Abstract
The dopamine D2 receptor exists in two different states, D2high and D2low; the former is the functional form of the D2 receptor and associates with intracellular G-proteins. The D2 agonist [3H]MCL-536 has high affinity for the D2 receptor (Kd 0.8 nM) and potently displaces the binding of (R-(−)-N-n-propylnorapomorphine (NPA; Ki 0.16 nM) and raclopride (Ki 0.9 nM) in competition binding assays. Here, we further characterize [3H]MCL-536. [3H]MCL-536 was metabolically stable, with about 75% of the compound remaining intact after 1 h incubation with human liver microsomes. Blood–brain barrier penetration in rats was good, attaining at 15 min a % injected dose per gram of wet tissue (%ID/g) of 0.28 in males versus 0.42 in females in the striatum. Specific uptake ratios ([%ID/g striatum]/[%ID/g cerebellum]) were stable in males during the first 60 min and in females up to 15–30 min. The D2-rich striatum exhibited the highest uptake and slowest washout compared to D2-poor cortex or cerebellum. In peripheral organs, uptake peaked at 15 min but declined to baseline at 60 min, indicating good clearance from the body. In vitro autoradiography on transaxial and coronal brain sections showed specific binding of [3H]MCL-536, which was abolished by preincubation with D2/D3 ligands sulpiride, NPA, and raclopride and in the presence of the stable GTP analogue guanylylimidodiphosphate. In amphetamine-sensitized animals, striatal binding was higher than in controls, indicating specificity for the D2high receptor state. [3H]MCL-536’s unique properties make it a valuable tool for research on neurological disorders involving the dopaminergic system like Parkinson’s disease or schizophrenia.
Keywords: dopamine D2high receptor, Parkinson’s disease, schizophrenia, aporphine, tritiated radioligand
Graphical Abstract
INTRODUCTION
The neurotransmitter dopamine is a key mediator of signaling in the brain and disturbance of the dopaminergic system, especially if it involves the D2 dopamine receptor, which has been closely associated with a variety of neurological disorders including Parkinson’s disease (PD), schizophrenia, restless leg syndrome, and attention deficit disorder, to name just a few.1-6 Some of these neurological and psychiatric diseases become symptomatic long after the first significant changes in brain physiology occur. An earlier diagnosis is desirable in these cases, since this would allow for earlier and more targeted intervention. Ultimately, broadening the arsenal of treatment options would greatly benefit patients with dopaminergic dysfunction.
Dopamine receptors are G-protein coupled receptors (GPCRs), divided into two subfamilies based on their signal transduction characteristics. The subfamily of D2-like receptors (D2, D3, and D4) inhibit cAMP production by adenylate cyclase.7-9 The D2 receptor subtype is a major target in the pathophysiology and treatment of schizophrenia and PD. Typical for GPCRs, it exhibits interconvertible high- and low-affinity states for agonists in vitro.10-12 In the high-affinity state, which is considered to be the active form of the receptor, D2 is coupled to the G-protein.13 In the low-affinity state, in contrast, the receptor is uncoupled from the G-protein and is inactive. A plethora of evidence indicates that alterations in the density of D2 receptors in the high-affinity state may be more relevant to the pathophysiology of neuropsychiatric disorders than alterations in the total receptor density.12
Currently, there are many high affinity antagonist D2/D3 receptor ligands, which are widely used, for example, in PET imaging. However, in light of the evidence above, focus has recently been redirected to the development of agonist D2 receptor ligands, which have great promise with regard to their potential to differentiate the high- and low-affinity states of the D2 receptor; notable examples include [11C]MNPA,14-19 [11C]NPA,20 and [11C]-(+)-PHNO.21,22
We have synthesized a highly promising high affinity dopamine D2 receptor ligand, R-(−)-2-(3-fluoropropanoxy-11-hydroxy-N-n-propyl(1,2-[3H])noraporphine (MCL-536), as a potential tritiated radioligand for applications in receptor binding assays and autoradiography studies in vitro.23 MCL-536 is an aporphine agonist that binds with high affinity to dopamine D2 receptors but not to other receptors of the D2 receptor family.24,25 MCL-536 also exhibits little to no affinity for other receptors found in the brain.25 MCL-536 has a high affinity for the D2 receptor in its active state, with a Kd value of 0.8 nM and Ki values of 0.16 nM for (R)-(−)-N-n-propylnorapomorphine (NPA) and 0.9 nM for raclopride, respectively, in competition binding assays.25
The ability to label a radiotracer with 18F instead of 11C greatly increases its utility and value to the research and medical communities, since 18F has a much longer half-life than 11C (110 min versus 20 min) and does not limit the use of the radiotracer to facilities with an on-site cyclotron.
Previous attempts to develop 18F-labeled D2/D3 ligands from nonfluorinated analogues were not successful, underscoring the fact that incorporation of 18F into a tracer and preserving its biological activity is not a trivial task.26-28 In contrast, MCL-536 possesses a fluoropropanoxy side chain rather than N-fluoroalkyl moiety, and it lacks the unstable catechol moiety found in [11C]MNPA and [11C]NPA.
