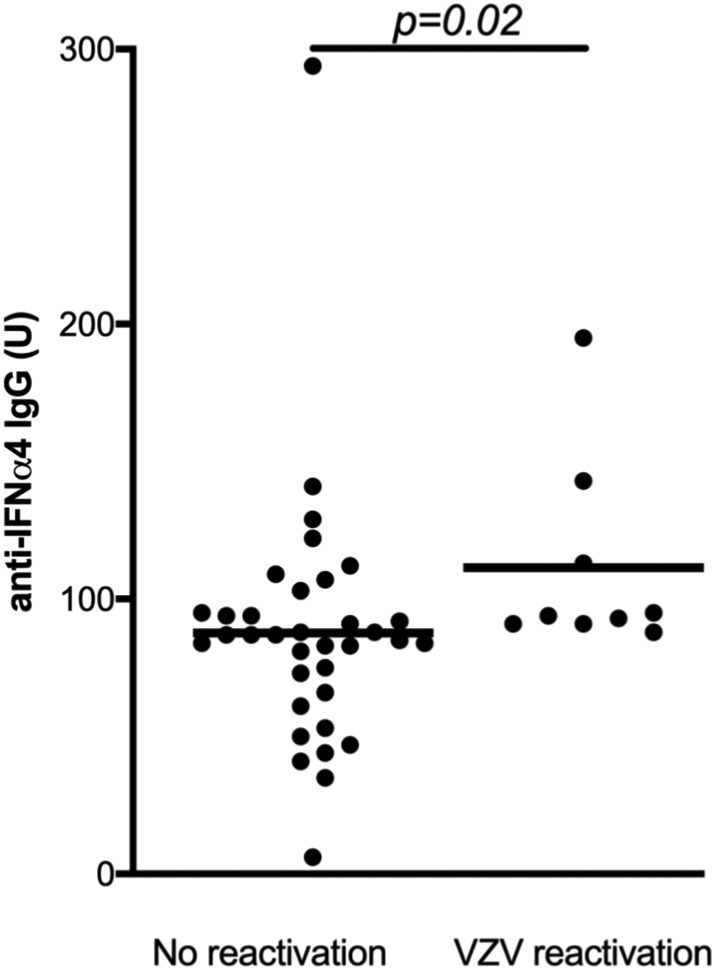
Neutralizing anti-IFN-α4 antibody levels in patients with APS-1 with or without a history of VZV reactivation (herpes zoster). The mean level is indicated by the horizontal bars. The data are shown on a relative scale. The statistical significance of the difference was calculated using the Mann-Whitney U test.
3.3. Anti-IL-22 antibodies or AIRE genotype are not associated with more severe clinical representation of herpesvirus infection
Autoantibodies to helper T cell-17 cytokines are found in the majority of patients with APS-1 and have been shown to impair antifungal defenses, causing chronic mucocutaneous candidiasis [7,20]. Th17 cytokines are also important for antiviral defense. The majority of patients in the present study (42/44, 95.5%) exhibited anti-IL22 antibodies, but their level did not differ between patient groups with HZ, atypical HSV or patients hospitalized due to herpesvirus infections compared with other patients (data not shown).
Most of our patients (34/44, 77.3%) were homozygous for the pathogenic AIRE variant c.769C > T, p.Arg257Ter. Others had the c.769C > T variant compounded with c.967_979del13, p.Leu323fs (n = 5), c.891C > A (p.Asp297Glu) (n = 2), c.932G > A (p.Cys311Tyr) (n = 2), or c.137C > G (p.Thr46Arg) (n = 1). There was no detectable link between the type of AIRE mutation and the incidence or severity of VZV or HSV infections or reactivations (Table I).
3.4. Patients with APS-1 have reduced anti-VZV antibody levels but normal frequency of VZV reactive T cells
Serum samples were available from 40 of the 44 patients. We used a clinically validated ELISA to measure anti-VZV-IgG antibody levels from these 40 patients with APS-1 and 46 healthy controls. All of our study subjects were VZV-seropositive. The VZV-specific IgG levels were, however, significantly lower in the patients than in the controls (Fig. 3A). VZV IgG levels were similar in patients with and without VZV reactivation (Fig. 3B). PBMC were available from 10 patients and 10 healthy controls and were used to measure the frequency of VZV-reactive T cells. Interferon- γ Elispot assay showed that the patients and healthy controls had comparable responses to stimulation with inactivated VZV (Fig. 3C).
