Abstract
Emotional memories are better remembered than neutral ones, but the mechanisms leading to this memory bias are not well understood in humans yet. Based on animal research, it is suggested that the memory-enhancing effect of emotion is based on central noradrenergic release, which is triggered by afferent vagal nerve activation. To test the causal link between vagus nerve activation and emotional memory in humans, we applied continuous noninvasive transcutaneous auricular vagus nerve stimulation (taVNS) during exposure to emotional arousing and neutral scenes and tested subsequent, long-term recognition memory after 1 week. We found that taVNS, compared with sham, increased recollection-based memory performance for emotional, but not neutral, material. These findings were complemented by larger recollection-related brain potentials (parietal ERP Old/New effect) during retrieval of emotional scenes encoded under taVNS, compared with sham. Furthermore, brain potentials recorded during encoding also revealed that taVNS facilitated early attentional discrimination between emotional and neutral scenes. Extending animal research, our behavioral and neural findings confirm a modulatory influence of the vagus nerve in emotional memory formation in humans.
SIGNIFICANCE STATEMENT Emotionally relevant information elicits stronger and more enduring memories than nonrelevant information. Animal research has shown that this memory-enhancing effect of emotion is related to the noradrenergic activation in the brain, which is triggered by afferent fibers of the vagus nerve (VN). In the current study, we show that noninvasive transcutaneous auricular VN stimulation enhances recollection-based memory formation specifically for emotionally relevant information as indicated by behavioral and electrophysiological indices. These human findings give novel insights into the mechanisms underlying the establishment of emotional episodic memories by confirming the causal link between the VN and memory formation which may help understand the neural mechanisms underlying disorders associated with altered memory functions and develop treatment options.
Keywords: emotion, ERPs, memory, Old/New effect, LPP, vagus nerve, tVNS
Introduction
Emotional salience influences the initial stages of processing (Dolan, 2002; Méndez-Bértolo et al., 2016; Dolcos et al., 2020), and exerts long-lasting effects, leading to better long-term episodic memory (Bradley et al., 1992; Weymar and Hamm, 2013). This mnemonic advantage for emotionally relevant, relative to neutral information, is mainly mediated by recollection which, compared with familiarity, reflects an elaborate memory process that includes the storage and retrieval of specific spatial, temporal, and/or other contextual information (Ochsner, 2000; Sharot et al., 2004; Dolcos et al., 2005, 2020).
As shown in animal (Roozendaal and McGaugh, 2011; Barsegyan et al., 2014, 2019; Atucha et al., 2017) and human studies (Cahill et al., 1994; Strange and Dolan, 2004), the amygdala (AMY), in interaction with the hippocampus (HC), plays a crucial role in the memory-enhancing effects of emotion (Dolcos et al., 2004, 2005; Kensinger and Schacter, 2005). Stress hormones, such as adrenaline and corticosterone, released during the encounter with an arousing event, facilitate the noradrenergic release in the AMY (Miyashita and Williams, 2006), which can strengthen further neuroplasticity and memory storage processing mediated by the HC (among other regions), a mechanistic AMY-HC interaction that seems critical during initial encoding and memory consolidation of the emotionally arousing event (e.g., Strange and Dolan, 2004; Van Stegeren et al., 2005; Weymar et al., 2010a; Ritchey et al., 2017; for review, see Roozendaal and Hermans, 2017).
Importantly, the vagus nerve (VN) is a critical path through which the stress hormones modulate activity in memory-sensitive brain regions via the nucleus of the solitary tract and locus coeruleus (LC) (Loughlin et al., 1986; Williams et al., 1998; Groves et al., 2005; Dorr and Debonnel, 2006), a key node that participates in the neural amplification of relevant information during attentional selection and memory formation (Mather et al., 2016). Animal research confirmed the causal role of ascending vagal fibers on emotional memory (Williams and McGaugh, 1993; Clark et al., 1998; McIntyre et al., 2012; Hulsey et al., 2017), but this link has not been demonstrated in humans yet. In an earlier study, it was found that invasive stimulation of the VN, during encoding of words, enhances immediate memory performance for such material (Clark et al., 1999). Although these results may provide support for a causal role of the VN on memory in humans, the fact that emotional episodic memory was not tested poses challenges for the generalization of the same neural path from animals to humans. Furthermore, because immediate memory was tested, it is uncertain whether VN stimulation modulates long-term retention.
To fill this gap, we investigated the influence of the VN on long-term emotional episodic memory (1 week delay) in humans, using noninvasive, transcutaneous auricular VN stimulation (taVNS) (Farmer et al., 2021; Weymar and Zaehle, 2021). Previous findings suggest that taVNS may be a good proxy for the current purpose, as it has been shown to enhance the P300b (e.g., Ventura-Bort et al., 2018; but see Warren, et al., 2019), an event-related potential (ERP) associated with phasic LC activity (Nieuwenhuis et al., 2005), as well as salivary α amylase (sAA) levels (Ventura-Bort et al., 2018; Warren et al., 2019; but see also Giraudier et al., 2020; Koenig et al., 2021), an indirect marker for central noradrenergic activation (Chatterton et al., 1996; Thoma et al., 2012; Warren et al., 2017). In a within-subject, 3-session design, participants viewed a series of unpleasant and neutral pictures while receiving either taVNS or sham stimulation, and 1 week later performed a recognition memory task. To assess brain dynamics, we recorded ERPs during encoding and retrieval. During encoding, we focused on the late positive potential (LPP), an electrophysiological index for motivated attention and elaborated (mnemonic) processing of salient information (Cuthbert et al., 2000; Schupp et al., 2004; Weymar et al., 2012). During retrieval, we analyzed the ERP Old/New effect, an index for recollection-based recognition memory (Rugg and Curran, 2007). Both ERP components have been shown to be modulated by emotional arousal (e.g., Cuthbert et al., 2000; Weymar et al., 2010a, 2011). If taVNS increases arousal in the brain, we expected that taVNS would increase the initial encoding and long-term consolidation of emotional information as shown by enhanced LPPs, better recollection memory performance, and enhanced ERP Old/New differences.
Materials and Methods
Participants
A total of 41 healthy students (21 women, 20 men; mean age = 23.08 years) from the University of Greifswald participated in the study in exchange of course credits or financial compensation. All participants had normal or corrected-to-normal vision and were native German speakers. Each individual provided written informed consent for a protocol approved by the Review Board of the German Psychological Society. Before the first session, participants were phone-screened and invited to participate if they did not match any of the following exclusion criteria: neurologic or mental disorders, brain surgery, undergoing chronic or acute medication, pregnancy, history of migraine and/or epilepsy, heart-related diseases, metal implants in the face or brain, implants in or physical alterations of the ear. Data from 4 participants (3 men, 1 woman) could not be analyzed because of technical problems related to the recording or the stimulation, leaving a total of 37 participants (20 women; mean age = 23.15 years). Table 1 summarizes the participants excluded for each analyzed dependent variable.
Table 1.
Summary of participants that were excluded for at least one dependent variable (including reason for exclusion)
| Participant | Behavioral performance | Saliva | HR | BP | ERP encoding | ERP retrieval |
|---|---|---|---|---|---|---|
| 09 | a | |||||
| 12 | a | a | a | |||
| 13 | b | |||||
| 15 | b | |||||
| 16 | c | |||||
| 18 | a | |||||
| 23 | b | |||||
| 32 | c | |||||
| 33 | b | |||||
| 34 | a | |||||
| 35 | a | |||||
| 38 | c | |||||
| 40 | a | b | ||||
| 41 | d |
a, Missing data; b, Low number of good trials (n < 9); c, technical problems during recording; d, technical problems during stimulation.
Procedure and tasks
To implement a randomized, single-blinded, within-subject, crossover design (taVNS-sham; sham-taVNS), the experiment consisted of 3 study days: two encoding sessions conducted on 2 consecutive days and a retrieval session which took place 1 week after the first encoding session1 (Fig. 1).
Figure 1.
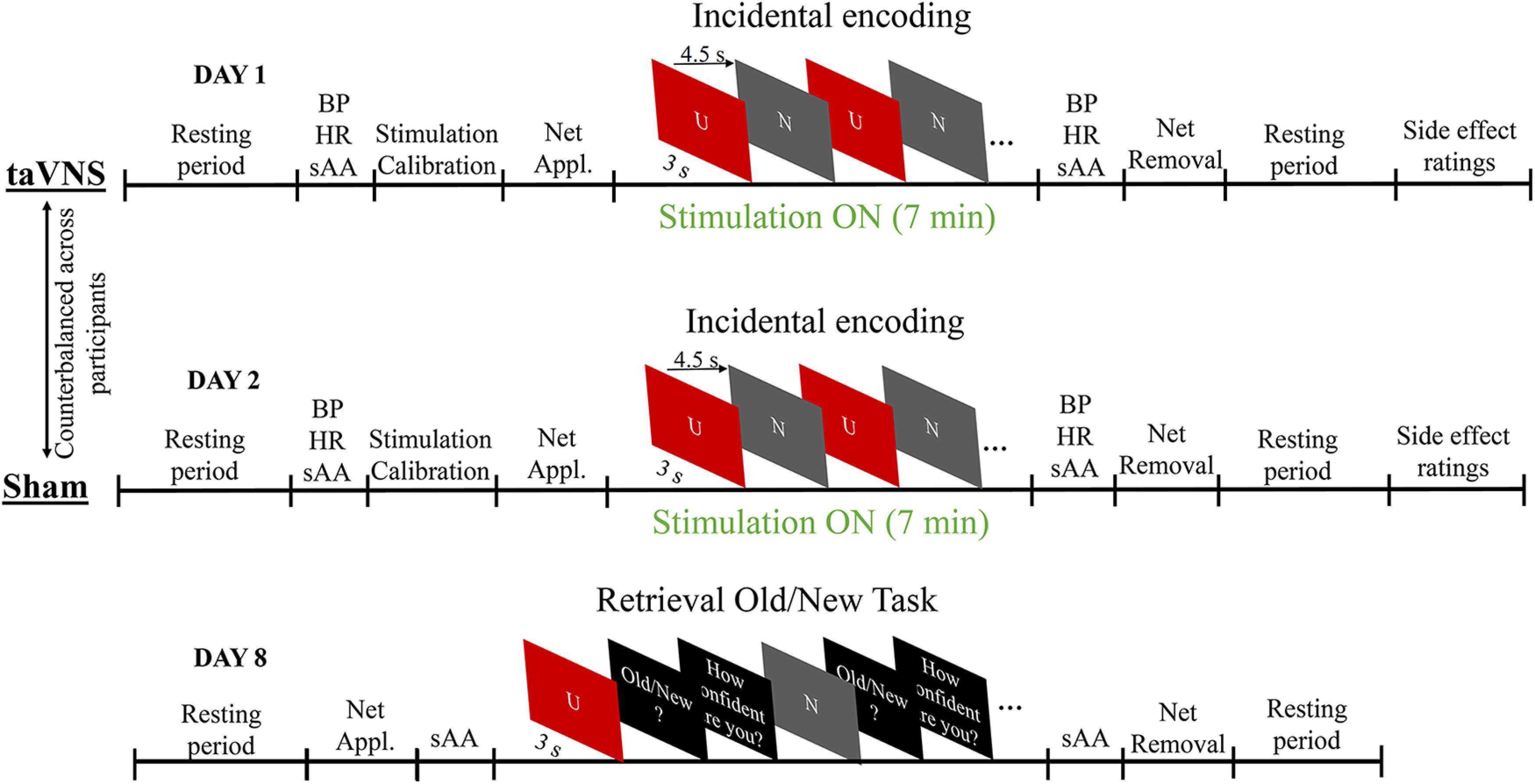
Schematic representation of the design. In a within-subject design, participants encoded unpleasant and neutral images on two consecutive sessions (1 day apart), in which sham and taVNS stimulation was alternated. The order of the stimulation condition was counterbalanced across participants. One week after the second session, participants came back to the laboratory and performed a retrieval session (recognition memory task) in which the encoded images (Old) were mixed with nonencoded (New) images. Participants performed an Old-New task and provided information about the confidence of their judgments. N, Neutral; U, unpleasant; taVNS, transcutaneous auricular vagus nerve stimulation; BP, blood pressure; HR, heart rate; sAA, salivary alpha amylase; Appl., Application.
