Abstract
The coronavirus disease 2019 (COVID-19), which emerged in December 2019, continues to be a serious health concern worldwide. There is an urgent need to develop effective drugs and vaccines to control the spread of this disease. In the current study, the main phytochemical compounds of Nigella sativa were screened for their binding affinity for the active site of the RNA-dependent RNA polymerase (RdRp) enzyme of the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). The binding affinity was investigated using molecular docking methods, and the interaction of phytochemicals with the RdRp active site was analyzed and visualized using suitable software. Out of the nine phytochemicals of N. sativa screened in this study, a significant docking score was observed for four compounds, namely α-hederin, dithymoquinone, nigellicine, and nigellidine. Based on the findings of our study, we report that α-hederin, which was found to possess the lowest binding energy (–8.6 kcal/mol) and hence the best binding affinity, is the best inhibitor of RdRp of SARS-CoV-2, among all the compounds screened here. Our results prove that the top four potential phytochemical molecules of N. sativa, especially α-hederin, could be considered for ongoing drug development strategies against SARS-CoV-2. However, further in vitro and in vivo testing are required to confirm the findings of this study.
Keywords: Nigella Sativa, SARS-CoV-2, Docking, In silico, Phytochemical, Drug, RNA polymerase
Abbreviations: COVID-19, coronavirus disease 2019; GUI, graphical user interface; MERS-CoV, Middle East respiratory syndrome coronavirus; RCSB PDB, Research Collaboratory for Structural Bioinformatics Protein Data Bank; RdRp, RNA-dependent RNA polymerase; RMSD, root mean square deviation; RMSF, root mean square fluctuations; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2
1. Introduction
The coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome-coronavirus (SARS-CoV)-2, has adversely affected the health of people across the globe (Mali et al., 2020). As of January 16, 2021, there are 92,262,621 confirmed cases of COVID-19 worldwide, including 1,995,037 deaths (https://www.who.int/emergencies/diseases/novel-coronavirus-2019). SARS-CoV-2 belongs to the Coronaviridae family and is closely related to the other members of the family, especially SARS-CoV and the Middle East respiratory syndrome coronavirus (MERS-CoV) (Hui et al., 2020, Mali et al., 2020). The positive stranded RNA genome of SARS-CoV-2 is surrounded by a lipid envelope which contains the spike proteins as well as the membrane proteins. The spike proteins of SARS-CoV-2 bind to the host cell receptors and help the virus release the viral genome into the host cell where it is translated into two polyproteins and the structural proteins, and replication of the viral genome is initiated (V’kovski et al., 2020). The two-third of the RNA genome of SARS-CoV-2 encodes viral RNA-dependent RNA polymerase (RdRp), the associated accessory proteins, and the two large non-structural polyproteins. The remaining one-third of the genome codes for the four structural proteins (spike, envelope, membrane and nucleocapsid), and the other helper proteins (Luk et al., 2019). RdRp is very important for replication and transcription of the viral genome and is highly conserved among different RNA viruses. The core protein of RdRp consisting of a single chain of approximately 900 amino acid residues, shows minimal activity. However, enhanced activity is achieved by the attachment of additional key subunits (Ahn et al., 2012, Kirchdoerfer and Ward, 2019, Subissi et al., 2014). Being a very essential enzyme in the life cycle of RNA viruses including SARS-CoV-2, the RdRp has already been targeted in various viral infections, including the hepatitis C virus, Zika virus and coronaviruses (Elfiky et al., 2013, Elfiky, 2016, Elfiky, 2017, Elfiky, 2019, Elfiky and Elshemey, 2016, Elfiky and Elshemey, 2018, Elfiky and Ismail, 2017, Elfiky and Ismail, 2019, Ganesan and Barakat, 2017). The active site of RdRp has has been reported to be highly conserved among the different corona viruses (Doublié and Ellenberger, 1998, Elfiky, 2020a, Elfiky and Ismail, 2018). Therefore, RdRp is the main drug target for SARS-Cov-2 and other coronaviruses (Aftab et al., 2020, Elfiky, 2021b, Elfiky, 2020b, Yin et al., 2020).