Here, we further characterize MCL-536 with regard to its metabolic stability using a human liver microsome assay, biodistribution in rats in vivo, and binding characteristics in rat brain tissue, evaluated by in vitro autoradiography in the presence or absence of competing ligands, and in amphetamine sensitized rats ex vivo, which have increased numbers of D2 receptors in the high affinity state.
RESULTS AND DISCUSSION
Microsomal Stability.
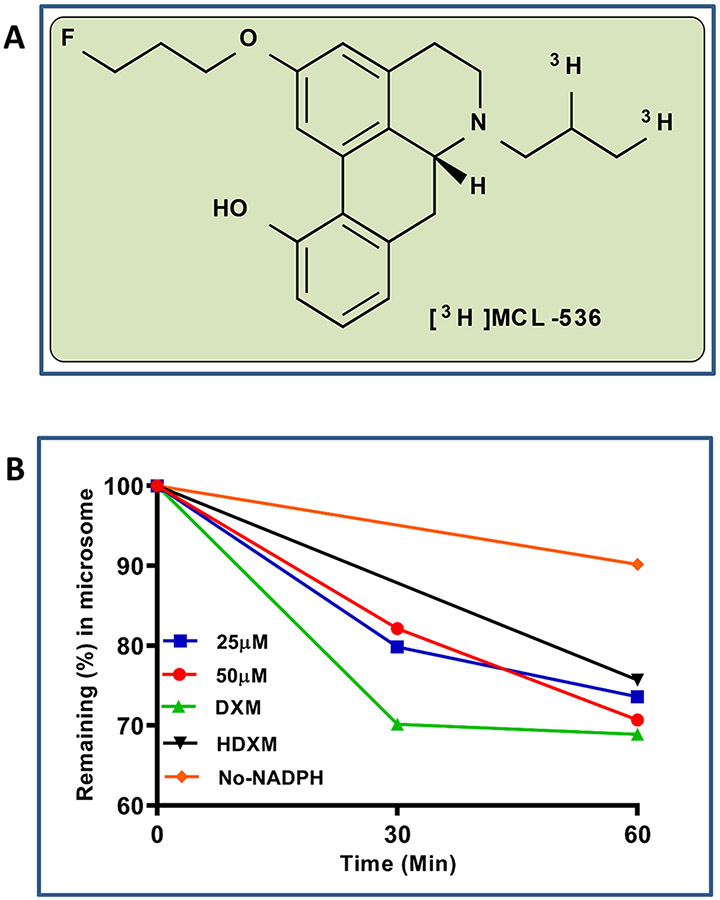
Investigation of metabolic stability of MCL-536 (Figure 1A) in human liver microsomes resulted in a high recovery at 1 h of about 75% of the ligand, indicating good metabolic stability (Figure 1B).
Figure 1.
Human liver microsome stability assay of MCL-536. Briefly, MCL-536 was preincubated with pooled human microsomes in 100 mM phosphate buffer; then NAPDH (final concentration 1 mM) was added. After 30 or 60 min incubation, the reaction was stopped, and samples were analyzed with a Varian Prostar HPLC system on Agilent Microsorb-MV 100 C18 columns fitted with a Microsorb 100-5 C18 MetaGuard column and a detector set at 265 nm. Dextromethorphan (DXM) was used as positive control (detection at 278 nm); negative controls included DXM incubated with heat-inactivated microsomes (HDXM) and one incubation mix without NADPH. The experiment was performed in triplicate. (A) Structure of MCL 536. (B) Microsome stability assay.
Biodistribution of MCL-536 in the Brain and Peripheral Organs.
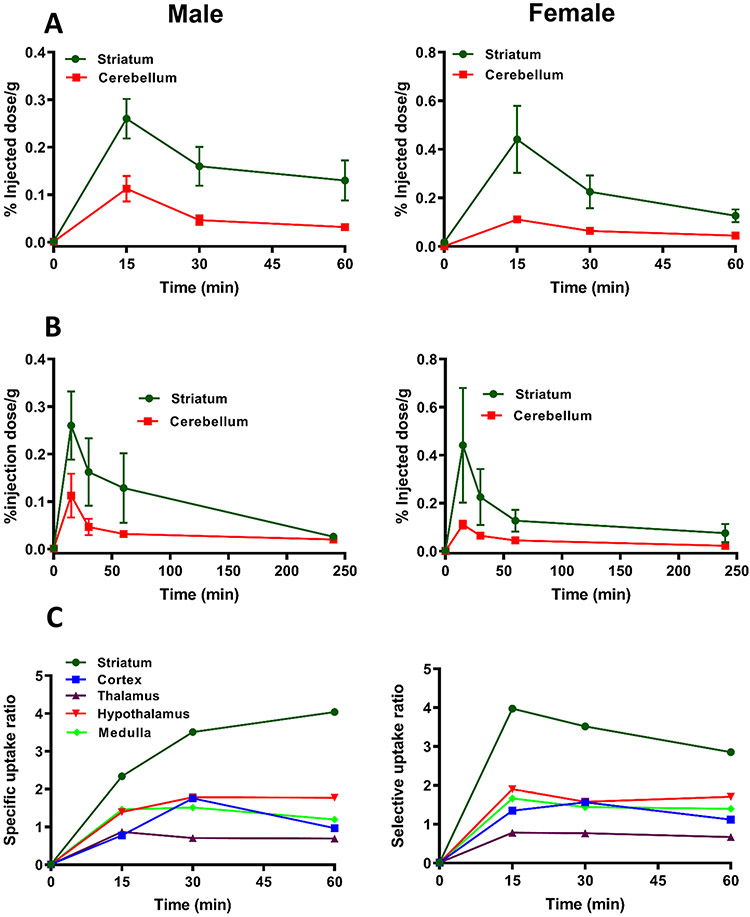
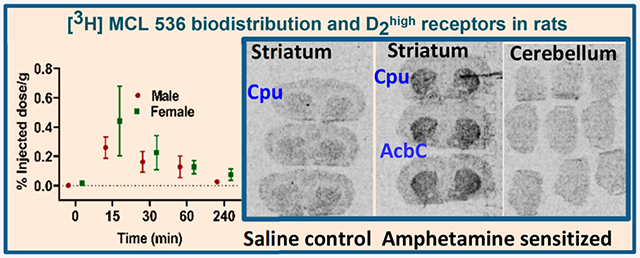
The highest brain uptake of [3H]MCL-536 was observed after 15 min in the striatum, with an uptake of 0.28% injected dose per gram wet tissue (%ID/g) in males and 0.42 %ID/g in females (Figure 2A,B). The difference in % ID/g between males and females was not significant. The striatum is known to be rich in D2 receptors.29 In contrast, the cerebellum, a region that contains almost no D2 receptors, showed a baseline binding between 0.1 and zero (Figure 2A,B). The washout rate of [3H]MCL-536 was comparatively fast in both sexes, showing a decrease in concentrations of about 50–70% by 60 min. Values reached almost baseline 4 h after injection of the ligand (Figure 2B).