Stimuli used in the tasks comprised a total of 240 images selected from the International Affective System (Lang et al., 2008) and from the Nencki Affective Picture System (Marchewka et al., 2014), consisting of 120 neutral (e.g., buildings, neutral views, neutral human faces) and 120 unpleasant pictures (e.g., depicting mutilations, attacks, disgusting content, accidents). All pictures were selected based on the normative valence (ranging from 1 [unpleasant] to 9 [pleasant]) and arousal ratings (ranging from 1 [calm] to 9 [excited]) with a mean (SD) value of 5.12 (0.35) and 3.27 (0.48) for neutral; and 2.86 (0.57) and 5.5 (0.86) for unpleasant contents. Pictures were selected to differ in both valence and arousal ratings (p < 0.001), but to match in physical attributes, such as complexity, brightness, and contrast (p > 0.44). Pictures were divided in four different sets consisting of 60 scenes each (30 neutral, 30 unpleasant). The four sets were arranged in four different encoding lists and counterbalanced across participants so that each picture set was assigned to the taVNS condition in one list, to the sham condition in another list (both lists used as the old condition in the later recognition memory task) and to be novel in the remaining two lists (used for new condition in the later recognition memory task).
In each of the encoding sessions, a total of 60 scenes (30 neutral, 30 unpleasant) were presented during 3000 ms with a varying intertrial interval (ITI) of 4000, 4500, or 5000 ms. Picture presentation was pseudorandomized with no more than two scenes of the same category presented consecutively. Participants were instructed to attentively watch the pictures presented on the screen, and no mention of a later memory test was made (i.e., incidental encoding). During the consecutive encoding sessions, either taVNS or sham stimulation was administered (randomized, single-blinded, taVNS-sham, within-subject, crossover design).
Both incidental encoding sessions followed an identical protocol. Participants entered the experimental room; and before undergoing stimulation, they were seated in a comfortable chair in a sound-attenuated, dimly lit room. After sitting at rest for 6 min (during this period, heart rate [HR] was continuously recorded; data are not reported because they do not contribute to answering this research question), different autonomic baseline measures were recorded, including HR, blood pressure (BP), and sAA levels (i.e., putative marker of noradrenergic activity). Thereafter, the stimulation electrodes were applied to the left ear and the intensity was adjusted as described in the following section (similar to Ventura-Bort et al., 2018). Following the stimulation intensity rating, the high-density EEG net was applied, and participants performed the encoding task, which lasted ∼7 min. After this experimental viewing task, the EEG net and the stimulation electrodes were removed, and autonomic measures (HR, BP, and sAA levels) were recorded again. Finally, after another resting period of 6 min without stimulation (HR measurement, see resting period before stimulation), participants were asked to report, on a 7-point scale (ranging from 1 [not at all] to 7 [very much]), how much they experienced the following symptoms during the stimulation: headache, nausea, dizziness, neck pain, muscle contractions in the neck, stinging sensations under the electrodes, skin irritation in the ear, fluctuation in concentration or feelings, and unpleasant feelings.
One week after the first of the two sessions, participants returned to the laboratory to perform a recognition memory task. After arrival, participants were seated in a comfortable chair in the same room as during encoding. First, participants rested during a 6 min interval while their HR was continuously recorded. Thereafter, the EEG electrodes were attached, a saliva sample was collected (data are not reported because they do not contribute to answering this research question), and the recognition memory task was performed, which lasted ∼26 min. In this task, all 120 previously seen pictures (i.e., 60 from each encoding session) were presented randomly intermixed with 120 new pictures. Each image was shown on the screen for 3000 ms, preceded by a 2000 ms fixation cross. Following picture offset, the question “Old/New?” was presented and participants were asked to make an Old or New judgment. If the image was recognized as previously seen during the encoding sessions, they were instructed to press the “Old” button on the keyboard, whereas if the image was identified as novel, they were instructed to press the “New” button. After the button press, participants were asked to rate the confidence of their recognition judgment on a Likert scale ranging from 0 (not confident) to 10 (absolutely confident). Finally, a saliva sample was collected, the net was removed, and HR was continuously recorded during a 6 min resting period (Fig. 1).
taVNS
The taVNS stimulator, which consisted of two titan electrodes attached to a mount, was located in the left auricle, and wired to a stimulation unit (CMO2, Cerbomed). In the taVNS condition, the stimulator was placed in the left cymba conchae, an area innervated exclusively by the auricular branch of the VN (Peuker and Filler, 2002; Ellrich, 2011). As in previous studies using taVNS (Kraus et al., 2007; Steenbergen et al., 2015; Burger et al., 2016; Szeska et al., 2020), for sham condition, the electrodes were positioned in the center of the left ear lobe, an area known to be free of vagal innervation (Peuker and Filler, 2002; Ellrich, 2011). The stimulation was delivered continuously (Fischer et al., 2018; Ventura-Bort et al., 2018) with a pulse width of 200-300 µs at 25 Hz. The auricular branch of the VN is related to touch sensation. Therefore, to ensure its activation, the stimulus intensity was set to be perceived, but without generating discomfort. Thus, the stimulation was adjusted above the detection threshold and below the pain threshold (Ellrich, 2011). In order to individually regulate the stimulation intensity, participants received increasing and decreasing series of 10 s stimulation trials, and rated the subjective sensation of the stimulation on a 11 point scale, ranging from nothing (0), light tingling (3), strong tingling (6), to painful (10). The increasing series of trials started from an intensity of 0.1 mA and increased 0.1 mA on a trial-by-trial basis until participants reported a “tingling” sensation of 9. Before starting the decreasing series, the same intensity was repeated and then reduced trial by trial in 0.01 mA steps until a subjective sensation of ≤6 was experienced. This procedure was repeated a second time. The final stimulation intensity used for the experimental procedure was calculated based on the average of the four intensities rated as 8 (i.e., 2 from increasing and 2 from decreasing series). The average stimulation intensity for both conditions was as follows: 1.34 mA (0.4-3.5 mA) for active and 1.58 mA (0.5-3.9 mA) for sham condition. The stimulation intensity did not differ between both conditions (t(36) = 1.46, p = 0.15, d = 0.24). The stimulation was administered continuously during each of the encoding tasks (∼7 min).
Autonomic measures
To evaluate the effects of stimulation on autonomic reactivity, we measured HR and BP (systolic and diastolic) before (baseline) and after stimulation in both experimental encoding sessions. HR was measured using a portable HR Monitor (RS800, Polar Electro Oy), and BP was assessed with an upper arm cuff placed on the left arm, using the Riva-Rocci method. In addition, sAA was also measured as a marker of endogenous noradrenergic activation (Chatterton et al., 1996; Warren et al., 2017). Saliva samples were collected using regular cotton Salivette sampling devices (Sarstedt). Participants were instructed to gently chew the swab in their mouths for 60 s. After removal, saliva samples were stored at −20°C. Analyses were performed by the Dresden LabService (http://www.labservice-dresden.de) using an enzyme kinetic method.
Electrophysiological recording
EEG signals were recorded continuously from 257 electrodes using an Electrical Geodesics HydroCel high-density EEG system with NetStation software on a Macintosh computer. The EEG recording was digitized at a rate of 250 Hz, using vertex sensor (Cz) as recording reference. Scalp impedance for each sensor was kept to <30 kΩ, as recommended by the manufacturer's guidelines. All channels were bandpass filtered online from 0.1 to 100 Hz. Offline reduction was performed using ElectroMagnetic EncephaloGraphy Software (Peyk et al., 2011) a well-suited software for EEG analyses using dense array sensor nets (Junghöfer et al., 2000), which included lowpass filtering (at 20 Hz for the encoding tasks and at 40 Hz for the retrieval task), artifact detection, sensor interpolation, baseline correction, and conversion to the average reference (Junghöfer et al., 2000). The MATLAB-based toolbox BioSig (Vidaurre et al., 2011) was used for eye movement and blink artifact corrections of the extracted epochs. This method is based on linear regression to reliably remove electro-oculogram activity from the EEG (Schlögl et al., 2007). If after artifact correction, a sensor within an individual trial was artifact-contaminated, activity on that sensor was replaced by means of spherical spline interpolation, statistically weighted on the basis of the remaining sensors. For both the encoding and the retrieval sessions, stimulus-synchronized epochs were extracted from 100 ms before to 1200 ms after picture onset and baseline-corrected (100 ms before stimulus onset).
Analyses
Self-report and autonomic measures
Because of technical issues (i.e., sample loss), data from 2 participants for sAA and BP, and from 1 participant for HR, could not be used, leaving a total sample of 35 and 36 participants for these analyses, respectively (Table 1). To test for potential side effects induced by the stimulation, t tests for the ratings comparing taVNS and sham stimulation for each reported subjective symptom were performed, separately. To test the effects of stimulation on autonomic reactivity and salivary levels, a repeated-measures ANOVA with the within-subject factors Time (pre- vs post-stimulation) and Stimulation (taVNS vs Sham) was performed for each variable, separately. The log transformation was successfully applied to the skewed sAA data (W = 0.98, p < 0.001) to achieve a normal distribution (W = 0.72, p = 0.07). If interaction effects were found (or exploratory analysis conducted), bootstrapped paired t tests were performed using 10,000 permutations with replacement. The bootstrap CIs are reported for each post hoc t-test.
Behavioral performance
Data from 1 participant could not be included in the analyses (the retrieval session did not take place), leaving a total of 36 participants.
Recognition memory
To evaluate the effects of taVNS on recognition memory performance, the discrimination index Pr, p(hit) – p(false alarm), and the bias index Br, p(false alarm)/(1 – Pr), were calculated for each stimulation condition and emotional category separately (Snodgrass and Corwin, 1988). Whereas Pr is an index of memory discrimination, with higher values associated with better discrimination, Br represents the measure of response bias, with values >0.5 representing a liberal response criterion (bias to respond “Old”) and lower values indicating a conservative response bias. In addition, d prime2 (d′), derived from signal detection theory, was calculated: z(p[hit]) – z(p[false alarm]). Pr, Br, and d′ were analyzed using a repeated-measures ANOVA, including the within-subject factors Emotion (Unpleasant vs Neutral) and Stimulation (taVNS vs Sham).
Recognition memory based on confidence ratings
Considerable evidence suggests that the extent of confidence may differentiate between processes involved in memory retrieval. Memory judgments based on familiarity may be reflected by a low but gradually increase of recognition confidence, whereas recollection-based memory judgments may be represented by the highest level of confidence (Yonelinas, 2001, 2002; Wixted and Stretch, 2004; Rimmele et al., 2012). This assumption is supported by evidence showing that confidence of a memory judgment is highly correlated with its accuracy (Mickes et al., 2009), and therefore related to recollection. In the same line, familiarity-driven memory decisions are associated with a wide range of recognition confidence (ranging from very low to very high recognition confidence), whereas recollection-driven memory decisions are related to the highest level of recognition confidence (Yonelinas, 2001). Similarly, electrophysiological markers of recollection memory are more pronounced for correct information retrieved with high, compared with low, confidence (Weymar et al., 2009; Wynn et al., 2019). Importantly, many studies found that memory for emotional information is, to a greater extent, modulated by the process of recollection rather than familiarity (e.g., Sharot et al., 2004; Dolcos et al., 2005). Neuroimaging studies suggest that this bias toward a better recollection of emotional memories is related to a greater involvement of regions, such as HC and AMY (Yonelinas et al., 2002; Dolcos et al., 2005; Rugg et al., 2012; Yonelinas and Ritchey, 2015). Given that these regions receive strong noradrenergic projection from the LC (McGaugh, 2015; McIntyre et al., 2012; Sara and Bouret, 2012), and are modulated by taVNS (Frangos et al., 2015), the effects of taVNS are expected to be more pronounced when memory is based on recollection, rather than familiarity. Hence, to explore the modulatory effects of stimulation on emotional memory performance based on recollection- and familiarity-based retrieval processes, hit rates were split based on their confidence ratings. Hit rates with a confidence rating of 10 (i.e., absolutely confident) were classified as Recollection-based hit rates, and hit rates with a confidence rating <10 were classified as Familiarity-based hit rates. The effects of taVNS on memory recognition based on confidence ratings were evaluated using a 3 × 2 repeated-measures ANOVA with the within-subject factors Emotion, Stimulation, and Memory (recollection- vs familiarity-based). Similarly, d′ indexes were calculated for recollection-based and familiarity-based judgments and submitted to a 3 × 2 repeated-measures ANOVA with the within-subject factors Emotion, Stimulation, and Memory.3
Receiver operating characteristic (ROC) analyses
In addition, to further investigate the differential effects of taVNS on familiarity and recollection processes, individual behavioral responses were used to create ROC curves. The ROCs were fit with the dual-process signal detection model (Yonelinas and Parks, 2007), using the ROC toolbox (Koen et al., 2017), to extract estimates of recollection and familiarity. To do so, responses were transformed into a 10 bin scale using the criteria in Table 2.
Table 2.