The virus generally spreads from the infected person through close contact along with the droplets spilled during talking, coughing and sneezing (Chan et al., 2020). The lack of approved drugs or vaccines for COVID-19 is the main concern of the ongoing pandemic. Therefore, developing a promising vaccine or drug intervention is of prime interest and importance in combating this viral disease (Vardhan and Sahoo 2020). Various efforts are being carried out by the researchers throughout the world for developing the effective treatment strategy for this infectious disease. As a result of these continued efforts various clinical interventions are on trial worldwide. In addition to drug repurposing and synthesis of new drug molecules, the herbal medicine system has gained global emphasis in providing favorable interventions to combat this pandemic viral disease (Patwardhan et al., 2020, Rastogi et al., 2020, Elfiky, 2021a). One of the herbs, Nigella sativa (also known as black seed or Prophetic medicine) has been reported to possess several pharmacological properties including anti-microbial, anti-inflammatory and immunostimulatory activities (Ahmad et al., 2013, Molla et al., 2019). N. sativa belongs to the plant family ‘Ranunculaceae’ and its seeds in have been consumed to treat various diseases and infirmities (Mazaheri et al., 2019, Majeed et al., 2021, Yimer et al., 2019). Besides its use as a spice and a food preservative, it has been traditionally used as a protective and curative remedy for several disorders (Majeed et al., 2021). Moreover, the beneficial effects and safety of N. sativa seeds in different diseases is well established in the literature (Daryabeygi-Khotbehsara et al., 2017, He and Xu, 2019, Koshak et al., 2017, Namazi et al., 2018, Sahebkar et al., 2016a, Sahebkar et al., 2016b, Tavakkoli et al., 2017) . Recently, various studies have highlighted the efficacy of N. sativa in the treatment of viral diseases (Ahmad et al., 2020, Barakat et al., 2013, Bouchentouf and Noureddine, 2020, Dorra et al., 2019, Elfiky, 2021a, Onifade et al., 2013a, Onifade et al., 2013b, Sekiou et al., 2020). N. sativa has been reported to inhibit cytomegalovirus, herpes simplex virus, human immunodeficiency virus and hepatitis A virus infections (Salem and Hossain, 2000, Barakat et al., 2010). Some studies in literature have revealed that N. sativa inhibited growth of influenza virus H5N1 and replication of Hepatitis C virus (Oyero et al., 2016, Dorra et al., 2019), in addition to decreasing the coronavirus load in infected HeLa cells (Ulasli et al., 2014). Few studies have also focused on the in silico screening of its main phytochemicals against some drug targets of SARS-CoV-2 (Ahmad et al., 2020, Bouchentouf and Noureddine, 2020, Molla et al., 2019, Maiti et al., 2020). However, the inhibitory potential of these phytochemicals against the prominent drug target, the RNA-dependent RNA polymerase of SARS-CoV-2 has not yet been reported. Therefore, based on some previous studies in literature (Akram Khan and Afzal, 2016, Ahmad et al., 2020, Bouchentouf and Noureddine, 2020, Molla et al., 2019, Maiti et al., 2020), here we selected the major phytochemicals of N. sativa and screened them for their potential to inhibit the RdRp of SARS-CoV-2 using computer aided molecular docking methods.
2. Material and methods
2.1. Preparation of RdRp for molecular docking
The crystallographic three-dimensional (3D) structure of the RdRp (PDB ID: 6 M71) of SARS-CoV-2 was downloaded from the Research Collaboratory for Structural Bioinformatics (RCSB) Protein Data Bank (PDB) (http://www.rscb.org) and saved as a PDB file (.pdb). AutoDock Tools 1.5.7 was used to prepare this target protein (PDB ID: 6 M71) for in silico molecular docking experiments (Goodsell and Olson 1990). The water molecules were removed, polar hydrogen atoms and the Kollman charges were added to the protein, and saved as a PDBQT file (.pdbqt). A grid box covering the active site pocket of 6 M71 was generated and the grid parameters were saved as a TXT file (.txt) for input during docking (Iheagwam and Rotimi, 2020, Vardhan and Sahoo, 2020).
2.2. Preparation of ligands
The 3D structures of the main chemical compounds of N. sativa as well as that of Remdesivir were downloaded as SDF files (.sdf) from PubChem (https://pubchem.ncbi.nlm.nih.gov/) and converted to the PDB format (.pdb) using Open Babel graphical user interface (GUI) tool (O’Boyle et al., 2011). The PDB files of all the ligands (compounds of N. sativa and the positive control) were prepared for docking using AutoDock Tools 1.5.7. Ligand preparation included the addition of gasteiger charges, setting torsion roots and merging non-polar hydrogens. The prepared ligands were saved as PDBQT files (.pdbqt) for input during the docking procedure. We did not investigate Lipinski’s physicochemical parameters of the ligands here because this information is already available in the literature (Ahmad et al., 2020, Bouchentouf and Noureddine, 2020).
2.3. Molecular docking and visualization of the docking complex
The molecular docking of individual ligands into the target protein was performed using the AutoDock Vina software (Trott and Olson, 2009). The grid dimensions (Å) for active site- specific docking were searched from the available literature and fixed at: x = 28, y = 26, z = 32 (Iheagwam and Rotimi, 2020). The default exhaustiveness value of 8 was uniformly fixed for all ligands during the docking procedure. The known drug candidate, remdesivir was used as a positive control for docking the RdRp active site. AutoDock Vina results represent the docking scores as the Gibbs free energy of binding (ΔG (kcal/mol)). The Gibbs free energy of binding (ΔG) obtained as a result of molecular docking by AutoDock Vina and expressed in kcal/mol represents the efficacy of ligand binding to the active site of the selected receptor (Ardra et al., 2020). Further, the output files generated from docking experiments were converted to protein–ligand complexes using the PyMOL software (https://pymol.org/2/) (DeLano 2002), and the interaction of the ligands with the receptor residues was visualized and analyzed using the BioVia Discovery Studio Software (https://discover.3ds.com/discovery-studio-visualizer-download).
3. Results and discussion
3.1. Molecular docking of the phytochemical compounds of N. Sativa
RdRp is crucial for the replication of SARS-CoV-2 and is therefore considered a key target for the development of antiviral drugs (Aftab et al., 2020, Elfiky, 2021b, Elfiky, 2020b, Yin et al., 2020). Recently, several studies have reported the in silico screening of phytochemicals from N. sativa against different drug targets of SARS-CoV-2 (Ahmad et al., 2020, Bouchentouf and Noureddine, 2020, Maiti et al., 2020, Rajapaksa et al., 2020, Sampangi-ramaiah et al., 2020, Silva et al., 2020), however, RdRp, an important drug target has not been included in these studies. Therefore, to fill the gap, the same strategy has been applied in the current study to screen the N. sativa phytochemicals for their potential to inhibit RdRp of SARS-CoV-2.