Fig. 3.
Adaptive anti-VZV responses in APS-1 patients. A) VZV-specific IgG levels, shown as enzyme immunoassay units on a relative scale. B) VZV-specific IgG levels in patients without and with VZV reactivation. C) The number of cells producing IFN-γ in healthy controls and APS-1 patients (both with no reactivation and with VZV reactivation) in response to stimulation with inactivated VZV, measured by γ -IFN Elispot. The data are shown as spots per 200,000 cells. Healthy controls are indicated by open circles, APS-1 patients by filled circles. The mean level is indicated by the horizontal bars. The statistical significance of the difference was calculated using the Mann-Whitney U test.
4. Discussion
In this study, we show in a cohort of 44 patients with genetically confirmed APS-1 that patients with APS-1 have an increased incidence and unusually early presentation of severe herpesvirus manifestations. The primary VZV infection hospitalization of three (6.8%) patients. In comparison, the hospitalization rate for varicella in the general population prior to the availability of vaccines was estimated to be approximately 6.2 per 10,000 varicella cases (0.06%) [21]. Furthermore, three out of 18 (16.7%) patients with APS-1 with symptomatic HSV infection, normally a self-limiting disease in the general population, required hospitalization. Moreover, reactivation of VZV was seen in patients with APS-1 twice as often as in the controls and at markedly younger age. The disease pattern in patients is in clear contrast with the pattern in the general population, where VZV reactivation is rare in children and adolescents [22].
Increased reactivation of VZV has been observed in patients receiving sifalimumab, a recombinant anti-IFNα antibody, and notably the reactivation is more common in patients receiving the highest dose of the drug [13]. The same was also true for patients treated with anifrolumab, a monoclonal anti-IFNα receptor antibody [14]. Further, an earlier study on patients with APS-1 exhibiting anti-IFNα antibodies indicated that the expression level of interferon-stimulated genes in PBMCs ex vivo depended on the titer of neutralizing antibodies in patients' sera [2]. These findings are consistent with our data showing that the patients with APS-1 with severe clinical representations of herpesvirus infections predominantly had levels of anti-IFNα4 antibodies above the median. Indigenous anti-IFNα antibodies have also been associated with severe VZV infection in few patients with RAG deficiency [23] and they have been detected in patients who had developed postherpetic neuralgia [24]. Nevertheless, the phenotype of APS-1 is complex, with skewed distribution and function of T cell subsets and autoantibodies to several cytokines.
Our data have some limitations. Our samples were taken after the primary VZV infection or reactivation, and do not necessarily reflect the anti-IFNα autoantibody levels at the time of the complications. Because practically all APS-1 patients have high levels of anti-IFNα antibodies [1,2,6], it is challenging to distinguish the role they play in the pathogenesis of the disease manifestations. However, anti-IFNα autoantibodies are usually the earliest immunopathological manifestation in APS-1, suggesting that our patients already had them at the time of VZV primary infection. Taken together, in our data the anti-IFNα antibodies are the factor with the strongest association with severe manifestations of VZV in APS-1 but longitudinal studies would be needed to validate these findings. Also, our findings do not exclude other immunological aberrations contributing to the increased susceptibility.
Indeed, we also found that antibody levels against VZV were significantly lower in the patients compared to controls. Post-vaccination IgG levels against VZV have been shown to correlate with neutralization of the virus, protection against infection, and the clinical representations of VZV breakthrough infections [25]. Passive immunization with anti-VZV immunoglobulin also modifies infection outcome and is used to treat immunocompromised individuals [26]. Thus antibodies to VZV clearly can have a protective role, and the decreased humoral responses in patients with APS-1 may contribute to the more severe course of VZV infection, and perhaps even more to the failure of immunosurveillance to prevent VZV reactivation. On the other hand, the cellular responses to VZV were at least quantitatively normal, although qualitative differences remain a possibility.
Together with previous studies [27], our data highlights the multifaceted immunodeficiency as an important component of APS-1, a syndrome generally regarded predominantly as an autoimmune disease. This was also emphasized by the recent demonstration of neutralizing anti-IFN antibodies, both in patients with APS-1 and more generally, as a risk factor for severe COVID-19 and for life threatening disease after yellow fever virus vaccination [3,4,[28], [29], [30]]. Finally, the data suggest that the predilection to more severe sequelae in herpesvirus infections should be taken into account when planning the treatment of patients with APS-1, and vaccination or preventive medication should be considered.