Adaptation of memory performance to a 10-bin scale based on confidence ratings for ROC analysis
| 10-bin scale | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Response (rating bins) | New (10-9) | New (8-7) | New (6-5) | New (4-3) | New (2-0) | Old (0-2) | Old (3-4) | Old (5-6) | Old (7-8) | Old (9-10) |
The recollection and familiarity estimates were analyzed using a 3 × 2 repeated-measures ANOVA with Emotion, Memory, and Stimulation as within-subject factors.
For each behavioral dependent variable, significant interactions were followed up by lower-level ANOVAs and paired t-test comparisons. Paired t tests were bootstrapped using 10,000 permutations with replacement. The CIs of the bootstrapping are reported for each post hoc t test.
Electrophysiology: cluster-based permutation test
To test for stimulation effects on encoding and recognition-related ERPs, data were submitted to a nonparametric statistical testing procedure that includes correction for multiple comparisons (Maris and Oostenveld, 2007), the so-called cluster-based permutation test. This test uses a two-step procedure to identify significant effects between conditions. In a first step (i.e., sensor-level criterion), F tests are performed for each time point and sensor. When those with a significant α value of p = 0.05 during at least five consecutive time points (i.e., 20 ms), and for at least five neighbor sensors are detected, their F values are summed in “cluster masses.” In a second step (cluster-level criterion), using Monte Carlo simulations of 1000 permuted drawings of experimental conditions and participants, random permutation cluster masses are extracted and compared against the original cluster masses with an α level of p = 0.05. Only cluster masses that surpassed the α level of p = 0.05 in a previously defined time epoch and electrode site are considered significant.
Encoding
Individual ERP averages were computed for each sensor, emotional category (Emotion: neutral and unpleasant), and stimulation type (Stimulation: taVNS and Sham). Three participants were excluded because of a low number of good trials per condition left after EEG-data preprocessing (n < 9), leaving a total of 34 participants for the analysis. Emotion effects are typically observed in the LPP components (Cuthbert et al., 2000). The LPP has been shown to be modulated by both emotionally arousing stimuli and stress contexts (e.g., Weymar et al., 2011). LPPs have been identified as more positive-going waveforms for emotional compared with neutral material over posterior regions at different time windows (Foti et al., 2009). Therefore, in line with previous studies (e.g., Pastor et al., 2015; Rehbein et al., 2015; Bublatzky et al., 2020), cluster-based permutation tests were performed over the posterior sites on an earlier (200-600 ms) and later (600-1200 ms) time window. First, the main effects of Emotion regardless of stimulation were tested to replicate the larger LPP toward emotional material (Foti et al., 2009). Second, to elucidate the effects of stimulation on emotion processing, the Emotion × Stimulation interaction was submitted to the cluster-based permutation analysis.
Retrieval
Previous ERP research has consistently found that recognition of old information produces an overall larger positivity compared with correctly identified new material, the so-called ERP Old/New effect (Rugg and Curran, 2007). Critically, it has been shown that recollection- and familiarity-based processes have different electrophysiological signatures (Rugg and Curran, 2007; Wilding and Ranganath, 2012). Whereas familiarity-related processes are typically identified in an early (300-500 ms) ERP Old/New effect over frontal regions (Woodruff et al., 2006; Rugg and Curran, 2007; Yu and Rugg, 2010), recollection-based processes are related to a late (>400 ms), more parietally located, ERP Old/New effect. In support of this differentiation, ERP studies on emotional memory have robustly shown that the memory-enhancing effects of emotion are specifically observed in the late, but not in the early, ERP Old/New effect (Weymar et al., 2009, 2010b, 2011), indicating recollection-mediated retrieval. Furthermore, the recollection sensitive ERP Old/New effect has been shown to be modulated by emotional arousal during encoding (e.g., Weymar et al., 2010a; Weymar and Hamm, 2013; Wirkner et al., 2013). Two sets of analysis were performed during retrieval.
First, we investigated the main effects of memory on familiarity- and recollection-based ERP components. To do so, individual ERP averages were computed for each sensor, emotional category (Emotion: neutral and unpleasant), and memory judgment (hits, correct rejections, false alarms, and misses). Two participants were excluded because of an insufficient number of good trials per condition after EEG-data preprocessing (n < 9), leaving a total of 34 participants for the analysis. The cluster-based permutation test was performed over both anterior and posterior sites, in an earlier (200-500 ms) and later (400-1000 ms) time window, separately, comparing the Memory (hits vs correct rejections) as well as the interacting Memory × Emotion effects.
Second, to test the effect of stimulation on emotional memory retrieval, ERPs from correctly identified old pictures were submitted to the cluster-based permutation test, comparing the interacting effects of Emotion (neutral and unpleasant) and Stimulation (taVNS and Sham), using the same time windows (200-500 ms and 400-1000 ms) and location (both anterior and posterior) as in the previous analysis.
Based on prior studies showing a positive relation between the increase of sAA as a putative marker of central NE (Ehlert et al., 2006; Warren et al., 2017), and the P3b ERP component (Ventura-Bort et al., 2018), we explored the effects of taVNS on changes in sAA levels and ERP amplitudes, both during encoding and retrieval, using bivariate and repeated-measures correlations (Bakdash and Marusich, 2017). Given the exploratory nature of this analysis, a Bonferroni correction was applied.
Results
Self-report and autonomic measures
Self-reported symptom ratings
Results from the self-reported symptom ratings after stimulation are shown in Table 3. Overall, reported symptoms were low and did not differ between taVNS and sham conditions in any of the screened symptoms (p > 0.19).
Table 3.
Mean (SD) subjective ratings for the stimulation side effects (rated from 1 [not at all] to 7 [very much]) in the active and sham condition (including t test comparing both conditions)
| Sham | taVNS | t | Degrees of freedom | p | |
|---|---|---|---|---|---|
| Headache | 1.13 (0.34) | 1.27 (0.73) | −1.22 | 36 | 0.23 |
| Nausea | 1.13 (0.42) | 1.16 (0.55) | −0.25 | 36 | 0.8 |
| Dizziness | 1.16 (0.44) | 1.3 (0.87) | −0.8 | 36 | 0.41 |
| Neck pain | 1.16 (0.5) | 1.35 (0.82) | −1.31 | 36 | 0.19 |
| Neck contraction | 1.37 (0.55) | 1.41 (0.68) | −0.27 | 36 | 0.78 |
| Stinging sensation | 2.27 (1.53) | 2 (1.41) | 0.76 | 36 | 0.45 |
| Ear irritation | 1.21 (0.62) | 1.21 (0.58) | 0 | 36 | 1 |
| Concentration | 1.70 (0.77) | 1.89 (1.19) | −1.07 | 36 | 0.29 |
| Fluctuation of feelings | 1.43 (1.12) | 1.57 (1.3) | −1.43 | 36 | 0.46 |
| Unpleasant feelings | 1.83 (1.07) | 1.86 (1.25) | -0.12 | 36 | 0.91 |
Autonomic measures
HR, BP, and salivary data are presented in Table 4. A main effect of Time for diastolic BP (F(1,34) = 6.72, p = 0. 014, ηp2 = 0.16) and HR (F(1,35) = 46.47, p = 0.014, ηp2 = 0.57) indicated habituation of the autonomic activation during the experiment. This reduction was not observed for systolic BP (F(1,34) = 1.58, p = 0.21, ηp2 = 0.04). A main effect of Stimulation was observed for both diastolic (F(1,34) = 5.58, p = 0.024, ηp2 = 0.14) and systolic BP (F(1,34) = 9.46, p = 0.004, ηp2 = 0.21), but not for HR (F < 1), with larger values in the taVNS than in the sham session. Most importantly, no Time × Stimulation interaction effects were observed for the diastolic (F < 1), the systolic BP (F(1,34) = 1.83 p = 0.19, ηp2 = 0.05), nor the HR (F < 1), suggesting that taVNS did not have any specific effects on autonomic changes over time.
Table 4.
Mean (SD) of the autonomic and salivary measures before and after the stimulation
| Time | HR (bpm) | Systolic BP (mmHg) | Diastolic BP (mmHg) | α-Amylase (μkatal/L) | Log(α-amylase) | |
|---|---|---|---|---|---|---|
| taVNS | Pre | 79.52 (20.10) | 117.02 (11.67) | 78.32 (8.59) | 113.71 (120.04) | 4.32 (0.94) |
| Post | 67.94 (14.81) | 115.65 (12.69) | 79.37 (7.48) | 175.25 (171.63) | 4.75 (0.96) | |
| Sham | Pre | 77.1 (14.03) | 114.13 (12.50) | 75.35 (7.48) | 116.88 (100.78) | 4.45 (0.82) |
| Post | 66.68 (13.14) | 114 (12.13) | 77.46 (8.58) | 167.98 (168.66) | 4.71 (0.99) |
For sAA, a main effect of Time was observed (F(1,34) = 12.22, p = 0.001, ηp2 = 0.26), indicating that the activation of the (central) noradrenergic system increased with time. However, no Stimulation (F < 1) or interaction effects (F(1,34) = 1.32, p = 0.26, ηp2 = 0.04) were observed (F < 1). Exploratory analysis to test for the effects of stimulation for both conditions, separately, revealed that taVNS significantly increased sAA levels (t(34) = 3.48, p < 0.001 d = 0.59; bootstrapped CI [0.16, 0.65]), whereas sAA increases after sham stimulation was only significant at trend level (t(34) = 1.95, p = 0.059, d = 0.33; bootstrapped CI [−0.01, 0.47]).
Behavioral performance
Table 5 summarizes the behavioral results (mean and SD) for the recognition memory task as a function of emotion and stimulation.
Table 5.
Mean (SD) of behavioral indices for unpleasant and neutral images encoded under sham and taVNS stimulation
| Sham |
taVNS |
|||
|---|---|---|---|---|
| Unpleasant | Neutral | Unpleasant | Neutral | |
| Item recognition | ||||
| Pr | 0.63 (0.13) | 0.52 (0.16) | 0.64 (0.15) | 0.55 (0.15) |
| Br | 0.35 (0.22) | 0.32 (0.20) | 0.47 (0.26) | 0.44 (0.25) |
| d′ | 1.99 (0.56) | 1.72 (0.63) | 2.12 (0.76) | 1.74 (0.59) |
| Recognition memory based on confidence ratings | ||||
| Familiarity-based hit rate | 0.45 (0.18) | 0.44 (0.16) | 0.42 (0.19) | 0.46 (0.17) |
| Recollection-based hit rate | 0.34 (0.17) | 0.24 (0.17) | 0.39 (0.22) | 0.24 (0.18) |
| Familiarity-based d′ | 0.99 (0.63) | 0.99 (0.64) | 0.91 (0.69) | 1.06 (0.66) |
| Recollection-based d′ | 1.76 (0.64) | 1.49 (0.70) | 1.9 (0.72) | 1.48 (0.73) |
| ROC analyses | ||||
| Familiarity estimates | 1.48 (0.63) | 1.1 (0.50) | 1.52 (0.65) | 1.35 (0.65) |
| Recollection estimates | 0.34 (0.21) | 0.31 (0.17) | 0.40 (0.26) | 0.27 (0.19) |
Recognition memory
For Pr, results revealed a main effect of Emotion (F(1,35) = 29.69, p < 0.001, ηp2 = 0.46), showing larger memory discrimination for emotional, compared with neutral images. However, no Stimulation (F(1,35) = 1.64, p = 0.208, ηp2 = 0.04) or interaction effects were observed (F < 1). For Br, a main effect of Emotion was observed (F(1,35) = 21.38, p < 0.001, ηp2 = 0.38), indicating a more conservative response bias for neutral than for emotional images. However, no Stimulation (F(1,35) = 2.3, p = 0.14, ηp2 = 0.06) or interaction effects were found (F < 1). For d′, a main effect of Emotion was also found (F(1,35) = 14.22, p < 0.001, ηp2 = 0.29), indicating better memory discrimination for emotional, compared with neutral images. No Stimulation (F(1,35) = 1.36, p = 0.25, ηp2 = 0.04) or interaction effects (F < 1) were found.
Recognition memory based on confidence ratings
When hit rates were split based on subjective confidence ratings to examine the differential contribution of recollection (rating = 10) and familiarity (rating: 1-9), a main effect of Emotion (F(1,35) = 46.03, p < 0.001, ηp2 = 0.57), indicating larger hit rates for emotional compared with neutral images, and a main effect of Memory (F(1,35) = 7.06, p = 0.01, ηp2 = 0.17), indicating larger hit rates for familiarity compared with recollection, were observed, but no main effect of Stimulation (F(1,35) = 1.47, p = 0.23, ηp2 = 0.04). The Stimulation × Emotion interaction (F < 1) and the Stimulation × Memory interaction (F(1,35) = 1.66, p = 0.21, ηp2 = 0.04) were not significant. Critically, both the Memory × Emotion (F(1,35) = 19.48, p < 0.001, ηp2 = 0.36) and the Memory × Emotion × Stimulation interaction were significant (F(1,35) = 7.01, p =0.012, ηp2 = 0.17).