Our results revealed that out of the total nine compounds of N. sativa that we screened by in silico analysis, four (α-hederin, dithymoquinone, nigellicine and nigellidine) had significant binding affinity towards the active site of RdRp as indicated by their considerably lower binding energies (ΔG values less than the cut-off value of –6 kcal/mol) (Shityakov and Foerster, 2014). α-hederin possessed the lowest binding energy (ΔG = –8.6 Kcal/mol) in complex with 6 M71, followed by dithymoquinone (ΔG = –6.1 kcal/mol), nigellicine (ΔG = –6.1 kcal/mol) and nigellidine (ΔG = –6.0 kcal/mol). The binding affinity (ΔG = –8.6 Kcal/mol) of α-hederin was found to be higher than that of remdesivir (–7.6 kcal/mol), the already known antiviral compound used as a positive control in this study. The binding energies of the other three compounds were lower, but close to that of remdesivir (Table 1).
Table 1.
Chemical structures of the main phytochemical compounds of Nigella Sativa and remdesivir (control drug) along with their respective docking scores upon molecular docking with RNA-dependent RNA polymerase (RdRp) of SARS-CoV-2 (PDB ID: 6 M71).
| Phytochemical compounds/ligands | Molecular weight (g/mol) | Chemical structure/PubChem CID | Docking Score (kcal/mol) |
|---|---|---|---|
| α- Hederin | 750.97 | 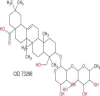 |
–8.6 |
| Dithymoquinone | 328.41 | 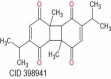 |
–6.1 |
| Nigellicine | 246.27 | 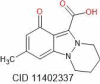 |
–6.1 |
| Nigellidine | 294.35 | 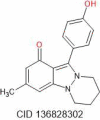 |
–6.0 |
| Nigellimine | 203.24 | 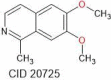 |
–5.1 |
| Thymohydroquinone | 166.22 | 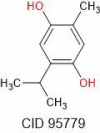 |
–4.6 |
| Thymoquinone | 164.20 | 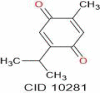 |
–4.6 |
| Carvacrol | 150.22 | 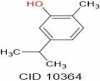 |
–4.5 |
| Thymol | 150.22 | 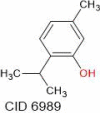 |
–4.3 |
| Remdesivir | 602.6 | 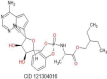 |
–7.6 |
3.2. In silico analysis of the docking complexes and visualization of the enzyme-ligand interactions
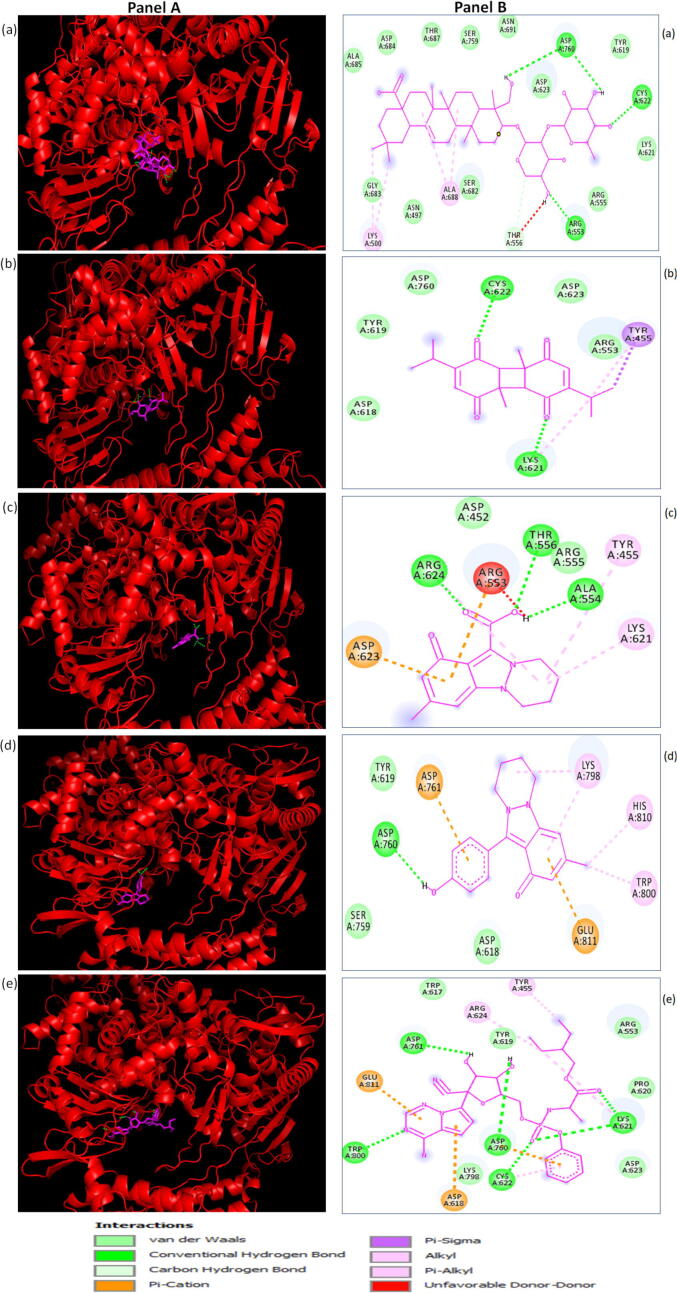
In the current study we have used the recently determined X-Ray crystal structure of RdRp (PDB ID: 6 M71) for screening the selected phytochemicals. According to the available literature, the key residues of RdRp involved in the interaction include; Y618, C622, N691, N695, M755, I756, L757, L758, S759, D760, D761, A762, V763, E811, F812, C813 and S814 (numbering for PDB ID: 6 M71). The key residues in the active site are D761 and D762. These are involved in the actual reaction of the RdRp enzyme (Elfiky, 2021b, Elfiky, 2020b, Yin et al., 2020). We used the RdRp holoenzyme for the initial screening of the selected phytochemicals. The structure of RdRp (PDB ID: 6 M71) and the binding of high-affinity phytochemicals/ligands to its active site is shown in Fig. 1. The 3D and 2D visualization of the interaction of the top four phytochemicals of N. sativa