Figure 2.
Biodistribution of [3H]MCL-536 in the rat brain. A total amount of 6 μCi of [3H]MCL-536 was injected into the tail vein of male or female Sprague–Dawley rats, and 15, 30, 60, and 240 min after injection, brain regions were dissected, and the tissue was weighed and dissolved in 1 mL of 0.8 N NaOH. After addition of 5 mL of scintillant, samples were counted in a β-counter and the %ID/g wet tissue was calculated. Data are presented as mean ± SEM of 4 animals per time point and ligand. (A, B) Biodistribution of [3H]MCL-536 in the striatum and cerebellum of female and male rats. (C) Biodistribution of [3H]MCL-536 presented as the brain region/cerebellum ratio of female and male rats. Uptake in brain regions was calculated as the ratio of percent dose per gram of the region versus the cerebellum, where D2 receptors are scarce.
Specific uptake ratios, defined as [%ID/g striatum]/[%ID/g cerebellum] reached a maximum of about 4 for both males and females. In males, the ratio was increased during the first 60 min after injection. In females, however, it reached the maximum at 15 min, followed by a slow decline to about 3 at 60 min (Figure 2C). For all other brain regions, namely, the cortex, thalamus, hypothalamus, and medulla, the specific uptake ratios were between about 0.5 and 2, confirming that in regions with low D2 receptor expression, binding of [3H]MCL-536 is low.
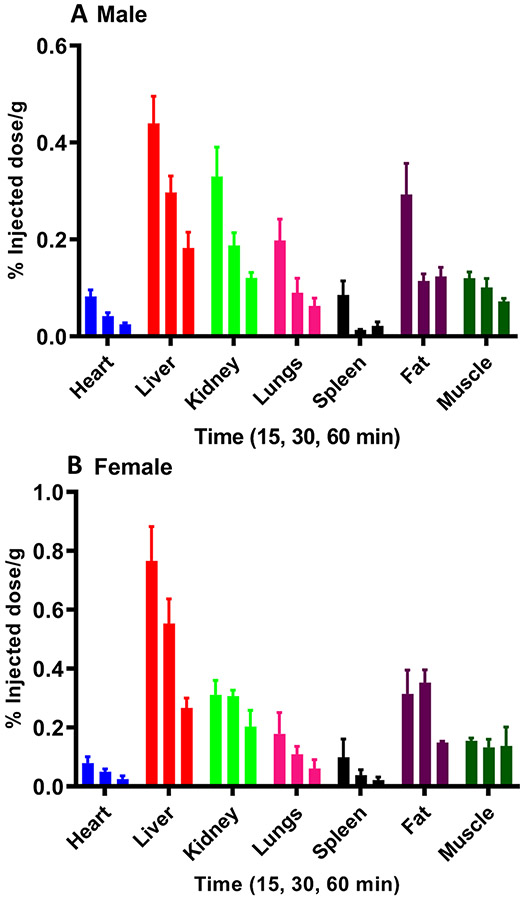
In peripheral organs and tissues, uptake of [3H]MCL-536 was comparatively low in heart, spleen, and muscle in both sexes, but higher in fat and lungs (Figure 3A,B). Highest %ID/ g was observed in liver and kidneys. In all peripheral tissues the maximum %ID/g was at 15 min, followed by a washout (Figure 3A,B).
Figure 3.