Repeated-measures 2 × 2 ANOVAs for recollection- (high confidence) and familiarity-based (low confidence) judgments were conducted. For familiarity-based judgments, no effect of Emotion (F(1,35) = 1.44, p = 0.238, ηp2 = 0.04) or Stimulation (F < 1) was observed. However, the interaction reached significance (F(1,35) = 5.77, p < 0.022, ηp2 = 0.14). Nevertheless, t tests comparing both stimulation conditions for each emotional category separately did not reveal any significant results (Unpleasant, t(35) = −1.80, p = 0.08, d= 0.3; bootstrapped CI [-0.06, 0.06]; Neutral t(35) = 1.12, p = 0.27, d = 0.18; bootstrapped CI [−0.16, 0.06]). Critically, for recollection-based judgments, a main effect of Emotion was observed (F(1,35) = 39.12, p < 0.001, ηp2 = 0.53), but no main effect of Stimulation was found (F(1,35) = 3.57, p = 0.06, ηp2 = 0.09). Most relevant, an Emotion × Stimulation interaction effect was significant (F(1,35) = 4.76, p = 0.036, ηp2 = 0.12). t tests comparing both stimulation conditions for each emotional category, separately, revealed significant differences between taVNS and sham conditions for unpleasant (t(35) = 2.81, p = 0.008, d = 0.46; bootstrapped CI [0.01, 0.08]) but not for neutral pictures (t < 1; bootstrapped CI [−0.03, 0.04]) (Fig. 2A).
Figure 2.
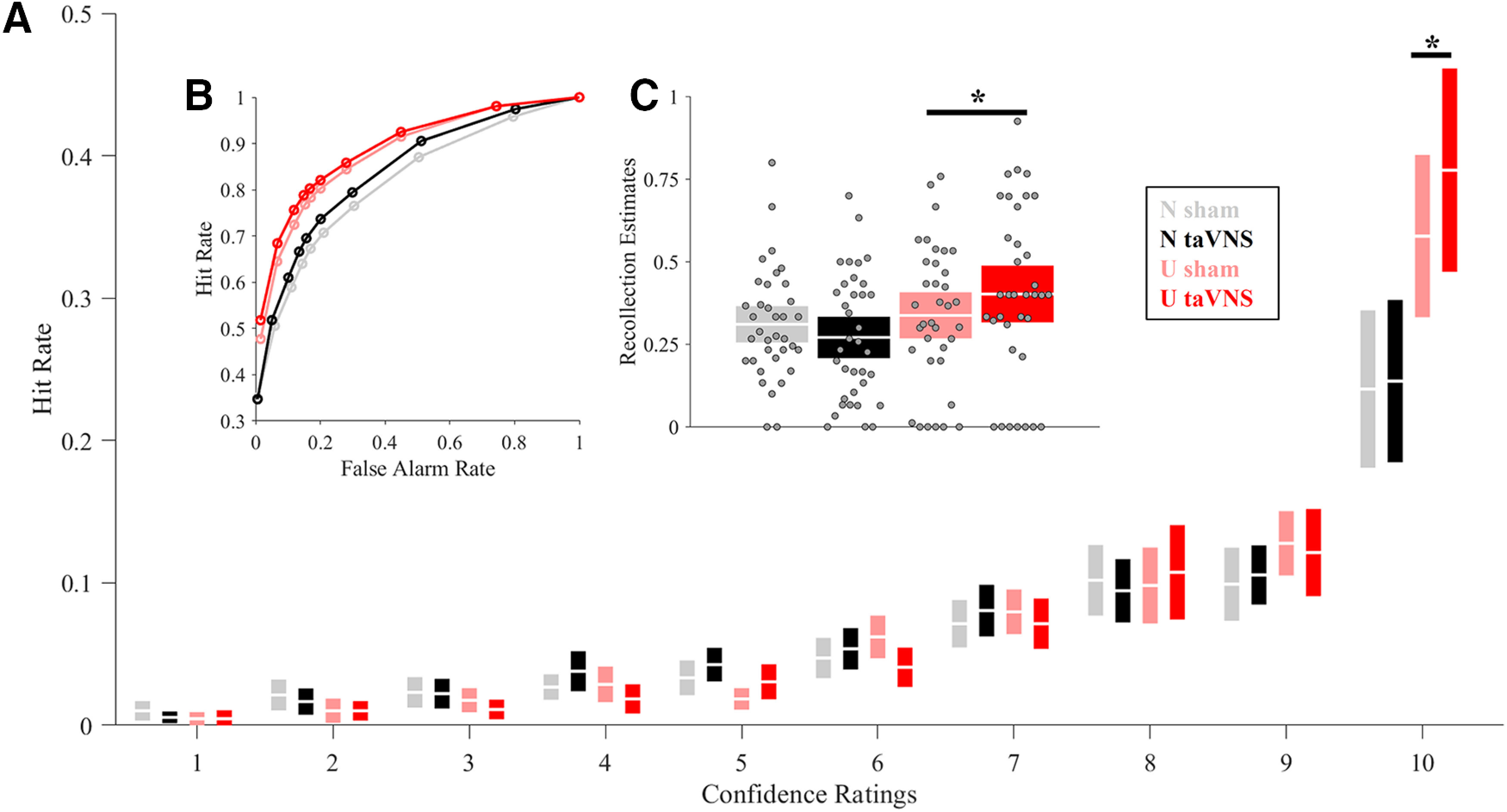
The main behavioral findings. A, Hit rates based on confidence ratings reflecting recollection and memory. Hit rates under high confidence ratings (recollection) were larger for unpleasant images encoded under taVNS, compared with sham condition. B, ROC curve for each condition. C, Recollection estimates extracted from the ROCs indicated larger recollection-based memory for unpleasant images under taVNS compared with sham. Boxplots represent the 95% CI. White line in boxplots indicates the mean. N, Neutral; U, unpleasant. *p < 0.05.
Analyses on d′ showed main effects of Emotion (F(1,35) = 10.24, p = 0.003, ηp2 = 0.22) and Memory (F(1,35) = 12.63, p = 0.003, ηp2 = 0.27), indicating better discrimination for emotional images, and for recollection-related judgments, respectively. However, no main Stimulation effect (F(1,35) < 1.05, p = 0.31, ηp2 < 0.03) or Stimulation × Emotion (F < 1) effects were found. An Emotion × Memory interaction was observed (F(1,35) = 12.13, p = 0.001, ηp2 = 0.26), as well as a three-way Emotion × Memory × Stimulation interaction (F(1,35) = 6.85, p = 0.013, ηp2 = 0.13). Post hoc testing for familiarity-based judgments showed no main effects of Emotion or Stimulation (F < 1). However, the interaction reached significance (F(1,35) = 5.72, p < 0.02, ηp2 = 0.14). Nevertheless, t tests comparing both stimulation conditions for each emotional category separately did not reveal any significant results (Unpleasant: t(35) = −1.74, p = 0.09, d = 0.29; bootstrapped CI [−0.009, 0.18]; Neutral: t(35) = 1.18, p = 0.25, d = 0.19; bootstrapped CI [−0.05, 0.18]). For recollection-based judgments, however, a main effect of Emotion was observed (F(1,35) = 16.64, p < 0.001, ηp2 = 0.32), but no main effect of Stimulation was found (F(1,35) = 1.99, p =0.17, ηp2 = 0.05). As for recollection-based hit rates, an Emotion × Stimulation interaction effect was significant (F(1,35) = 4.25, p = 0.04, ηp2 = 0.11). t test comparing both stimulation conditions for each emotional category separately revealed significant differences between taVNS and Sham conditions for Unpleasant (t(35) = 2.71, p = 0.01, d = 0.45; bootstrapped CI [0.03, 0.23]), but not for Neutral pictures (t < 1; bootstrapped CI [−0.13, 0.12]).
ROC analyses
Figure 2B depicts the ROCs for each condition. Results from the three-way ANOVA showed a main effect of Memory (F(1,35) = 142.6, p < 0.001, ηp2 = 0.8), a main effect of Stimulation (F(1,35) = 4.91, p = 0.03, ηp2 = 0.12), and a main effect of Emotion (F(1,35) = 20.71, p < 0.001, ηp2 = 0.37). The Emotion × Memory approached significance (F(1,35) = 3.9, p = 0.056, ηp2 = 0.1); however, Stimulation interacted neither with Emotion (F(1,35) = 1.23, p = 0.27, ηp2 = 0.03) nor with Memory (F(1,35) = 2.67, p = 0.11, ηp2 = 0.07). Critically, the triple interaction Emotion × Memory × Stimulation was significant (F(1,35) = 6.43, p = 0.02, ηp2 = 0.15). Subsequently, the effect of stimulation on emotional memory was separately tested for the familiarity and recollection estimates extracted from the ROC curves. For the familiarity estimates, no Stimulation effect was found (F(1,35) = 3.75, p = 0.06, ηp2 = 0.1). However, a significant Emotion effect was observed (F(1,35) = 10.84, p = 0.002 ηp2 = 0.24). Also, a significant Emotion × Stimulation interaction was found (F(1,35) = 4.32, p = 0.045, ηp2 = 0.11). Follow-up t tests showed significant differences between conditions for neutral images (t(35) = −3.37, p = 0.002, d = 0.56; bootstrapped CI [−0.39, −0.11]) but not for unpleasant ones (t < 1; bootstrapped CI [-0.24, 0.17]).
For the recollection estimates, no effect of Stimulation was observed (F < 1), but a significant Emotion effect (F(1,35) = 5.35, p = 0.027, ηp2 = 0.13). Additionally, a significant Emotion × Stimulation was observed (F(1,35) = 5.83, p = 0.021, ηp2 = 0.14). Follow-up t tests showed no differences between condition for neutral images (t(35) = −1.72, p = 0.093, d = 0.29; bootstrapped CI –0.005, 0.08]), but larger recollection estimates for unpleasant images encoded under taVNS compared with sham (t(35) = 2.24, p = 0.032, d = 0.37; bootstrapped CI [0.009, 0.12]; Fig. 2C).
In summary, our results replicate previous findings, showing a general memory advantage for emotional compared with neutral material. Furthermore, when the contribution of familiarity and recollection was assessed (via confidence ratings), the memory-enhancing effect of emotion was particularly driven by the process of recollection (replicating prior studies). In the absence of a stimulation effect on overall behavioral performance, we consistently found across behavioral measures (i.e., hit rate, d′ and ROC analyses) that noninvasive VN stimulation, compared with sham, specifically facilitated emotional episodic recollection memory.
Electrophysiological analyses: permutation test
Figure 3 depicts the main ERP findings during encoding and recognition of emotional and neutral scenes.
Figure 3.
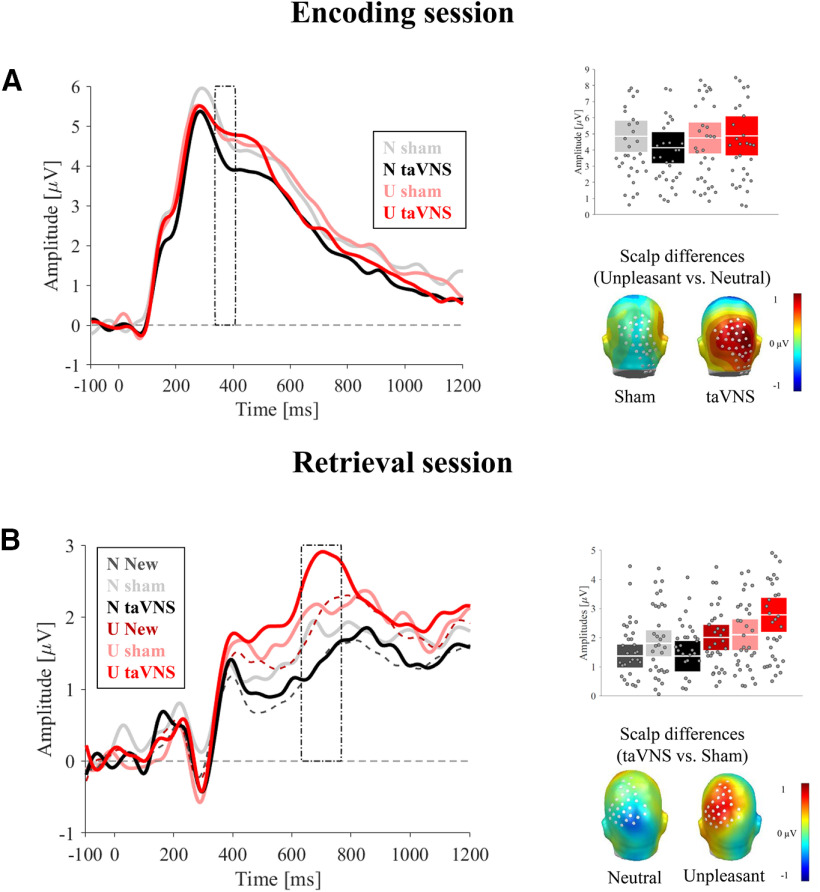
ERP results during encoding (A) and retrieval (B). A, Left, ERP-averaged waveforms across the significant sensor cluster. A, Top right, Mean averaged ERPs during the significant time window (332-400 ms) and sensor cluster, showing a larger emotion discrimination during taVNS than sham condition. Boxplots represent the 95% CI. White line in boxplots indicates the mean. A, Bottom right, Scalp topographies showing emotional differences during the taVNS and sham condition. B, Left, ERP-averaged waveforms across the significant sensor cluster. A, Top right, Mean averaged ERPs during the significant time window (628-760 ms) and sensor cluster, showing a larger activity for correctly retrieved unpleasant images encoded during taVNS, compared with sham. Boxplots represent the 95% CI. White line in boxplots indicates the mean. A, Bottom right, Topographical differences (taVNS-sham) for unpleasant and neutral images. N, Neutral; U, unpleasant.