with the active site residues of RdRp is shown in Fig. 2. The phytochemicals α-hederin and negillicine interacted with the active site residues through four and three conventional hydrogen bonds, respectively, whereas the other two phytochemicals, dithymoquinone and negillidine interacted with the active site residues through two and one hydrogen bonds, respectively. There are also some non-bonded interactions, such as van der Waals forces, pi-alkyl, pi-cation, etc., between the phytochemicals and active site amino acid residues of RdRp as depicted in the 2D illustration (Fig. 2). The control drug, remdesivir is a nucleotide analog that binds to RdRp in a similar manner as a nucleotide does, thereby inhibiting viral RdRp activity through RNA chain termination. The interaction of various active site amino acid residues of RdRp with remdesivir is available in the literature (Elfiky, 2020b, Yin et al., 2020). In the present study, we observed that upon in silico screening of the selected phytochemicals, the top four compounds (α-hederin, dithymoquinone, negillicine and negillidine), especially α-hederin efficiently bound to the binding pocket of RdRp and interacted with one or more interacting residues present in the RNA binding tunnel of RdRP (Fig. 2) which might lead to the inhibition of its activity. Our results support those of earlier studies and suggest that N. sativa and its phytochemicals are worth studying further and could be recommended as an antiviral herbal supplement against COVID-19.
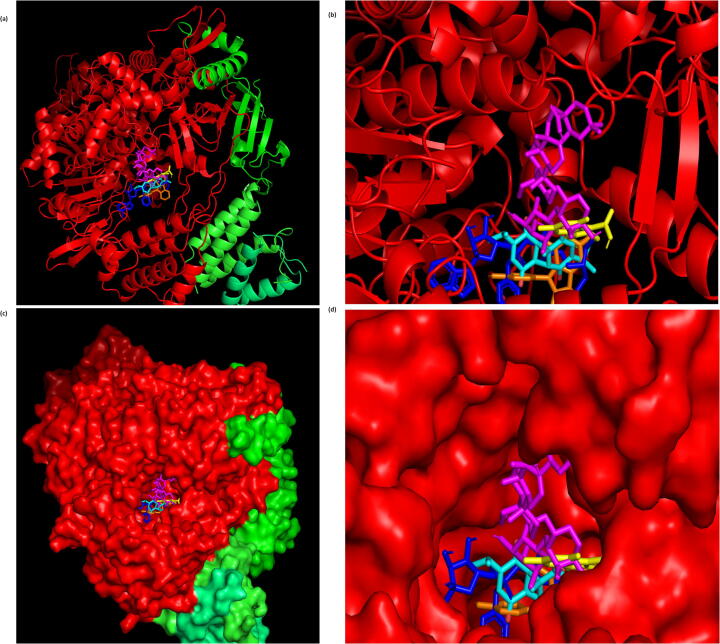
Fig. 1.
Structure of RNA-dependent RNA polymerase (RdRp) (PDB ID: 6 M71) demonstrating the binding of the high affinity phytochemicals to its active site (binding pocket). Chains A, B, C, and D of RdRp are shown in red, green, tv-green, and lime-green color, respectively, whereas, the ligands, α-hederin, dithymoquinone, negillicine, negillidine, and remdesivir, are shown in magenta, cyan, yellow, orange, and blue color, respectively.(a) Cartoon representation of RdRp with the phytochemicals bound to its active site. (b) Magnified view (Cartoon representation) of the active site of RdRp occupied by the phytochemicals. (c) Surface representation of RdRp with the phytochemicals bound to its active site. (d) Magnified view (Surface representation) of the active site of RdRp occupied by the phytochemicals.
Fig. 2.
Molecular docking analysis revealing the binding positions of the selected top 4 compounds of Nigella Sativa [α-hederin (a), dithymoquinone (b), nigellicine (c), nigellidine (d)] and remdesivir (e) to the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) RdRp (PDB ID: 6 M71; shown as a ribbon structure in red). Panel A shows the three-dimensional illustration of the interaction of ligands to the 6 M71 structure and Panel B shows the two-dimensional diagrams displaying the interactions with specific amino acid residues in the active site. All the ligands (α-hederin, dithymoquinone, nigellicine, nigellidine, and remdesivir) are shown in magenta color.