Biodistribution of [3H]MCL-536 in peripheral organs in male and female rats. [3H]MCL-536 (6 μCi/300 g body weight) was injected into the tail vein; 15, 30, and 60 min after injection, peripheral organs were dissected, and the tissue weighed and dissolved in 1 mL of 0.8 N NaOH. After addition of 5 mL of scintillant, samples were counted in a β-counter and the %ID/g wet tissue was calculated. Data are presented as mean ± SEM of 4 animals per time point and ligand. (A) Male rats. (B) Female rats.
The biodistribution studies showed that the uptake of [3H]MCL-536 was highest in the striatum and in the kidneys, with comparable maxima. However, %ID/g in the striatum was stable in females and declined only by about 25% in males at 60 min postinjection, while in the kidneys, the decline was about 60% by 60 min in both sexes. The data suggest an efficient passage of the radioligand through the blood–brain barrier as well as an efficient excretion from the body.
In Vitro Autoradiography.
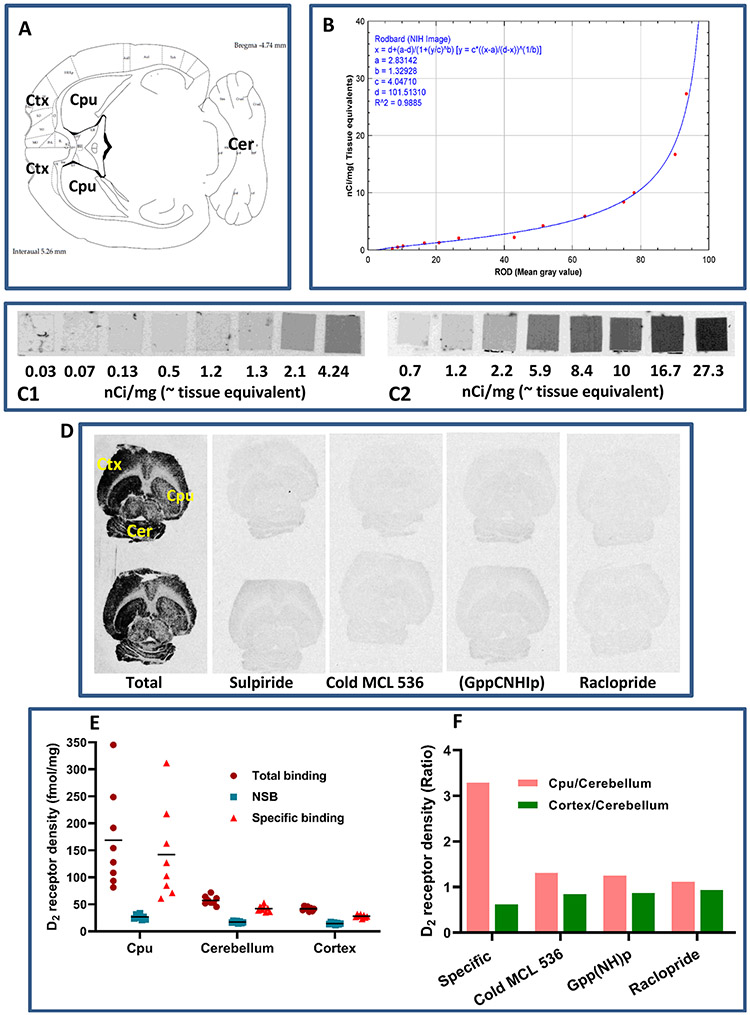
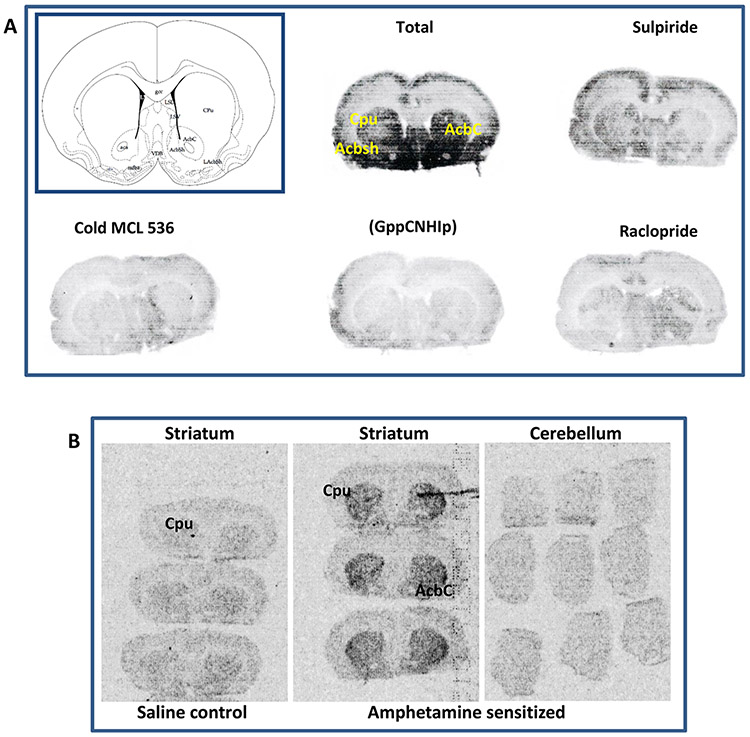
In vitro autoradiography on transaxial brain sections showed specific binding of [3H]MCL-536 (Figure 4D) in the striatum, which could be abolished by preincubation with receptor ligand sulpiride (10 nM) and by blocking with cold ligand MCL-536 (100 nM), as well as in the presence of guanylylimidodiphosphate (Gpp(NH)p, 200 μM) and raclopride (100 nM). Without preincubation with buffer, the presence of endogenous ligand at the receptors inhibited binding of [3H]MCL-536.