Acknowledgements
We would like to thank Olle Kämpe for providing the anti-cytokine antibody measurements as well as Nea Boman, Åsa Hallgren and Tamás Bazsinka for their technical assistance. This work was funded by Helsinki University research funds, the Helsinki University Doctoral Programme in Biomedicine, Sigrid Jusélius Foundation, European Joint Program for Rare Diseases and Academy of Finland (grants 334813 and 334812), Novo Nordisk Foundation, Foundation for Pediatric Research and the Swedish Childhood Cancer Foundation. All authors have read the journal's authorship agreement and policy on disclosure of potential conflicts of interest. No conflict of interest to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clim.2021.108851.
Appendix A. Supplementary data
Supplementary material
References
- 1.Meager A., Visvalingam K., Peterson P., Moll K., Murumagi A., Krohn K., et al. Anti-interferon autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Med. 2006;3(7) doi: 10.1371/journal.pmed.0030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kisand K., Link M., Wolff A.S., Meager A., Tserel L., Org T., et al. Interferon autoantibodies associated with AIRE deficiency decrease the expression of IFN-stimulated genes. Blood. 2008;112(7):2657–2666. doi: 10.1182/blood-2008-03-144634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastard P., Orlova E., Sozaeva L., Lévy R., James A., Schmitt M.M., et al. Preexisting autoantibodies to type I IFNs underlie critical COVID-19 pneumonia in patients with APS-1. J. Exp. Med. 2021;218(7) doi: 10.1084/jem.20210554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemarquis A., Campbell T., Aranda-Guillén M., Hennings V., Brodin P., Kämpe O., et al. Severe COVID-19 in an APS1 patient with interferon autoantibodies treated with plasmapheresis. J. Allergy Clin. Immunol. 2021;148(1):96–98. doi: 10.1016/j.jaci.2021.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer S., Woodward M., Hertel C., Vlaicu P., Haque Y., Karner J., et al. AIRE-deficient patients harbor unique high-affinity disease-ameliorating autoantibodies. Cell. 2016;166(3):582–595. doi: 10.1016/j.cell.2016.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kisand K., Peterson P. Autoimmune polyendocrinopathy candidiasis ectodermal dystrophy: known and novel aspects of the syndrome. Ann. N. Y. Acad. Sci. 2011;1246:77–91. doi: 10.1111/j.1749-6632.2011.06308.x. [DOI] [PubMed] [Google Scholar]
- 7.Kisand K., Boe Wolff A.S., Podkrajsek K.T., Tserel L., Link M., Kisand K.V., et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J. Exp. Med. 2010;207(2):299–308. doi: 10.1084/jem.20091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puel A., Doffinger R., Natividad A., Chrabieh M., Barcenas-Morales G., Picard C., et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J. Exp. Med. 2010;207(2):291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bastard P., Manry J., Chen J., Rosain J., Seeleuthner Y., AbuZaitun O., et al. Herpes simplex encephalitis in a patient with a distinctive form of inherited IFNAR1 deficiency. J. Clin. Invest. 2021;131(1) doi: 10.1172/JCI139980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyts I., Casanova J.L. Viral infections in humans and mice with genetic deficiencies of the type I IFN response pathway. Eur. J. Immunol. 2021;51(5):1039–1061. doi: 10.1002/eji.202048793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Q., Bastard P., Liu Z., Le Pen J., Moncada-Velez M., Chen J., et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370(6515) doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winston D.J., Eron L.J., Ho M., Pazin G., Kessler H., Pottage J.C., Jr., et al. Recombinant interferon alpha-2a for treatment of herpes zoster in immunosuppressed patients with cancer. Am. J. Med. 1988;85(2):147–151. doi: 10.1016/s0002-9343(88)80333-4. [DOI] [PubMed] [Google Scholar]
- 13.Khamashta M., Merrill J.T., Werth V.P., Furie R., Kalunian K., Illei G.G., et al. Sifalimumab, an anti-interferon-alpha monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 2016;75(11):1909–1916. doi: 10.1136/annrheumdis-2015-208562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furie R., Khamashta M., Merrill J.T., Werth V.P., Kalunian K., Brohawn P., et al. Anifrolumab, an anti-interferon-alpha receptor monoclonal antibody, in moderate-to-severe systemic lupus erythematosus. Arthritis Rheumatol. (Hoboken, NJ) 2017;69(2):376–386. doi: 10.1002/art.39962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Batra A., Davison S., Rajwal S., Hale A., Stringer M.D., McClean P. Varicella recurrence complicated by pneumonia after liver transplantation for APECED. J. Pediatr. Gastroenterol. Nutr. 2007;44(5):637–639. doi: 10.1097/01.mpg.0000243432.09216.aa. [DOI] [PubMed] [Google Scholar]