Based on its lowest docking energy on docking with RdRp (PDB ID: 6 M71) and its efficient interaction with the active site residues of 6 M71, we report here α-hederin as the most potent inhibitor of SARS-CoV-2 RdRp, among the nine compounds screened in this study. For further confirmation, we docked the top-ranked phytochemicals (α-hederin, dithymoquinone, negillicine and negillidine) with a single chain of RdRp (Chain A, the core enzyme). We observed that α-hederin, dithymoquinone, negillidine and negillicine bind into the active site of RdRp (Chain A) with a docking score of –8.6, –6.7, –6.6 and –6.1 kcal/mol, respectively. The stability of the docking complex of the top-ranked compound (α-hederin) was confirmed by molecular dynamic simulation using the Molecular Dynamics on Web (MDWEB) server (https://mmb.irbbarcelona.org/MDWeb/) and CABS flex server (http://212.87.3.12/CABSflex2/index) as shown in Fig. 3.
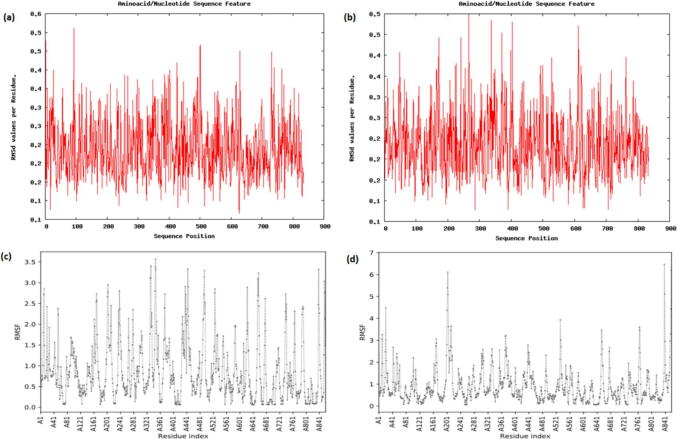
Fig. 3.
Molecular dynamic simulations of the RdRp/α-hederin docking complex. (a) Root mean square deviation (RMSD) profile of the RdRp chain A alone. (b) RMSD profile of the RdRp chain A/α-hederin complex. (c) Root mean square fluctuations (RMSF) profile of the RdRp chain A alone (d) RMSF profile of the RdRp chain A/α-hederin complex.
4. Conclusion
In this in silico study, we identified four phytochemicals from N. sativa that have the potential to inhibit the RdRp of SARS-CoV-2. Of the four compounds, α-hederin, dithymoquinone, negillicine, and negillidine, α-hederin had the highest binding affinity towards the active site residues of RdRp. Our docking results prove that the top four potential phytochemical molecules of N. sativa, especially α-hederin, could be considered for ongoing drug development strategies against SARS-CoV-2. However, further in vitro, in vivo, and clinical studies are warranted to establish the comprehensive pharmacological roles of these phytochemicals. Our study also supports the available literature regarding the traditional use of N. sativa to prevent viral diseases.
Funding information
This study was financially supported by the Deanship of Scientific Research (DSR) at Majmaah University, Al Majma’ah, Saudi Arabia [Grant no. RGP-2019–30].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are highly thankful to the Deanship of Scientific Research (DSR) at Majmaah University for financially supporting this study under project number RGP-2019-30 and for their support in proofreading the manuscript through professional editors at Editage, a division of Cactus Communications.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aftab S.O., Ghouri M.Z., Masood M.U., Haider Z., Khan Z., Ahmad A., Munawar N. Analysis of SARS-CoV-2 RNA-dependent RNA polymerase as a potential therapeutic drug target using a computational approach. J. Transl. Med. 2020;18(1) doi: 10.1186/s12967-020-02439-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad A., Husain A., Mujeeb M., Khan S.A., Najmi A.K., Siddique N.A., Damanhouri Z.A., Anwar F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013;3(5):337–352. doi: 10.1016/S2221-1691(13)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S., Abbasi H.W., Shahid S., Gul S., Abbasi S.W. Molecular docking, simulation and MM-PBSA studies of Nigella sativa compounds: A computational quest to identify potential natural antiviral for COVID-19 treatment. J. Biomol. Struct. Dyn. 2020;12:1–9. doi: 10.1080/07391102.2020.1775129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn D.-G., Choi J.-K., Taylor D.R., Oh J.-W. Biochemical characterization of a recombinant SARS coronavirus nsp12 RNA-dependent RNA polymerase capable of copying viral RNA templates. Arch. Virol. 2012;157(11):2095–2104. doi: 10.1007/s00705-012-1404-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akram Khan M., Afzal M. Chemical composition of Nigella sativa Linn: Part 2 Recent advances. Inflammopharmacology. 2016;24(2-3):67–79. doi: 10.1007/s10787-016-0262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardra, P., Singh, P., Hariprasad, V.R., Babu, U.V., Rafiq, M., Rao, R.P., 2020. Potential phytochemical inhibitors of the coronavirus RNA-dependent RNA polymerase: A molecular docking study. Res. Sq. DOI: https://doi.org/10.21203/rs.3.rs-35334/v1.