Figure 4.
In vitro autoradiography showing the binding of [3H]MCL-536 in the rat brain. Transaxial brain sections were preincubated with buffer to eliminate nonspecific binding. Afterward, sections were incubated with [3H]MCL-536 alone or in combination with D2 receptor antagonists or cold MCL-536 to show specificity. Dried sections were exposed to autoradiography film for visualization. (A) Schematic of a transaxial brain section; CPU, caudate putamen; Ctx, cortex; Cer, cerebellum. (B) Conversion of gray scale values to relative optical densities (ROD). (C) 3H microscales were coexposed on the same films as the experimental samples to allow quantification. (D) In vitro binding of radioligand to 10 μm thick rat brain transaxial slices in the presence or absence of 10 M sulpiride, 100 nM cold ligand, 200 μM Gpp(NH)p, or 100 nM raclopride. (E) Quantification of receptor density for D2 (fmol/mg). (F) D2 receptor density ratio in striatum versus cerebellum and cortex versus cerebellum in the presence or absence of other radioligands or cold MCL 536.
3H microscales were exposed simultaneously as a reference for total radioactivity quantitative analysis (Figure 4C), and the gray values were converted into relative optical densities (ROD, Figure 4B). Quantitative analysis of D2 receptors (fmol/mg) and the receptor density ratios between D2-rich caudate putamen versus D2 poor cerebellum as well as D2 poor cortex versus D2 poor cerebellum are shown in Figure 4E,F, respectively. From the data, it is clear that [3H]MCL-536 is able to detect the elevated number of D2 receptors in the striatum as compared to cerebellum or cortex.
Figure 5A shows that MCL-536 specifically detects a dense population of D2 receptors in the caudate putamen and nucleus accumbens. Again, this binding could be abolished by co-incubation with D2 receptor antagonists sulpiride and raclopride, as well as by presence of cold MCL-536 and (Gpp(NH)p.
Figure 5.
In vitro and ex vivo autoradiography of [3H]MCL-536 binding on transverse brain sections of untreated and amphetamine-sensitized rats. Transverse brain sections of control or amphetamine-sensitized rats were preincubated with buffer to eliminate nonspecific binding. Afterward, sections were incubated with [3H]MCL-536 alone or in combination with D2 receptor antagonists or cold MCL-536 to show specificity. Dried sections were exposed to autoradiography film for visualization. (A) Schematic of a transverse brain section at the level of the anterior commissure (CPU, caudate putamen; AcbC, nucleus accumbens, core; Acbsh, nucleus accumbens, shell) and in vitro binding of [3H]MCL-536 to transverse rat brain slices at the level of the nucleus accumbens in the absence or presence of D2 receptor antagonists sulpiride and raclopride, cold ligand, or Gpp(NH)p. (B) Ex vivo binding of [3H]MCL-536 to transverse rat brain sections of amphetamine-sensitized rats in comparison with control rats. Note that panels A and B are separate experiments and that exposure times of the autoradiographs on the film differ between panels A and B for better visualization.
Ex Vivo Autoradiography in D2high Rat Model.
Since amphetamine sensitization markedly up-regulates D2 receptors in the high affinity state in vitro,12 this model was used to determine whether [3H]MCL-536 detects such an elevation in D2high. As shown in Figure 5B, [3H]MCL-536 detected more D2 receptors in the striatum of amphetamine sensitized animals compared to controls. In the cerebellum, a D2 poor region, as expected, no increase in the binding of [3H]MCL-536 was detected.
Results confirm that [3H]MCL-536 binds preferentially at D2high receptors in D2 rich regions like the striatum, especially the area of the caudate putamen and nucleus accumbens, confirming selectivity of the ligand in brain tissue.
Most of the commonly used antipsychotic drugs act at dopamine D2 receptors. Binding and blocking of these receptors is important in the efficacy of these drugs toward psychiatric illness, for example, against the positive symptoms of schizophrenia. Since drugs with very different pharmacological and therapeutic profiles act at D2 receptors, these receptors are still subjected to intense research both in vitro and in vivo. Tools that enable a comprehensive study of D2 receptors are therefore in demand. An agonist ligand is preferable in this context because it is able to distinguish between the active D2high state of the receptor and its inactive D2low state, while antagonist ligands fail to do that.
There are many examples of radioligands for D2/D3 receptors, including the antagonists [3H]spiperone and its more lipophilic derivative [3H]N-methylspiperone (NMSP),30 [3H]domperidone,31 [3H]raclopride,32 [3H]nemonapride,33 and [3H]remoxipride.34 Among the agonists are [3H]-apomorphine35 [3H]-N-propyl-norapomorphine ([3H]-NPA),36 and [3H]quinpirole.37 However, the usefulness of these substances is limited, either because of their antagonist nature, which excludes distinction between high and low affinity D2 receptors, or because of their cross-reactivity with other members of the dopamine receptor family or even other receptor families, for example, adrenergic or serotonergic receptors, which compromises specificity.