- 16.Nagafuchi S., Umene K., Yamanaka F., Oohashi S., Shindo M., Kurisaki H., et al. Recurrent herpes simplex virus infection in a patient with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy associated with L29P and IVS9-1G>C compound heterozygous autoimmune regulator gene mutations. J. Intern. Med. 2007;261(6):605–610. doi: 10.1111/j.1365-2796.2007.01786.x. [DOI] [PubMed] [Google Scholar]
- 17.Laakso S., Borchers J., Toiviainen-Salo S., Pekkinen M., Mäkitie O. Severe phenotype of APECED (APS1) increases risk for structural bone alterations. Front. Endocrinol. (Lausanne) 2020;11:109. doi: 10.3389/fendo.2020.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vakkilainen S., Kleino I., Honkanen J., Salo H., Kainulainen L., Gräsbeck M., et al. The safety and efficacy of live viral vaccines in patients with cartilage-hair hypoplasia. Front. Immunol. 2020;11:2020. doi: 10.3389/fimmu.2020.02020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landegren N., Sharon D., Freyhult E., Hallgren A., Eriksson D., Edqvist P.H., et al. Proteome-wide survey of the autoimmune target repertoire in autoimmune polyendocrine syndrome type 1. Sci. Rep. 2016;6:20104. doi: 10.1038/srep20104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puel A., Doffinger R., Natividad A., Chrabieh M., Barcenas-Morales G., Picard C., et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J. Exp. Med. 2010;207(2):291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liese J.G., Grote V., Rosenfeld E., Fischer R., Belohradsky B.H., Kries R.V. The burden of varicella complications before the introduction of routine varicella vaccination in Germany. Pediatr. Infect. Dis. J. 2008;27(2):119–124. doi: 10.1097/INF.0b013e3181586665. [DOI] [PubMed] [Google Scholar]
- 22.Kawai K., Gebremeskel B.G., Acosta C.J. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6) doi: 10.1136/bmjopen-2014-004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walter J.E., Rosen L.B., Csomos K., Rosenberg J.M., Mathew D., Keszei M., et al. Broad-spectrum antibodies against self-antigens and cytokines in RAG deficiency. J. Clin. Invest. 2016;126(11):4389. doi: 10.1172/JCI91162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bayat A., Burbelo P.D., Browne S.K., Quinlivan M., Martinez B., Holland S.M., et al. Anti-cytokine autoantibodies in postherpetic neuralgia. J. Transl. Med. 2015;13:333. doi: 10.1186/s12967-015-0695-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marin M., Guris D., Chaves S.S., Schmid S., Seward J.F. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recommend. Rep. Morbid. Mortal. Weekly Rep. Recommend. Rep. 2007;56(Rr-4):1–40. [PubMed] [Google Scholar]
- 26.Keller M.A., Stiehm E.R. Passive immunity in prevention and treatment of infectious diseases. Clin. Microbiol. Rev. 2000;13(4):602–614. doi: 10.1128/cmr.13.4.602-614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borchers J., Pukkala E., Mäkitie O., Laakso S. Patients with APECED have increased early mortality due to endocrine causes, malignancies and infections. J. Clin. Endocrinol. Metab. 2020;105(6):e2207–e2213. doi: 10.1210/clinem/dgaa140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.H., Zhang Y., et al. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020:e2207–e2213. doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beccuti G., Ghizzoni L., Cambria V., Codullo V., Sacchi P., Lovati E., et al. A COVID-19 pneumonia case report of autoimmune polyendocrine syndrome type 1 in Lombardy, Italy: letter to the editor. J. Endocrinol. Investig. 2020;43(8):1175–1177. doi: 10.1007/s40618-020-01323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bastard P., Michailidis E., Hoffmann H.H., Chbihi M., Le Voyer T., Rosain J., et al. Auto-antibodies to type I IFNs can underlie adverse reactions to yellow fever live attenuated vaccine. J. Exp. Med. 2021;218(4) doi: 10.1084/jem.20202486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material