- Barakat A.B., Shoman S.A., Dina N., Alfarouk O.R. Antiviral activity and mode of action of Dianthus caryophyllus L. and Lupinustermes L. seed extracts against in vitro herpes simplex and hepatitis: A viruses infection. J. Microbiol. Antimicrob. 2010;2:23–29. doi: 10.5897/JMA.9000011. [DOI] [Google Scholar]
- Barakat E.M.F., El Wakeel L.M., Hagag R.S. Effects of Nigella sativa on outcome of hepatitis C in Egypt. World J. Gastroenterol. 2013;19:2529–2536. doi: 10.3748/wjg.v19.i16.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchentouf S., Noureddine M. Identification of compounds from Nigella sativa as new potential inhibitors of 2019 novel coronavirus (COVID-19): Molecular docking study. ChemRxiv. 2020 doi: 10.26434/chemrxiv.12055716.v1. [DOI] [Google Scholar]
- Chan J.-W., Yuan S., Kok K.-H., To K.-W., Chu H., Yang J., Xing F., Liu J., Yip C.-Y., Poon R.-S., Tsoi H.-W., Lo S.-F., Chan K.-H., Poon V.-M., Chan W.-M., Ip J.D., Cai J.-P., Cheng V.-C., Chen H., Hui C.-M., Yuen K.-Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daryabeygi-Khotbehsara R., Golzarand M., Ghaffari M.P., Djafarian K. Nigella sativa improves glucose homeostasis and serum lipids in type 2 diabetes: A systematic review and meta-analysis. Complement. Ther. Med. 2017;35:6–13. doi: 10.1016/j.ctim.2017.08.016. [DOI] [PubMed] [Google Scholar]
- DeLano W.L. Pymol: An open-source molecular graphics tool. CCP4 Newsl on Protein Crystallogr. 2002;40:82–92. [Google Scholar]
- Dorra N., El-Berrawy M., Sallam S., Mahmoud R. Evaluation of antiviral and antioxidant activity of selected herbal extracts. J. High. Inst. Public. Heal. 2019;49(1):36–40. doi: 10.21608/jhiph.2019.29464. [DOI] [Google Scholar]
- Doublié S., Ellenberger T. The mechanism of action of T7 DNA polymerase. Curr. Opin. Struct. Biol. 1998;8(6):704–712. doi: 10.1016/S0959-440X(98)80089-4. [DOI] [PubMed] [Google Scholar]
- Elfiky A.A. Zika viral polymerase inhibition using anti-HCV drugs both in market and under clinical trials. J. Med. Virol. 2016;88(12):2044–2051. doi: 10.1002/jmv.v88.1210.1002/jmv.24678. [DOI] [PubMed] [Google Scholar]
- Elfiky A.A. Zika virus: Novel guanosine derivatives revealed strong binding and possible inhibition of the polymerase. Future Virol. 2017;12(12):721–728. doi: 10.2217/fvl-2017-0081. [DOI] [Google Scholar]
- Elfiky A.A. Novel guanosine derivatives as anti-HCV NS5b polymerase: A QSAR and molecular docking study. Med. Chem. 2019;15(2):130–137. doi: 10.2174/1573406414666181015152511. [DOI] [PubMed] [Google Scholar]
- Elfiky A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248:117477. doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfiky A.A. Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA-dependent RNA polymerase (RdRp): A molecular docking study. Life Sci. 2020;253:117592. doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfiky A.A. Natural products may interfere with SARS-CoV-2 attachment to the host cell. J. Biomol. Struct. Dyn. 2021;39:3194–3203. doi: 10.1080/07391102.2020.1761881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfiky A.A. SARS-CoV-2 RNA dependent RNA polymerase (RdRp) targeting: an in silico perspective. Journal of Biomolecular Structure and Dynamics. 2021;39(9):3204–3212. doi: 10.1080/07391102.2020.1761882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfiky A.A., Elshemey W.M., Gawad W.A., Desoky O.S. Molecular modeling comparison of the performance of NS5b polymerase inhibitor (PSI -7977) on prevalent HCV genotypes. Protein J. 2013;32(1):75–80. doi: 10.1007/s10930-013-9462-9. [DOI] [PubMed] [Google Scholar]
- Elfiky A.A., Elshemey W.M. IDX -184 is a superior HCV direct-acting antiviral drug: A QSAR study. Med. Chem. Res. 2016;25(5):1005–1008. doi: 10.1007/s00044-016-1533-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfiky A.A., Elshemey W.M. Molecular dynamics simulation revealed binding of nucleotide inhibitors to ZIKV polymerase over 444 nanoseconds. J. Med. Virol. 2018;90(1):13–18. doi: 10.1002/jmv.v90.110.1002/jmv.24934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfiky A.A., Ismail A.M. Molecular modeling and docking revealed superiority of IDX-184 as HCV polymerase inhibitor. Future Virol. 2017;12(7):339–347. doi: 10.2217/fvl-2017-0027. [DOI] [Google Scholar]
- Elfiky A.A., Ismail A.M. Molecular docking revealed the binding of nucleotide/side inhibitors to Zika viral polymerase solved structures. SAR QSAR Environ Res. 2018;29(5):409–418. doi: 10.1080/1062936X.2018.1454981. [DOI] [PubMed] [Google Scholar]
- Elfiky A.A., Ismail A. Molecular dynamics and docking reveal the potency of novel GTP derivatives against RNA-dependent RNA polymerase of genotype 4a HCV. Life Sci. 2019;238:116958. doi: 10.1016/j.lfs.2019.116958. [DOI] [PubMed] [Google Scholar]