Encoding
LPPs
In the early time window of the LPP (200-600 ms), the cluster-based permutation test revealed a main effect of emotion over posterior sites, as indicated by a significant cluster (mass of 30,648.3) surpassing the critical cluster mass of 2118. The cluster extended from 356 to 600 ms (sensors: 45, 53, 59, 60, 65, 66, 70, 71, 72, 75, 76, 77, 78, 79, 80, 81, 84, 85, 86, 87, 88, 89, 90, 96, 97, 98, 99, 100, 101, 106, 107, 108, 109, 110, 115, 116, 117, 118, 119, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 137, 138, 139, 140, 141, 142, 143, 144, 149, 150, 151, 152, 153, 154, 155, 158, 159, 160, 161, 162, 163, 164, 168, 169, 170, 171, 172, 173, 177, 178, 179, 180, 181, 182, 190, 191, 192, 193, and 257). Subsequent t tests showed that emotional pictures produced larger amplitudes (mean = 2.89 µV, SD = 1.66) than neutral pictures (mean = 2.45 µV, SD = 1.26; t(33) = 4.49, p < 0.001). No main effects of stimulation were observed.
In the late time window, three clusters were found over posterior sites with a mass larger than the critical mass of 2102.5 (Cluster 1: 37,272.4; Cluster 2: 4059.1; Cluster 3: 2757.1) for the time windows from 600-904 (Cluster 1), from 1088-1200 (Cluster 2), and from 660-892 (Cluster 3). Only Cluster 1 (encompassing 45, 53, 59, 60, 65, 66, 70, 71, 72, 74, 75, 76, 77, 78, 79, 80, 81, 84, 85, 86, 87, 88, 89, 90, 96, 97, 98, 99, 100, 101, 106, 107, 108, 109, 110, 116, 117, 118, 119, 126, 127, 128, 129, 130, 131, 132, 139, 140, 141, 142, 143, 144, 151, 152, 153, 154, 155, 160, 161, 162, 163, 164, 170, 171, 172, 173, 180, 181, 182, and 257) showed enhanced ERP amplitudes toward emotional pictures (mean = 2.07 µV, SD = 1) compared with neutral pictures (mean = 1.6 µV, SD = 0.71; t(33) = 4.98, p < 0.001, d = 0.85).
Effects of stimulation on emotion processing4
In the early time window (200–600 ms), the cluster-based permutation test comparing the differential effects of stimulation on emotional processing over posterior regions revealed a cluster exceeding the critical cluster mass of 1596.5. The significant cluster (mass of 1793.1) was found between 332 and 400 ms (sensors: 98, 101, 108, 109, 110, 116, 117, 118, 119, 125, 126, 127, 128, 129, 137, 138, 139, 140, 141, 148, 149, 150, 151, 157, 158, 159, 160, 166, 167, 168, 169, 174, 175, and 176). Subsequent t tests showed larger positivity for unpleasant (mean = 4.87 µV, SD = 3.59), relative to neutral scenes under taVNS (mean = 4.11 µV, SD = 2.86; t(33) = 3.45, p = 0.001, d = 0.59), whereas no differences between picture categories were observed under sham stimulation (mean = 4.73 µV, SD = 2.85 for unpleasant; mean = 4.83 µV, SD = 2.9 for neutral; t < 1). Furthermore, neutral images during taVNS, compared with sham, showed reduced LPP amplitudes (t(33) = −2.23, p = 0.032, d = 0.38). Unpleasant images, however, prompted comparable LPP amplitudes across conditions (t < 1).
The cluster-based permutation test in the late time window (600-1200 ms) over posterior electrodes did not reveal any cluster exceeding the critical cluster mass of 1718.5.
Recognition
Old/New effects
Significant overall memory effects were found in the late time window (400-1000 ms) between 428 and 700 ms (cluster mass: 17,540; critical cluster mass: 3607) over central sensors: 5, 6, 7, 8, 9, 14, 15, 16, 17, 20, 21, 22, 23, 24, 28, 29, 30, 42, 43, 44, 45, 51, 52, 53, 60, 66, 77, 78, 79, 80, 81, 87, 88, 89, 90, 129, 130, 131, 132, 141, 142, 143, 144, 153, 154, 155, 163, 164, 173, 183, 184, 185, 186, 196, 197, 198, 206, 207, 214, 215, 224, and 257. Subsequent testing revealed larger activity for correctly retrieved old images (mean = −0.26 µV, SD = 1.07), compared with correctly identified new ones (mean = −0.65 µV, SD = 1.15; t(33) = 4.71, p < 0.001, d = 0.81). A second significant cluster was further obtained in the time window 660-892 ms over frontal regions (sensors: 16, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 42, and 43), reflecting larger activity for correctly identified old (mean = −1.1 µV, SD = 1.66), compared with new images (mean = −1.45 µV, SD = 1.47; t(33) = 2.26, p = 0.031, d = 0.0.39).
In the early time window, a significant cluster emerged (cluster mass: 3830.9; critical cluster mass: 1789.5), during 428-500 ms (sensors: 5, 6, 7, 8, 9, 14, 15, 16, 17, 21, 22, 23, 24, 28, 29, 30, 42, 43, 44, 45, 51, 52, 53, 60, 80, 81, 90, 130, 131, 132, 142, 143, 144, 154, 155, 164, 183, 184, 185, 186, 196, 197, 198, 206, 207, 214, 215, 224, and 257). The significant main effect indicated larger activity for correctly identified old (mean = −1.11 µV, SD = 1.67) than new images (mean = −1.52 µV, SD = 1.8; t(33) = 3.68, p < 0.001, d =0.63).
The cluster-based permutation test revealed significant Emotion × Memory interaction neither in the early (200-500 ms; critical cluster mass: 1719) nor in the later time window (400-1000 ms; critical cluster mass: 3138).
Effects of stimulation on emotional Old/New effect5
To elucidate the effects of stimulation on ERP correlates of emotional episodic memory, the interacting effect of Stimulation and Emotion was calculated for the correctly retrieved trials. The cluster-based permutation test found a significant cluster with a mass of 2853.8 (critical mass = 2694.5) only in the later time window between 628 and 760 ms, over posterior regions, which matches the late Old/New effect in time and space (sensors: 8, 9, 17, 44, 45, 53, 60, 66, 72, 76, 77, 78, 79, 80, 81, 86, 87, 88, 89, 90, 98, 99, 100, 101, 110, 129, 130, and 257). Subsequent t test revealed that, while correctly retrieved neutral pictures encoded under taVNS (mean = 1.35 µV, SD = 1.55) and sham (mean = 1.8 µV, SD = 1.31) evoked comparable ERP amplitudes (t(33) = 1.71, p = 0.096, d = 0.29), the retrieval of encoded unpleasant images was enhanced under taVNS (mean = 2.78 µV, SD = 1.73) compared with sham (mean = 2.09 µV, SD = 1.54) condition (t(33) = 2.78, p = 0.009, d = 0.48). Furthermore, comparing amplitudes for old and new images, we found increased ERP Old/New differences for unpleasant images under taVNS (Unpleasant new images: mean = 1.99 µV, SD = 1.28; t(33) = 2.89, p = 0.007, d = 0.5, bootstrapped CI [0.30, 1.28]), compared with sham (t < 1, bootstrapped CI [−0.31, 0.50]), whereas the opposite pattern was observed for the neutral images (Neutral new images: mean = 1.35 µV, SD = 1.18; Old/New effect for taVNS,: t < 1, bootstrapped CI [−0.41, 0.37], Old/New effect for sham: t(33) = 2.18, p = 0.036, d= 0.37, bootstrapped CI [0.059, 0.8]).
Correlational analysis
Exploratory correlational analysis was performed to reveal stimulation-specific associations between sAA-level changes and ERP changes related to encoding and retrieval. To correct for multiple comparison, we applied the Bonferroni correction (p = 0.05/9 = 0.005). For encoding, bivariate correlations showed no relation between the increase of sAA levels and the LPP difference (negative vs neutral) either during taVNS (r = 0.27, p < 0.13) or during sham (r = 0.08, p < 0.66). However, LPP difference between unpleasant and neutral scenes during taVNS, compared with sham, was positively correlated with the increase of sAA levels during taVNS, compared with sham stimulation at a trend level (r(32) = 0.46, p = 0.009). Additionally, a significant repeated-measures correlation was observed between the increase of the sAA levels and the increase of LPP (r = 0.48, p = 0.004). For retrieval, bivariate correlations showed no relation between the increase of sAA levels and the ERP Old/New effect for any of the stimulation conditions and emotional categories (–0.11< r < 0.15. p > 0.41). Repeated-measures correlations did not show any significant relation between the increase of sAA and the emotional or neutral ERP Old/New effects (–0.01 < r < 0.01, p > 0.57).
In summary, we found enhanced ERP amplitudes toward emotional compared with neutral scenes in early (356-600 ms) and late (600-904 ms) time windows, replicating the well-known LPP effect from prior studies (e.g., Hajcak et al., 2012). Furthermore, an interaction with stimulation in an early LPP time window (332-400 ms) revealed that taVNS modulated the processing of emotional and neutral stimuli. During retrieval, overall Old/New effects were found over central and frontal electrode clusters, indicating that both familiarity and recollection-based processes took place during recognition. Most strikingly, emotional images encoded under taVNS evoked larger activation than those encoded under sham, reflecting an enhancement of recollection-based memory, which is also in line with our behavioral data.
Discussion
Previous animal research has demonstrated that afferent vagal fibers mediate the noradrenergic AMY activation, which causes an HC-dependent memory-enhancing effect for emotional experiences. In the current study, we used taVNS in the context of an emotion recognition memory paradigm to investigate whether the modulation of VN activity during encoding influences the later retrieval of emotional episodic memories in humans. We observed that taVNS during encoding, compared with sham, enhanced recollection-based memory for emotional information as indexed by behavioral and electrophysiological measures.