The D2 receptor agonist MCL-536 has shown highly favorable in vitro properties toward D2 receptors, with a subnanomolar binding affinity (Kd 0.8 nM) for human cloned D2 long receptors expressed in CHO cells but not for the other receptors of the D2 subfamily. The biphasic nature of its curves shows that MCL-536 clearly differentiates between the active high affinity and the inactive low affinity state of the receptor.25 In in vitro competition experiments, [3H]MCL-536 had a Ki of 0.16 nM against the D2/D3 agonist (R-(−)-N-n-propylnor-apomorphine, NPA) and a Ki of 0.9 nM against the D2/D3 antagonist raclopride. Co-incubation with guanylylimidodiphosphate abolished binding to D2high. MCL-536 exhibited little to no affinity for other receptors tested.25,38 These properties make MCL-536 superior to other agonist ligands, which are less specific and usually have high binding affinities to more than one receptor.
One of the conditions for the suitability of MCL-536 as a D2high receptor radioligand for application in research, diagnosis, and treatment is metabolic stability. When tested in an assay with human liver microsomes, MCL-536 showed good metabolic stability with about 75% of the ligand still intact after 60 min of incubation. The rationale of this strategy is that the in vitro metabolic stability in the preparations from humans should reasonably well predict in vivo clearance in humans.
Another prerequisite for the usefulness of a radioligand is a good pharmacological profile, especially for in vivo applications, and good penetration of the blood–brain barrier, since neurological and psychiatric symptoms originate in D2 rich areas in the brain. Figure 2 shows the results of the ex vivo biodistribution studies in rodent brain. [3H]MCL-536 exhibited a rapid (15 min after injection into the tail vein) and highly specific binding in the striatum, a region enriched in D2 receptors, which persisted for at least 60 min at a high level in both male and female rats. If compared to the binding in the cerebellum, a region expressing a low number of D2 receptors, the ratio striatum/cerebellum was much higher than the ratios for other brain regions such as the cortex, thalamus, hypothalamus, and medulla, all regions expressing low numbers of D2 receptors.29 This not only suggests good penetration through the blood–brain barrier, which is a crucial property of a potential radioligand for receptors located in the brain but also confirms the preference of the ligand for D2 receptors in tissue.
A good pharmacological profile, as mentioned above, especially for applications that involve humans, also requires that the ligand be excreted rapidly from the body. Figure 3 shows that after an initial peak, [3H]MCL-536 is excreted rapidly from the body in both male and female rats. Highest concentrations were found in the body organs that mediate excretion, namely, the liver and kidney. In all other organs examined, heart, lungs, spleen, and muscle, initial uptake was much lower and excretion was rapid. A bit of an exception is the fatty tissue, where initial peaks were a bit higher; however, at 60 min, [3H]MCL-536 levels were reduced by about 2/3 in this compartment as well. From all these data, it can be concluded that [3H]MCL-536 has a very good ADME (absorption, distribution, metabolism and excretion) profile, suggesting its superiority to other D2 receptor ligands in reaching the target tissue while limiting or minimizing the dose to other organs.
Examination of the binding of [3H]MCL-536 to D2 rich versus D2 poor tissues in the brain in situ in the presence or absence of D2 receptor antagonists provided valuable information concerning the specific displaceable binding to the dopamine D2 receptor. Using autoradiography on transaxial and transversal brain slices, we could demonstrate enhanced binding in the D2 receptor rich striatum, especially the caudate putamen and nucleus accumbens. In contrast, binding at other dopamine innervated regions including the cortex, thalamus, hypothalamus, and medulla was much lower.
Co-incubation with an excess of D2/D3 receptor antagonists sulpiride or raclopride completely abolished binding of [3H]MCL-536. Raclopride, similar to other antagonists, has a high affinity to D2 receptors but does not have the capability to distinguish between D2high and D2low in whole, unfrozen rat anterior pituitary cells ex vivo.4,5,32
Likewise, competition with nonradioactive MCL-536 prevented binding of the ligand. It has been shown before that the percentage of D2 sites in the high-affinity agonist state is high in tissue sections;39 with [3H]spiperone (competing with dopamine), 90% of the dopamine receptors were shown to be in the high-affinity state on rat striatal slices. Addition of the GTP analogue GmP-PnP resulted in a total shift to the low-affinity state.39 In more recent studies on both fresh and frozen and thawed rat brain sections, 13% and 22% of the receptors, respectively, were determined to be in the high-affinity state in striatal slices.5 This contrasts with homogenate binding where the percentage is usually much lower, varying from 28% to 56% when using dopamine as a competitor.40-42 The first formal demonstration of a greater displaceability of an agonist ligand compared to an antagonist in the amphetamine challenge model was performed in mouse striatum using N-[3H]-n-propylnorapomorphine and [11C]-raclopride.43 It appears that higher proportions of D2high receptors are found for in vivo experiments44 or in brain sections,39,45 where the tissue structure is more intact, than in disrupted and homogenized tissue. The abundance of agonist binding sites in striatum seems to be lower than that of antagonist binding sites: agonist Bmax was found to be about 75–80% of antagonist Bmax in two independent studies.44,46 However, this may be due to experimental differences in the molar activity determination of various radioligands or an experimental artifact caused by depletion of G-protein in brain sections, which may cause the D2high receptor to revert to D2low in the presence of high agonist concentrations. A review of available data did not strongly argue for a substantial difference between agonist and antagonist Bmax.45
The dopamine D2 receptor is a G protein-coupled receptor. Its G protein alpha subunit binds GTP in the activated state, and the receptor becomes inactivated when GTP is metabolized to GDP and Pi. In our previous binding studies, the specific binding of [3H]MCL-536 was found to separate into two sites in the presence of GppNHp. The binding component removed by the guanine nucleotide represented the dopamine D2High receptors, confirming that [3H]MCL-536 is a D2high agonist.25 In our present study, co-incubation with GppNHp abolished binding of [3H]MCL-536; this was especially clear in the caudate putamen and nucleus accumbens, which depicted an intense labeling that was reduced to background levels by GDP. This is strong evidence for the specific binding of MCL-536 to D2high receptors.