- Ganesan A., Barakat K. Applications of computer-aided approaches in the development of hepatitis C antiviral agents. Expert Opin. Drug Discover. 2017;12(4):407–425. doi: 10.1080/17460441.2017.1291628. [DOI] [PubMed] [Google Scholar]
- Goodsell D.S., Olson A.J. Automated docking of substrates to proteins by simulated annealing. Proteins. 1990;8(3):195–202. doi: 10.1002/prot.340080302. [DOI] [PubMed] [Google Scholar]
- He T., Xu X. The influency of Nigella sativa for asthma control: A meta-analysis. Am. J. Emerg. Med. 2019;38:589–593. doi: 10.1016/j.ajem.2019.11.036. [DOI] [PubMed] [Google Scholar]
- Hui D.S., I Azhar E., Madani T.A., Ntoumi F., Kock R., Dar O., Ippolito G., Mchugh T.D., Memish Z.A., Drosten C., Zumla A., Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan. China. Int. J. Infect. Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iheagwam F.N., Rotimi S.O. Computer-aided analysis of multiple SARS-CoV-2 therapeutic targets: Identification of potent molecules from African medicinal plants. Scientifica. 2020;2020:1–25. doi: 10.1155/2020/1878410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchdoerfer R.N., Ward A.B. Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors. Nat. Commun. 2019;10:2342. doi: 10.1038/s41467-019-10280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshak A., Wei L.i., Koshak E., Wali S., Alamoudi O., Demerdash A., Qutub M., Pushparaj P.N., Heinrich M. Nigella sativa supplementation improves asthma control and biomarkers: A randomized, double-blind, placebo-controlled trial. Phys. Ther. Res. 2017;31(3):403–409. doi: 10.1002/ptr.5761. [DOI] [PubMed] [Google Scholar]
- Luk H.K.H., Li X., Fung J., Lau S.K.P., Woo P.C.Y. Molecular epidemiology, evolution and phylogeny of SARS coronavirus. Infect. Genet. Evol. 2019;71:21–30. doi: 10.1016/j.meegid.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti S., Banerjee A., Nazmeen A., Kanwar M., Das S. Active-site Molecular docking of Nigellidine with nucleocapsid- NSP2-MPro of COVID-19 and to human IL1R-IL6R and strong antioxidant role of Nigella-sativa in experimental rats. J Drug Target. 2020;2:1–23. doi: 10.1080/1061186X.2020.1817040. [DOI] [PubMed] [Google Scholar]
- Majeed A., Muhammad Z., Ahmad H., Rehmanullah, Hayat S.S.S., Inayat N., Siyyar S. Nigella sativa L.: Uses in traditional and contemporary medicines–An overview. Acta. Ecol. Sin. 2021;41(4):253–258. doi: 10.1016/j.chnaes.2020.02.001. [DOI] [Google Scholar]
- Mali S.N., Pratap A., Thorat B. The rise of new coronavirus infection-(COVID-19): A recent update. Eur. J. Med. Oncol. 2020;4:35–41. doi: 10.14744/ejmo.2020.22222. [DOI] [Google Scholar]
- Mazaheri Y., Torbati M., Azadmard-Damirchi S., Savage G.P. A comprehensive review of the physicochemical, quality, and nutritional properties of Nigella sativa oil. Food Rev. Int. 2019;35(4):342–362. doi: 10.1080/87559129.2018.1563793. [DOI] [Google Scholar]
- Molla S., Azad A., Hasib A., Hossain M., Ahammed S., Rana S., Islam M. A review on antiviral effects of nigella sativa L. Pharmacol. OnLine. 2019;2:47–53. [Google Scholar]
- Namazi N., Larijani B., Ayati M.H., Abdollahi M. The effects of Nigella sativa L. on obesity: A systematic review and meta-analysis. J. Ethnopharmacol. 2018;219:173–181. doi: 10.1016/j.jep.2018.03.001. [DOI] [PubMed] [Google Scholar]
- O’Boyle N.M., Banck M., James C.A., Morley C., Vandermeersch T., Hutchison G.R. Open Babel: An Open chemical toolbox. J. Cheminform. 2011;3:33. doi: 10.1186/1758-2946-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onifade A.A., Jewell A.P., Adedeji W.A. Nigella sativa concoction induced sustained seroreversion in HIV patient. Afr. J. Tradit. Complement. Altern. Med. 2013;10:332–335. doi: 10.4314/ajtcam.v10i5.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onifade A.A., Jewell A.P., Ajadi T.A., Rahamon S.K., Ogunrin O.O. Effectiveness of a herbal remedy in six HIV patients in Nigeria. J. Herb. Med. 2013;3(3):99–103. doi: 10.1016/j.hermed.2013.04.006. [DOI] [Google Scholar]
- Oyero O.G., Toyama M., Mitsuhiro N., Onifade A.A., Hidaka A., Okamoto M., Baba M. Selective inhibition of hepatitis C virus replication by alpha-zam, a nigella sativa seed formulation. Afr. J. Tradit. Complement. Altern. Med. 2016;13(6):144–148. doi: 10.21010/ajtcam.v13i6.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan B., Chavan-Gautam P., Gautam M., Tillu G., Chopra A., Gairola S., Jadhav S. Ayurveda rasayana in prophylaxis of COVID-19. Curr. Sci. 2020;118:1158–1160. [Google Scholar]
- Rajapaksa R.M.H., Perera B.T., Nisansala M.J., Perera W.P.R.T., Dissanayake K.G.C. Potential of inhibiting the receptor binding mechanism of sarscov-2 using phytochemical extracts of medicinal herb; moleculer docking study. Glob. J. Eng. Sci. Res. Manag. 2020;7:51–61. doi: 10.5281/zenodo.3766184. [DOI] [Google Scholar]