The memory-enhancing effect of taVNS was observed particularly for contents that were remembered with high levels of confidence and only reflected in the parietal ERP Old/New effect, suggesting that taVNS particularly improved recollection-based memory processes. This finding is consistent with previous literature generally showing a stronger involvement of recollection processes in the retrieval of emotional memories (for review, see Dolcos et al., 2017, 2020). It has been suggested, based on neurobiological and theoretical models, that the VN and the LC are involved in processing emotional memories (McIntyre et al., 2012; McGaugh, 2015; Mather et al., 2016). The VN is one of the main paths through which the peripherally released stress hormones reach back to the brain. Specifically, it has been assumed that, during emotional experiences, the afferent vagal fibers translate emotional arousal to the brain via central noradrenergic release from neurons of the LC, which mediates synaptic communication in regions important for emotional memory formation, such AMY, HC, and frontal cortex (McIntyre et al., 2012, their Fig. 1). In this line, animal research found that invasive VNS increases LC firing rates in rats (Dorr and Debonnel, 2006) and hippocampal activity (Roosevelt et al., 2006; Raedt et al., 2011). It is important to note that the HC plays a crucial role in recollection processes (Brown and Aggleton, 2001; Diana et al., 2007; Eichenbaum et al., 2007; Ranganath and Ritchey, 2012). For instance, it has been observed that hippocampal lesions specifically affect the recollective memory experience (Yonelinas et al., 2002) and the associated electrophysiological activity (Düzel et al., 2001). Similarly, in healthy participants, recollection-based judgments are related to larger hippocampal activation (Yonelinas et al., 2005; Eichenbaum et al., 2007; Diana et al., 2009). The current taVNS effects on recollection-based memory therefore suggest that the VN modulates the activation of this neural circuitry (see also Jacobs et al., 2015; Giraudier et al., 2020; but see Mertens et al., 2020). Interestingly, extending invasive VNS (Clark et al., 1999) and recent taVNS studies (Jacobs et al., 2015; Giraudier et al., 2020) showing increased item and associative memory for lower arousing stimuli following stimulation, we found that taVNS enhanced the recollective experience for emotionally arousing stimuli when presented among neutral stimuli, compared with sham stimulation.6 This selective recollection enhancement for emotional contents suggests that taVNS, likely via the LC, modulated the AMY-HC crosstalk. Thus, our findings support the recent glutamate amplifies noradrenergic effects (GANE) model postulating that emotional arousal enhances LC and AMY function (Mather et al., 2016). This enhancement, in interaction with perceptual regions (Pourtois et al., 2013), promotes attentional processing of salient and motivationally relevant information and, by influencing regions, such as the HC, memory encoding, and consolidation processes (Sara, 2009; McIntyre et al., 2012). Previous data (Ritchey et al., 2008) indicate that the recollection for emotional stimuli is stronger after a longer retention delay (1 week vs immediate; for ERP findings, see also Wirkner et al., 2018), which are also associated with stronger AMY-MTL connectivity. Because we also tested long-term memory (1 week delay), our data may therefore suggest that taVNS influenced recollection for emotional information via enhanced memory consolidation. We, however, did not test immediate recognition memory and did not stimulate during the postencoding phase. Thus, to delineate the differential effects of VN stimulation on memory encoding and consolidation, future studies are needed, testing, for instance, the effects of taVNS during and after encoding on immediate and long-term memory.
During encoding, we expected that taVNS would enhance the processing of emotionally arousing material, compared with sham stimulation. However, LPP amplitudes for emotional scenes encoded during taVNS and sham stimulation did not differ. Instead, early LPP amplitudes for neutral material were diminished when encoded during taVNS compared with sham. Although this result was somewhat unexpected, it may still be accounted for by GANE model (Mather et al., 2016). A key aspect of the Mather et al. (2016) model is the assumption that emotional arousal can increase attention toward information that receives high priority (by top-down and bottom-up processes) while diminishing attention toward low priority information. Within this framework, our findings suggest that taVNS, via activation of the LC-noradrenaline (NA) system, may enhance detection of salient signals (e.g., Colzato et al., 2017; Sellaro et al., 2018) at the cost of processing irrelevant neutral information. Of note, the early LPP, which among other cortical and subcortical regions, also correlates with AMY activity (Sabatinelli et al., 2013), may share the same neuromodulatory activity with the P300, as both components seem to be related to the LC-NA system (Hajcak and Foti, 2020). In line with this assumption, we observed that the increase of sAA levels was related to larger LPP differences (at specific for taVNS at trend level), suggesting that the increase of the central NA facilitated the differentiation between unpleasant and neutral material. Interestingly, prior taVNS studies also found modulatory effects on P300 amplitudes (Rufener et al., 2018; Ventura-Bort et al., 2018; but see Warren et al., 2019) which, consistent with the Adaptive Gain Theory (Aston-Jones and Cohen, 2005; Nieuwenhuis et al., 2005), may reflect a modulatory influence of the LC to increase the neural gain to facilitate the response to task and motivationally relevant events. Our findings therefore suggest that taVNS may facilitate the amplification of relevant information, by means of inhibiting the processing of neutral, nonrelevant material, likely because of (LC-)NA modulation.
Although we observed that taVNS modulates the initial processing and the subsequent retrieval of emotional information, from the current design it is difficult to discern how the initial decrease in processing of neutral information translates to a greater recollection of unpleasant material. It might be that both an enhanced processing of emotional information and a decreased processing of neutral information lead to similar memory effects, given that, in both cases, encoding and consolidation processes may be prioritized for emotional compared with neanrutral information. However, this interpretation is merely speculative and warrants further research.
One question that may arise is whether taVNS may induce a sustained increase of autonomic arousal (e.g., HR or BP) as it is observed during the experience of acute stress events (compare Weymar et al., 2011; Schwabe and Schächinger, 2018). Our autonomic and hormonal data suggest that taVNS did not increase the tonic levels of autonomic arousal. Similarly, although the sAA levels increase with time, taVNS did not specifically modulate this increase.7 With the current design, we measured HR and BP before and after stimulation, but we could not continuously track changes of the autonomic response through the task to investigate whether the autonomic reactivity fluctuated differently during taVNS compared with sham. A recent paper by Sharon et al. (2021) suggests that taVNS may induce phasic increases in pupil dilation (also assumed to correlate with LC-NE activity). Future studies recording continuous autonomic reactivity are needed, however, to clarify the role of tonic and phasic effects of taVNS on autonomic arousal and their contributing role on encoding and consolidation processes.
Together, in the current study, we demonstrated the causal role of VN activation in enhancing recollection-based, emotional memory in humans. Extending prior animal findings (Clark et al., 1998), we showed that noninvasive stimulation of the VN enhances the long-term recollective experience for emotionally relevant information. In addition, taVNS also facilitated emotional discrimination during encoding which may be related to an increase of central NA release. These findings confirm the causal link between vagal nerve activation and emotional memory formation in humans, which is derived from previous animal models. Although animal models may provide insights about the mechanisms underlying taVNS (Farmer et al., 2021), they have been discussed as partly limited for human disease mechanisms in translational research in general (Jucker, 2010); our findings may thus open new venues to investigate the potential use of taVNS to understand the neural underpinning of various psychological disorders associated with altered memory processes, such as Alzheimer's disease or depression, and to develop effective treatment options (Rong et al., 2016; Broncel et al., 2020). In this direction, future studies may also consider alternative pathways, for example, noninvasive stimulation of the greater occipital nerve (Vanneste et al., 2020), which shows very similar effects on the LC-NE system (proxy measures) and memory to those of taVNS.
Footnotes
1The implementation of this design was based on a previous study in which the suitability of two encoding sessions for memory paradigms was investigated (Jaworek, 2015). In this study, no evidence was found for psychological carryover effects from Session 1 to Session 2 that could possibly moderate the consecutive encoding processes.
2For those cases in which false alarms were 0, the value was substituted by 1/(total of trials [240] × 2).
3To ensure that our confidence-based data-split was not exclusively theory-driven, but also supported by the data, we tested whether the highest confidence bin reflected qualitatively different recollection processes than other high-confidence bins (i.e., 9). To do so, we examined the differences between these two bins on memory performance (i.e., hit rates, d′) for emotional and neutral material, irrespective of stimulation, using a within-subject repeated-measures ANOVA with the factors Emotion and Confidence. Based on the considerable amount of evidence suggesting that the memory advantage for emotional material is driven by recollection rather than familiarity processes (e.g., Dolcos et al., 2005, 2020; Sharot and Yonelinas, 2008; Schümann et al., 2017), we assumed that, if no Emotion × Confidence interaction emerges, recollection similarly influences both confidence judgments. However, a significant interaction between Emotion and Confidence judgments would indicate that the influence of recollection differs across confidence bins, providing support for the current confidence-based data-split. For hit rates, results showed a main effect of Confidence (F(1,35) = 34.38, p < 0.001, ηp2 = 0.49), a main effect of Emotion (F(1,35) = 66.14, p < 0.001, ηp2 = 0.65), and critically, a significant Confidence × Emotion interaction (F(1,35) = 17.42, p < 0.001, ηp2 = 0.33). For d′, results showed a main effect of Confidence (F(1,35) = 22.75, p < 0.001, ηp2 = 0.39) and Emotion (F(1,35) = 22.87, p < 0.001, ηp2 = 0.4) and a significant Confidence × Emotion interaction (F(1,35) = 5.64, p = 0.023, ηp2 = 0.14). Thus, these subsequent analyses further support the assumption that recognition judgments of the highest confidence reflecting recollection are qualitatively different from recognition judgments with lower confidence (e.g., 9).
4We also analyzed the data over the whole post-stimulus time window (0-1200 ms), using a sensor- and cluster-level criterion of p = 0.05. We observed that the significant cluster with a mass of 1793.1 did not surpass the critical cluster mass of 2749. However, when a more liberal cluster-level criterion was used (p = 0.1), the significant cluster became larger than the critical cluster mass of 1757.
5We also analyzed the data using the whole post-stimulus time window (0-1200 ms). Using a sensor- and cluster-level criterion of p = 0.05, we observed that the significant cluster with a mass of 2853.8 did not surpass the critical cluster mass of 3577. However, when a more liberal cluster-level criterion was used (p = 0.1), the significance became larger than the critical cluster mass of 2485.5.
6The recollection-based advantage for emotional material was found across different behavioral indices and was robust as indicated by the bootstrapped CI. However, it must be acknowledged that more stringent significant thresholds (i.e., Bonferroni-corrected p value: 0.05/8 = 0.007) would have rendered main significant findings at trend level.
7In the current study, we did not observe a specific increase of sAA (as marker for noradrenergic activity) during taVNS stimulation contrasting some prior findings (Ventura-Bort et al., 2018; Warren et al., 2019). The nonsignificant interaction effect of stimulation may be related to the length of stimulation, which was at least 35-40 minutes in previous studies. In the current study, however, we only stimulated for 7 minutes. Thus, it is possible that longer time of stimulation is required to capture the modulatory effect of taVNS on sAA. Protocols with longer stimulation duration that monitor changes in sAA at a higher rate would provide valuable insights on this matter in future studies.
This work was supported by German Research Foundation (DFG) Grant WE 4801/3-1 to M.W. We thank Nancy Gürtler and Sarah Schulz for assistance in data collection.
The authors declare no competing financial interests.