Quantification of the receptor binding in the caudate putamen yielded a mean specific binding of [3H]MCL-536 of about 145 fmol/mg protein. Seeman and Tallerico47 found three sets of D2 receptors in homogenates of rat striatum: D2 low-affinity sites (9.8 pmol/g), D2 high-affinity sites that were unmasked in the presence of D1 receptor blockade by SCH-23390 (4.2 pmol/g), and D2 high-affinity sites that were not unmasked by SCH-23390 but were converted to low-affinity sites in the presence of guanilylimidodiphosphate (3 pmol/g). In an in vivo study in contrast, the D2 receptor density for the caudate putamen was determined to be 784 ± 60 fmol/mg protein. 48
Further evidence for the specificity of [3H]MCL-536 for D2high receptors could be demonstrated in our experiments with amphetamine-sensitized rats, which display elevated D2 receptors in the high affinity state.12 Amphetamine typically induces about a 2.36-fold increase in D2high, measured in the striatum in vivo, which corresponds nicely to a 2.44-fold elevation of D2high in vitro in the same tissue.49 As expected, in the present experiment, binding of [3H]MCL-536 in the striatum of amphetamine sensitized rats was much higher than that in control animals. This suggests that in its tritiated form, MCL-536 can be used experimentally to investigate D2 receptor activity and dynamics more effectively as a function of neurological conditions. A recent study compared healthy volunteers who received a mildly sensitizing regimen of repeated oral amphetamine to unmedicated schizophrenia patients with a first-episode psychosis.50 Amphetamine sensitization of healthy volunteers significantly increased dopamine release in the prefrontal cortex as evaluated by PET using the D2/D3 receptor agonist ligand [11C]-(+)-PHNO. The dopamine release in amphetamine-sensitized volunteers was similar to that seen in the patient group. This study not only confirms the hypothesis of “endogenous sensitization” but at the same time also seconds the concept of impairment of prefrontal dopaminergic system in schizophrenia.
In summary as discussed in detail above, agonist ligands are superior, because their preferential binding to the receptors in their high affinity state makes them an ideal tool for studying the role of the receptor state in the pathogenesis of disorders associated with the dopaminergic system. MCL-536 with its high affinity to D2high receptors in vitro and in vivo with its good ADME profile is an excellent tool in this regard. Thus, MCL-536 will be enabling more detailed studies and enhancing the knowledge of dopamine transmission and D2 receptor dynamics in these disorders.
METHODS
Microsomal Stability.
The NADPH-dependent metabolism of MCL-536 was studied using human liver microsomes (pooled) according to the supplier’s instructions (Life Technologies; HMMCPL). Briefly, MCL-536 (2 μL of 100× stock) was preincubated with pooled human liver microsomes (5 μL of 20 mg/mL) in 183 μL of 100 mM phosphate buffer (pH 7.4) for 5 min at 37 °C; then the reaction was initiated with 10 μL of NAPDH (20 mM; final concentration 1 mM). After 30 or 60 min incubation at 37 °C (water bath), the reaction was stopped by addition of 200 μL of cold acetonitrile (MeCN). The samples were vortexed and then centrifuged at 3000 rpm (4 °C) for 5 min to precipitate proteins. The supernatant (about 180 μL) was transferred to a vial and filtered through a 0.2 μm syringe filter prior to injection; then 100 μL of each filtrate was analyzed with a Varian Prostar HPLC system on Agilent Microsorb-MV 100 C18 columns 150 mm × 4.6 mm, 5 μm particle size, fitted with a Microsorb 100–5 C18 MetaGuard column, 100 mm × 4.6 mm. Samples were analyzed using a 1 mL flow rate and gradient method beginning at 20% B going to 90% B for 4 min, going to 20% in 2 min, and holding for 4 min (20 min total time). The mobile phases used were phase A, 100 mM phosphate buffer, pH 2.9, and mobile phase B, acetonitrile, with detector set at 265 nm to determine remaining MCL-536. Dextromethorphan (DXM) was used as positive control (detector set at 278 nm). Negative controls included DXM incubated with heat-inactivated microsomes (HDXM) and one incubation mix without NADPH. The metabolic stability was calculated based on the 0 min sample. Each experiment was performed in triplicate.