- Rastogi, S., Pandey, D.N., Singh, R.H., 2020. COVID-19 pandemic: A pragmatic plan for ayurveda intervention. J. Ayurveda Integr. Med. S0975-9476(20)30019-X. DOI: 10.1016/j.jaim.2020.04.002. [DOI] [PMC free article] [PubMed]
- Sahebkar A., Beccuti G., Simental-Mendía L.E., Nobili V., Bo S. Nigella sativa (black seed) effects on plasma lipid concentrations in humans: A systematic review and meta-analysis of randomized placebo-controlled trials. Pharmacol. Res. 2016;106:37–50. doi: 10.1016/j.phrs.2016.02.008. [DOI] [PubMed] [Google Scholar]
- Sahebkar A., Soranna D., Liu X., et al. A systematic review and meta-analysis of randomized controlled trials investigating the effects of supplementation with Nigella sativa (black seed) on blood pressure. J. Hypertens. 2016;34(11):2127–2135. doi: 10.1016/j.clnu.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Salem M.L., Hossain M.S. Protective effect of black seed oil from Nigella sativa against murine cytomegalovirus infection. Int J Immunopharmacol. 2000;22(9):729–740. doi: 10.1016/S0192-0561(00)00036-9. [DOI] [PubMed] [Google Scholar]
- Sampangi-ramaiah M.H., Vishwakarma R., Shaanker R.U. Molecular docking analysis of selected natural products from plants for inhibition of SARS-CoV-2 main protease. Curr. Sci. 2020;118:1087–1092. doi: 10.18520/cs/v118/i7/1087-109. [DOI] [Google Scholar]
- Sekiou, O., Ismail, B., Zihad, B., Abdelhak, D., 2020. In-silico identification of potent inhibitors of COVID-19 main protease (Mpro) and Angiotensin Converting Enzyme 2 (ACE2) from Natural Products: Quercetin, Hispidulin, and Cirsimaritin Exhibited Better Potential Inhibition than Hydroxy-Chloroquine Against COVID-19 Main Protease Active Site and ACE2. ChemRxiv. Cambridge: Cambridge Open Engage; This content is a preprint and has not been peer-reviewed. DOI:10.26434/chemrxiv.12181404.v1.
- Shityakov S., Foerster C. In silico predictive model to determine vector-mediated transport properties for the blood-brain barrier choline transporter. Adv. Appl. Bioinform. Chem. 2014;7:23. doi: 10.2147/AABC.S63749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J.K.R., Figueiredo P.L.B., Byler K.G., Setzer W.N. Essential oils as antiviral agents. Potential of essential oils to treat SARS-CoV-2 infection: An in-silico investigation. Int. J. Mol. Sci. 2020;21:3426. doi: 10.3390/ijms21103426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subissi L., Posthuma C.C., Collet A., Zevenhoven-Dobbe J.C., Gorbalenya A.E., Decroly E., Snijder E.J., Canard B., Imbert I. One severe acute respiratory syndrome coronavirus protein complex integrates processive RNA polymerase and exonuclease activities. Proc. Natl. Acad. Sci. U. S. A. 2014;111(37):E3900–E3909. doi: 10.1073/pnas.1323705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakkoli A., Mahdian V., Razavi B.M., Hosseinzadeh H. Review on clinical trials of black seed (Nigella sativa) and its active constituent, thymoquinone. J. Pharmacopuncture. 2017;20(3):179–193. doi: 10.3831/KPI.2017.20.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott O., Olson A.J. AutoDockVina: Improving the speed and accuracy of docking with a new scoring function, ecient optimization, and multithreading. J. Comput. Chem. 2009;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulasli M., Gurses S.A., Bayraktar R., Yumrutas O., Oztuzcu S., Igci M., Igci Y.Z., Cakmak E.A., Arslan A. The effects of Nigella sativa (Ns), Anthemishyalina (Ah) and Citrus sinensis (Cs) extracts on the replication of coronavirus and the expression of TRP genes family. Mol. Biol. Rep. 2014;41(3):1703–1711. doi: 10.1007/s11033-014-3019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- V’kovski P., Kratzel A., Steiner S., Stalder H., Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 2020;19(3):155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardhan, S., Sahoo, S.K., 2020. Searching inhibitors for three important proteins of COVID-19 through molecular docking studies. arXiv;
- Yimer E.M., Tuem K.B., Karim A., Ur-Rehman N., Anwar F. Nigella sativa L. (Black Cumin): A Promising Natural Remedy for Wide Range of Illnesses. Evid Based Complement Alternat Med. 2019;2019:1–16. doi: 10.1155/2019/1528635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W., Mao C., Luan X., Shen D.-D., Shen Q., Su H., Wang X., Zhou F., Zhao W., Gao M., Chang S., Xie Y.-C., Tian G., Jiang H.-W., Tao S.-C., Shen J., Jiang Y.i., Jiang H., Xu Y., Zhang S., Zhang Y., Xu H.E. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science. 2020;368(6498):1499–1504. doi: 10.1126/science:abc1560. [DOI] [PMC free article] [PubMed] [Google Scholar]