References
- Aston-Jones G, Cohen JD (2005) An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28:403–450. 10.1146/annurev.neuro.28.061604.135709 [DOI] [PubMed] [Google Scholar]
- Atucha E, Vukojevic V, Fornari RV, Ronzoni G, Demougin P, Peter F (2017) Noradrenergic activation of the basolateral amygdala maintains hippocampus-dependent accuracy of remote memory. Proc Natl Acad Sci USA 114:9176–9181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakdash JZ, Marusich LR (2017) Repeated measures correlation. Front Psychol 8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsegyan A, McGaugh JL, Roozendaal B (2014) Noradrenergic activation of the basolateral amygdala modulates the consolidation of object-in-context recognition memory. Front Behav Neurosci 8:160. 10.3389/fnbeh.2014.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsegyan A, Mirone G, Ronzoni G, Guo C, Song Q, van Kuppeveld D, Schut EH, Atsak P, Teurlings S, McGaugh JL, Schubert D, Roozendaal B (2019) Glucocorticoid enhancement of recognition memory via basolateral amygdala-driven facilitation of prelimbic cortex interactions. Proc Natl Acad Sci USA 116:7077–7082. 10.1073/pnas.1901513116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Greenwald MK, Petry MC, Lang PJ (1992) Remembering pictures: pleasure and arousal in memory. J Exp Psychol Learn Mem Cogn 18:379–390. 10.1037/0278-7393.18.2.379 [DOI] [PubMed] [Google Scholar]
- Broncel A, Bocian R, Kulbat-Warycha K, Konopacki J (2020) Vagal nerve stimulation as a promising tool in the improvement of cognitive disorders. Brain Res Bull 155:37–47. 10.1016/j.brainresbull.2019.11.011 [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP (2001) Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci 2:51–61. 10.1038/35049064 [DOI] [PubMed] [Google Scholar]
- Bublatzky F, Kavcioğlu F, Guerra P, Doll S, Junghöfer M (2020) Contextual information resolves uncertainty about ambiguous facial emotions: Behavioral and magnetoencephalographic correlates. NeuroImage 215:116814. 10.1016/j.nlm.2016.05.007 [DOI] [PubMed] [Google Scholar]
- Burger AM, Verkuil B, Van Diest I, Van der Does W, Thayer JF, Brosschot JF (2016) The effects of transcutaneous vagus nerve stimulation on conditioned fear extinction in humans. Neurobiol Learn Mem 132:49–56. 10.1016/j.nlm.2016.05.007 [DOI] [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL (1994) β-Adrenergic activation and memory for emotional events. Nature 371:702–704. 10.1038/371702a0 [DOI] [PubMed] [Google Scholar]
- Chatterton RT, Vogelsong KM, Lu Y, Ellman AB, Hudgens GA (1996) Salivary α-amylase as a measure of endogenous adrenergic activity. Clin Physiol 16:433–448. 10.1111/j.1475-097X.1996.tb00731.x [DOI] [PubMed] [Google Scholar]
- Clark KB, Smith DC, Hassert DL, Browning RA, Naritoku DK, Jensen RA (1998) Posttraining electrical stimulation of vagal afferents with concomitant vagal efferent inactivation enhances memory storage processes in the rat. Neurobiol Learn Mem 70:364–373. 10.1006/nlme.1998.3863 [DOI] [PubMed] [Google Scholar]
- Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA (1999) Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci 2:94–98. 10.1038/4600 [DOI] [PubMed] [Google Scholar]
- Colzato LS, Sellaro R, Beste C (2017) Darwin revisited: The vagus nerve is a causal element in controlling recognition of other's emotions. Cortex 92:95–102. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ (2000) Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biol Psychol 52:95–111. 10.1016/S0301-0511(99)00044-7 [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C (2007) Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci 11:379–386. 10.1016/j.tics.2007.08.001 [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C (2009) Medial Temporal lobe activity during source retrieval reflects information type, not memory strength. J Cogn Neurosci 22:1808–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ (2002) Emotion, cognition, and behavior. Science 298:1191–1194. 10.1126/science.1076358 [DOI] [PubMed] [Google Scholar]
- Dolcos F, Labar KS, Cabeza R (2004) Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: an event-related fMRI study. Neuroimage 23:64–74. 10.1016/j.neuroimage.2004.05.015 [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R (2005) Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci USA 102:2626–2631. 10.1073/pnas.0409848102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, Katsumi Y, Weymar M, Moore M, Tsukiura T, Dolcos S (2017) Emerging directions in emotional episodic memory. Front Psychol 8:1867–1825. 10.3389/fpsyg.2017.01867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, Katsumi Y, Moore M, Berggren N, de Gelder B, Derakshan N, Hamm AO, Koster EH, Ladouceur CD, Okon-Singer H, Pegna AJ, Richter T, Schweizer S, Van den Stock J, Ventura-Bort C, Weymar M, Dolcos S (2020) Neural correlates of emotion-attention interactions: from perception, learning, and memory to social cognition, individual differences, and training interventions. Neurosci Biobehav Rev 108:559–601. [DOI] [PubMed] [Google Scholar]
- Dorr AE, Debonnel G (2006) Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. J Pharmacol Exp Ther 318:890–898. 10.1124/jpet.106.104166 [DOI] [PubMed] [Google Scholar]
- Düzel E, Vargha-Khadem F, Heinze HJ, Mishkin M (2001) Brain activity evidence for recognition without recollection after early hippocampal damage. Proc Natl Acad Sci U S A 98:8101–8106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlert U, Erni K, Hebisch G, Nater U (2006) Salivary α-amylase levels after yohimbine challenge in healthy men. J Clin Endocrinol Metab 91:5130–5133. 10.1210/jc.2006-0461 [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C (2007) The medial temporal lobe and recognition memory. Annu Rev Neurosci 30:123–152. 10.1146/annurev.neuro.30.051606.094328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellrich J (2011) Epilepsy transcutaneous vagus nerve stimulation isolated vagus nerve palsy with herpes zoster. Eur Neurol Rev 6:262–264. [Google Scholar]
- Farmer AD, Strzelczyk A, Finisguerra A, Gourine AV, Gharabaghi A, Hasan A, Burger AM, Jaramillo AM, Mertens A, Majid A, Verkuil B, Badran BW, Ventura-Bort C, Gaul C, Beste C, Warren CM, Quintana DS, Hämmerer D, Freri E, Frangos E, et al. (2021) International consensus based review and recommendations for minimum reporting standards in research on transcutaneous vagus nerve stimulation (version 2020). Front Hum Neurosci 14:568051. 10.3389/fnhum.2020.568051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer R, Ventura-Bort C, Hamm A, Weymar M (2018) Transcutaneous vagus nerve stimulation (tVNS) enhances conflict-triggered adjustment of cognitive control. Cogn Affect Behav Neurosci 18:680–693. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G, Dien J (2009) Differentiating neural responses to emotional pictures: evidence from temporal-spatial PCA. Psychophysiology 46:521–530. 10.1111/j.1469-8986.2009.00796.x [DOI] [PubMed] [Google Scholar]
- Frangos E, Ellrich J, Komisaruk BR (2015) Noninvasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul 8:624–636. 10.1016/j.brs.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudier M, Ventura-Bort C, Weymar M (2020) Transcutaneous vagus nerve stimulation (tVNS) improves high-confidence recognition memory but not emotional word processing. Front Psychol 11:1276. 10.3389/fpsyg.2020.01276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves DA, Bowman EM, Brown VJ (2005) Recordings from the rat locus coeruleus during acute vagal nerve stimulation in the anaesthetised rat. Neurosci Lett 379:174–179. 10.1016/j.neulet.2004.12.055 [DOI] [PubMed] [Google Scholar]
- Hajcak G, Weinberg A, MacNamara A, Foti D (2012) ERPs and the study of emotion. In: The Oxford Handbook of Event-related Potential Components (Luck SJ, Kappenman ES eds), pp 441–472. New York: Oxford University Press. [Google Scholar]
- Hajcak G, Foti D (2020) Significance?… Significance! Empirical, methodological, and theoretical connections between the late positive potential and P300 as neural responses to stimulus significance: an integrative review. Psychophysiology 57:e13570. 10.1111/psyp.13570 [DOI] [PubMed] [Google Scholar]
- Hulsey DR, Riley JR, Loerwald KW, Rennaker RL, Kilgard MP, Hays SA (2017) Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp Neurol 289:21–30. 10.1016/j.expneurol.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs HI, Riphagen JM, Razat CM, Wiese S, Sack AT (2015) Transcutaneous vagus nerve stimulation boosts associative memory in older individuals. Neurobiol Aging 36:1860–1867. 10.1016/j.neurobiolaging.2015.02.023 [DOI] [PubMed] [Google Scholar]
- Jaworek A (2015). Modulation of emotional episodic memory in humans. Evidence from event-related potential studies (Doctoral thesis). University of Greifswald, Greifswald, Germany. [Google Scholar]
- Jucker M (2010) The benefits and limitations of animal models for translational research in neurodegenerative diseases. Nat Med 16:1210–1214. [DOI] [PubMed] [Google Scholar]
- Junghöfer M, Elbert T, Tucker DM, Rockstroh B (2000) Statistical control of artifacts in dense array EEG/MEG studies. Psychophysiology 37:523–532. 10.1111/1469-8986.3740523 [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL (2005) Retrieving accurate and distorted memories: neuroimaging evidence for effects of emotion. Neuroimage 27:167–177. 10.1016/j.neuroimage.2005.03.038 [DOI] [PubMed] [Google Scholar]
- Koen JD, Barrett FS, Harlow IM, Yonelinas AP (2017) The ROC Toolbox: A toolbox for analyzing receiver-operating characteristics derived from confidence ratings. Behav Res Methods 49:1399–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig J, Parzer P, Haigis N, Liebemann J, Jung T, Resch F, Kaess M (2021) Effects of acute transcutaneous vagus nerve stimulation on emotion recognition in adolescent depression. Psychol Med 51:511–520. 10.1017/S0033291719003490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus T, Hösl K, Kiess O, Schanze A, Kornhuber J, Forster C (2007) BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J Neural Transm (Vienna) 114:1485–1493. 10.1007/s00702-007-0755-z [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN (2008) International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. University of Florida, Gainesville, FL. [Google Scholar]
- Loughlin SE, Foote SL, Bloom FE (1986) Efferent projections of nucleus locus coeruleus: topographic organization of cells of origin demonstrated by three-dimensional reconstruction. Neuroscience 18:291–306. 10.1016/0306-4522(86)90155-7 [DOI] [PubMed] [Google Scholar]
- Marchewka A, Jednoróg K, Grabowska A (2014) The Nencki Affective Picture System (NAPS): introduction to a novel, standardized, wide-range, high-quality, realistic picture database. Behav Res Methods 46:596–610. 10.3758/s13428-013-0379-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R (2007) Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 164:177–190. 10.1016/j.jneumeth.2007.03.024 [DOI] [PubMed] [Google Scholar]
- Mather M, Clewett D, Sakaki M, Harley CW (2016) Norepinephrine ignites local hotspots of neuronal excitation: how arousal amplifies selectivity in perception and memory. Behav Brain Sci 39:e200. 10.1017/S0140525X15000667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL (2015) Consolidating memories. Annu Rev Psychol 66:1–24. 10.1146/annurev-psych-010814-014954 [DOI] [PubMed] [Google Scholar]
- McIntyre CK, McGaugh JL, Williams CL (2012) Interacting brain systems modulate memory consolidation. Neurosci Biobehav Rev 36:1750–1762. 10.1016/j.neubiorev.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez-Bértolo C, Moratti S, Toledano R, Lopez-Sosa F, Martínez-Alvarez R, Mah YH, Vuilleumier P, Gil-Nagel A, Strange BA (2016) A fast pathway for fear in human amygdala. Nat Neurosci 19:1041–1049. 10.1038/nn.4324 [DOI] [PubMed] [Google Scholar]
- Mertens A, Naert L, Miatton M, Poppa T, Carrette E, Gadeyne S, Raedt R, Boon P, Vonck K (2020) Transcutaneous vagus nerve stimulation does not affect verbal memory performance in healthy volunteers. Front Psychol 11:551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickes L, Wais PE, Wixted JT (2009) Recollection is a continuous process. Psychol Sci 20:509–515. 10.1111/j.1467-9280.2009.02324.x [DOI] [PubMed] [Google Scholar]
- Miyashita T, Williams C (2006) Epinephrine administration increases neural impulses propagated along the vagus nerve: role of peripheral β-adrenergic receptors. Neurobiol Learn Mem 85:116–124. 10.1016/j.nlm.2005.08.013 [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD (2005) Decision making, the P3, and the locus coeruleus–norepinephrine system. Psychol Bull 131:510–532. 10.1037/0033-2909.131.4.510 [DOI] [PubMed] [Google Scholar]
- Ochsner KN (2000) Are affective events richly recollected or simply familiar? The experience and process of recognizing feelings past. J Exp Psychol Gen 129:242–261. 10.1037/0096-3445.129.2.242 [DOI] [PubMed] [Google Scholar]
- Pastor MC, Rehbein M, Junghoefer M, Poy R, Lopez R, Moltó J (2015) Facing challenges in differential classical conditioning research: Benefits of a hybrid design for simultaneous electrodermal and electroencephalographic recording. Front Hum Neurosci 9:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuker ET, Filler TJ (2002) The nerve supply of the human auricle. Clin Anat 15:35–37. 10.1002/ca.1089 [DOI] [PubMed] [Google Scholar]
- Peyk P, De Cesarei A, Junghöfer M (2011) Electromagnetic encephalography software: overview and integration with other EEG/MEG toolboxes. Comput Intell Neurosci 2011:861705. 10.1155/2011/861705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourtois G, Schettino A, Vuilleumier P (2013) Brain mechanisms for emotional influences on perception and attention: What is magic and what is not. Biol Psychol 92:492–512. [DOI] [PubMed] [Google Scholar]
- Raedt R, Clinckers R, Mollet L, Vonck K, El Tahry R, Wyckhuys T, De Herdt V, Carrette E, Wadman W, Michotte Y, Smolders I, Boon P, Meurs A (2011) Increased hippocampal noradrenaline is a biomarker for efficacy of vagus nerve stimulation in a limbic seizure model. J Neurochem 117:461–469. 10.1111/j.1471-4159.2011.07214.x [DOI] [PubMed] [Google Scholar]
- Ranganath C, Ritchey M (2012) Two cortical systems for memory-guided behaviour. Nat Rev Neurosci 13:713–726. 10.1038/nrn3338 [DOI] [PubMed] [Google Scholar]
- Rehbein M, Wessing I, Zwitserlood P, Steinberg C, Eden AS, Dobel C, Junghoefer M (2015) Cortex activation towards aversively paired faces and enhanced contingency detection are observed in highly trait-anxious women under challenging conditions. Front Behav Neurosci 9:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmele U, Davachi L, Phelps EA (2012) Memory for time and place contributes to enhanced confidence in memories for emotional events. Emotion 12:834–846. 10.1037/a0028003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M, Dolcos F, Cabeza R (2008) Role of amygdala connectivity in the persistence of emotional memories over time: an event-related fMRI investigation. Cereb Cortex 18:2494–2504. 10.1093/cercor/bhm262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M, McCullough AM, Ranganath C, Andrew P (2017) Stress as a mnemonic filter: interactions between medial temporal lobe encoding processes and post-encoding stress. Hippocampus 27:77–88. 10.1002/hipo.22674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong P, Liu J, Wang L, Liu R, Fang J, Zhao J, Zhao Y, Wang H, Vangel M, Sun S, Ben H, Park J, Li S, Meng H, Zhu B, Kong J (2016) Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: a nonrandomized controlled pilot study. J Affect Disord 195:172–179. 10.1016/j.jad.2016.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosevelt RW, Smith DC, Clough RW, Jensen RA, Browning RA (2006) Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res 1119:124–132. 10.1016/j.brainres.2006.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Hermans EJ (2017) Norepinephrine effects on the encoding and consolidation of emotional memory: improving synergy between animal and human studies. Curr Opin Behav Sci 14:115–122. 10.1016/j.cobeha.2017.02.001 [DOI] [Google Scholar]
- Roozendaal B, McGaugh JL (2011) Memory modulation. Behav Neurosci 125:797–824. 10.1037/a0026187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufener KS, Geyer U, Janitzky K, Heinze HJ, Zaehle T (2018) Modulating auditory selective attention by noninvasive brain stimulation: differential effects of transcutaneous vagal nerve stimulation and transcranial random noise stimulation. Eur J Neurosci 48:2301–2309. 10.1111/ejn.14128 [DOI] [PubMed] [Google Scholar]
- Rugg MD, Curran T (2007) Event-related potentials and recognition memory. Trends Cogn Sci 11:251–257. 10.1016/j.tics.2007.04.004 [DOI] [PubMed] [Google Scholar]
- Rugg MD, Vilberg KL, Mattson JT, Yu SS, Johnson JD, Suzuki M (2012) Item memory, context memory and the hippocampus: fMRI evidence. Neuropsychologia 50:3070–3079. 10.1016/j.neuropsychologia.2012.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D, Keil A, Frank DW, Lang PJ (2013) Emotional perception: correspondence of early and late event-related potentials with cortical and subcortical functional MRI. Biol Psychol 92:513–519. 10.1016/j.biopsycho.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ (2009) The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci 10:211–223. 10.1038/nrn2573 [DOI] [PubMed] [Google Scholar]
- Sara SJ, Bouret S (2012) Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron 76:130–141. 10.1016/j.neuron.2012.09.011 [DOI] [PubMed] [Google Scholar]
- Schlögl A, Keinrath C, Zimmermann D, Scherer R, Leeb R, Pfurtscheller G (2007) A fully automated correction method of EOG artifacts in EEG recordings. Clin Neurophysiol 118:98–104. [DOI] [PubMed] [Google Scholar]
- Schupp H, Cuthbert B, Bradley M, Hillman C, Hamm A, Lang P (2004) Brain processes in emotional perception: motivated attention. Cogn Emot 18:593–611. 10.1080/02699930341000239 [DOI] [Google Scholar]
- Schwabe L, Schächinger H (2018) Ten years of research with the Socially Evaluated Cold Pressor Test: data from the past and guidelines for the future. Psychoneuroendocrinology 92:155–161. 10.1016/j.psyneuen.2018.03.010 [DOI] [PubMed] [Google Scholar]
- Sellaro R, de Gelder B, Finisguerra A, Colzato LS (2018) Transcutaneous vagus nerve stimulation (tVNS) enhances recognition of emotions in faces but not bodies. Cortex 99:213–223. [DOI] [PubMed] [Google Scholar]
- Sharon O, Fahoum F, Nir Y (2021) Transcutaneous vagus nerve stimulation in humans induces pupil dilation and attenuates alpha oscillations. J Neurosci 41:320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharot T, Delgado MR, Phelps EA (2004) How emotion enhances the feeling of remembering. Nat Neurosci 7:1376–1380. 10.1038/nn1353 [DOI] [PubMed] [Google Scholar]
- Sharot T, Yonelinas AP (2008) Differential time-dependent effects of emotion on recollective experience and memory for contextual information. Cognition 106:538–547. [DOI] [PubMed] [Google Scholar]
- Snodgrass JS, Corwin J (1988) Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen 117:34–50. [DOI] [PubMed] [Google Scholar]
- Steenbergen L, Sellaro R, Stock AK, Verkuil B, Beste C, Colzato LS (2015) Transcutaneous vagus nerve stimulation (taVNS) enhances response selection during action cascading processes. Eur Neuropsychopharmacol 25:773–778. 10.1016/j.euroneuro.2015.03.015 [DOI] [PubMed] [Google Scholar]
- Strange BA, Dolan RJ (2004) Beta-adrenergic modulation of emotional memory-evoked human amygdala and hippocampal responses. Proc Natl Acad Sci USA 101:11454–11458. 10.1073/pnas.0404282101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schümann D, Bayer J, Talmi D, Sommer T (2017) Dissociation of immediate and delayed effects of emotional arousal on episodic memory. Neurobiol Learn Mem 148:11–19. [DOI] [PubMed] [Google Scholar]
- Szeska C, Richter J, Wendt J, Weymar M, Hamm AO (2020) Promoting long-term inhibition of human fear responses by noninvasive transcutaneous vagus nerve stimulation during extinction training. Sci Rep 10:1–16. 10.1038/s41598-020-58412-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma MV, Kirschbaum C, Wolf JM, Rohleder N (2012) Acute stress responses in salivary alpha-amylase predict increases of plasma norepinephrine. Biol Psychol 91:342–348. 10.1016/j.biopsycho.2012.07.008 [DOI] [PubMed] [Google Scholar]
- Vanneste S, Mohan A, Yoo HB, Huang Y, Luckey AM, McLeod LS, Tabet MN, Souza RR, McIntyre CK, Chapman S, Robertson IH, To WT (2020) The peripheral effect of direct current stimulation on brain circuits involving memory. Sci Adv 6:eaax9538–20. 10.1126/sciadv.aax9538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Stegeren AH, Goekoop R, Everaerd W, Scheltens P, Barkhof F, Kuijer JP, Rombouts SA (2005) Noradrenaline mediates amygdala activation in men and women during encoding of emotional material. Neuroimage 24:898–909. 10.1016/j.neuroimage.2004.09.011 [DOI] [PubMed] [Google Scholar]
- Ventura-Bort C, Wirkner J, Genheimer H, Wendt J, Hamm AO, Weymar M (2018) Effects of transcutaneous vagus nerve stimulation (tVNS) on the P300 and alpha-amylase level: a pilot study. Front Hum Neurosci 12:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaurre C, Sander TH, Schlögl A (2011) BioSig: the free and open source software library for biomedical signal processing. Comput Intell Neurosci 2011:935364. 10.1155/2011/935364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren CM, Tona KD, Ouwerkerk L, van Paridon J, Poletiek F, van Steenbergen H, Bosch JA, Nieuwenhuis S (2019) The neuromodulatory and hormonal effects of transcutaneous vagus nerve stimulation as evidenced by salivary alpha amylase, salivary cortisol, pupil diameter, and the P3 event-related potential. Brain Stimul 12:635–642. 10.1016/j.brs.2018.12.224 [DOI] [PubMed] [Google Scholar]
- Warren CM, van den Brink RL, Nieuwenhuis S, Bosch JA (2017) Norepinephrine transporter blocker atomoxetine increases salivary alpha amylase. Psychoneuroendocrinology 78:233–236. 10.1016/j.psyneuen.2017.01.029 [DOI] [PubMed] [Google Scholar]
- Weymar M, Hamm A (2013) Electrophysiological signature of emotional memories. In: Hurting memories and beneficial forgetting: Posttraumatic stress disorders, biographical developments, and social conflicts (Linden M, Rutkowski K eds), pp 21–35. Saint Louis:Elsevier. [Google Scholar]
- Weymar M, Löw A, Melzig CA, Hamm AO (2009) Enhanced long-term recollection for emotional pictures: evidence from high-density ERPs. Psychophysiology 46:1200–1207. 10.1111/j.1469-8986.2009.00869.x [DOI] [PubMed] [Google Scholar]
- Weymar M, Löw A, Modess C, Engel G, Gründling M, Petersmann A, Siegmund W, Hamm AO (2010a) Propranolol selectively blocks the enhanced parietal old/new effect during long-term recollection of unpleasant pictures: a high density ERP study. Neuroimage 49:2800–2806. 10.1016/j.neuroimage.2009.10.025 [DOI] [PubMed] [Google Scholar]
- Weymar M, Löw A, Schwabe L, Hamm AO (2010b) Brain dynamics associated with recollective experiences of emotional events. Neuroreport 21:827–831. 10.1097/WNR.0b013e32833d180a [DOI] [PubMed] [Google Scholar]
- Weymar M, Löw A, Hamm AO (2011) Emotional memories are resilient to time: evidence from the parietal ERP old/new effect. Hum Brain Mapp 32:632–640. 10.1002/hbm.21051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weymar M, Schwabe L, Löw A, Hamm AO (2012) Stress sensitizes the brain: increased processing of unpleasant pictures after exposure to acute stress. J Cogn Neurosci 24:1511–1518. 10.1162/jocn_a_00174 [DOI] [PubMed] [Google Scholar]
- Weymar M, Zaehle T (2021) Editorial: new frontiers in noninvasive brain stimulation: cognitive, affective and neurobiological effects of transcutaneous vagus nerve stimulation. Front Psychol 12:694723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding EL, Ranganath C (2012) Electrophysiological correlates of episodic memory processes. In: The Oxford Handbook of Event-Related Potential Components, pp 373–395. New York: Oxford University Press. [Google Scholar]
- Williams CL, McGaugh JL (1993) Reversible lesions of the nucleus of the solitary tract attenuate the memory-modulating effects of posttraining epinephrine. Behav Neurosci 107:955–962. 10.1037/0735-7044.107.6.955 [DOI] [PubMed] [Google Scholar]
- Williams CL, Men D, Clayton EC, Gold PE (1998) Norepinephrine release in the amygdala after systemic injection of epinephrine or escapable footshock: contribution of the nucleus of the solitary tract. Behav Neurosci 112:1414–1422. 10.1037/0735-7044.112.6.1414 [DOI] [PubMed] [Google Scholar]
- Wirkner J, Weymar M, Löw A, Hamm AO (2013) Effects of pre-encoding stress on brain correlates associated with the long-term memory for emotional scenes. PLoS ONE 8:e68212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirkner J, Ventura-Bort C, Schulz P, Hamm AO, Weymar M (2018) Event-related potentials of emotional and neutral memories: the role of encoding position and delayed testing. Psychophysiology 55:e13069. 10.1111/psyp.13069 [DOI] [PubMed] [Google Scholar]
- Wixted JT, Stretch V (2004) In defense of the signal detection interpretation of remember/know judgments. Psychon Bull Rev 11:616–641. 10.3758/bf03196616 [DOI] [PubMed] [Google Scholar]
- Woodruff CC, Hayama HR, Rugg MD (2006) Electrophysiological dissociation of the neural correlates of recollection and familiarity. Brain Res 1100:125–135. 10.1016/j.brainres.2006.05.019 [DOI] [PubMed] [Google Scholar]
- Wynn SC, Daselaar SM, Kessels RP, Schutter DJ (2019) The electrophysiology of subjectively perceived memory confidence in relation to recollection and familiarity. Brain Cogn 130:20–27. 10.1016/j.bandc.2018.07.003 [DOI] [PubMed] [Google Scholar]
- Yonelinas AP (2001) Components of episodic memory: the contribution of recollection and familiarity. Philos Trans R Soc Lond B Biol Sci 356:1363–1374. 10.1098/rstb.2001.0939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP (2002) The nature of recollection and familiarity: a review of 30 years of research. J Mem Lang 46:441–517. 10.1006/jmla.2002.2864 [DOI] [Google Scholar]
- Yonelinas AP, Parks CM (2007) Receiver operating characteristics (ROCs) in recognition memory: A review. Psychol Bull 133:800–832. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Ritchey M (2015) The slow forgetting of emotional episodic memories: an emotional binding account. Trends Cogn Sci 19:259–267. 10.1016/j.tics.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauvé MJ, Widaman KF, Knight RT (2002) Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nat Neurosci 5:1236–1241. 10.1038/nn961 [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD (2005) Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci 25:3002–3008. 10.1523/JNEUROSCI.5295-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SS, Rugg MD (2010) Dissociation of the electrophysiological correlates of familiarity strength and item repetition. Brain Res 1320:74–84. 10.1016/j.brainres.2009.12.071 [DOI] [PMC free article] [PubMed] [Google Scholar]