Ligand Studies.
[3H]MCL-536 was custom synthesized (maximum specific activity 60 Ci/mmol; American Radiolabeled Chemicals, Inc. St. Louis, MO 63146 USA).
Animals.
For biodistribution studies, a total of 60 adult (30 male and 30 female) Sprague–Dawley rats (Charles River Laboratories) were used for evaluation of the tritiated ligand. Animals were housed four rats per cage and allowed access to food and water ad libitum. The animal room was maintained on a reverse 12:12 light/dark cycle with lights off from 8:00 AM to 8:00 PM. Ten animals (5 male, 5 female) were used as controls; 50 animals (25 male, 25 female) were used for studies with [3H]MCL-536. All procedures involving animals were approved by the McLean Hospital IACUC Committee and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Biodistribution Studies.
[3H]MCL-536 in saline (6 μCi/300 g body weight, 200 μL total volume, n = 3–5 per group) was injected into the tail vein of male or female SD rats. Saline was used as a control. At 15, 30, 60, and 240 min after injection, selected organs (heart, liver, kidneys, lungs, spleen, fat, muscle, and brain) were removed. The striatum, cortex, thalamus, hypothalamus, medulla, and cerebellum were dissected. The organs and brain regions were weighed and dissolved in 1 mL of 0.8 N NaOH. After addition of 5 mL of scintillant, radioactivity was measured in a β-counter. The percent injected dose per gram (%ID/g) was calculated by normalizing the total counts of the organ by the weight of the organ as a percentage of the total injected radioactivity. Uptake in brain regions was calculated as the ratio of %ID/g of the region versus 5 the cerebellum, where D2 receptors are scarce.
Autoradiography In Vitro.
Brains were sectioned at −12 °C into transaxial and transversal 20 μm sections using a cryostat (HM560 Microm), and the sections were mounted onto Superfrost plus Gold adhesive slides (Fisher Scientific). Slides were organized in pairs, and for each pair, new sections were mounted alternatively on the first or second slide of the pair so that sections on the corresponding positions on the two slides forming a pair would represent adjacent tissue layers. The sections were allowed to dry and were stored at −80 °C with silica gel desiccant until used.
On the day of experiment, the slides were equilibrated 20 min at −20 °C and then allowed to dry at room temperature for 5–10 min. For binding studies, slides were equilibrated for 30 min with buffer (50 mM Tris-HCl, 1 mM EDTA, 5 mM KCl, 1.5 mM CaCl2, 4 mM MgCl2, 120 mM NaCl, 0.001% ascorbic acid and protease inhibitor cocktail tablet (Roche) pH = 7.4) at room temperature (RT).
Following preincubation, sections were incubated with 2 nM of [3H]MCL-536 alone or in combination with other ligands: nonspecific binding was determined in adjacent sections by addition of 10 μM sulpiride (D2 receptor antagonist), cold ligand MCL-536, or raclopride (D2 receptor antagonist, 100 nM).
In order to examine the effects of guanine nucleotides on [3H]MCL-536 binding, a separate set of slices was incubated with 2 nM [3H]MCL-536, as described above, in the presence of 200 μM guanilylimidodiphosphate (Gpp[NHlp), Millipore-Sigma, St. Louis, MO).
In a final set of slides, the preincubation step was omitted in the [3H]MCL-536 assay in order to verify the effects of endogenous ligands (data not shown).
After 2 h incubation at room temperature, sections were washed in 5 the appropriate buffer (2 × 5 min at 4 °C followed by a quick dip in ice cold distilled water) and left to dry at room temperature for 1 h. For visualization and optical density analysis, the sections were opposed to BioMax MR Film (Carestream) for 2–3 weeks alongside microscale-calibrated 3H standards ART0123C (0.03–4.24 nCi/mg, tissue equivalents) and ART0123B (0.7–27.3 nCi/mg, tissue equivalents) (American Radiolabeled Chemicals, St. Louis, MO).
Quantitative Analysis.
Analysis of optical density was carried out using the public NIH ImageJ program. The binding to the D2 receptor was quantified by determining the gray levels of the pixels (relative optical density (ROD)). The binding density was quantified by setting the optical densities of the tissue autoradiograms in relation to those of the radioactive microscale standards, which were the same thickness as the tissue sections and for which the amounts of 7 radioactivity are known. A ROD measured in a region was interpolated to a value expressed as nCi/mg protein using the calibration curve. Specific binding was determined by the following relationship: fmol/mg protein = (X nCi/mg protein) × (fmol/V nCi), where X = tissue equivalent value over the region being studied and V = specific activity of the radiolabeled ligand. The left and right side of the brain was quantified separately on three consecutive sections, and the background level on each section was measured and subtracted. For each brain area, three consecutive sections were processed to determine the total binding, and one was processed to evaluate the nonspecific binding. Both total and nonspecific binding were calculated in the same way, and nonspecific binding was subtracted from total binding to give specific binding. The number of animals was n = 5 for each experimental group.
Amphetamine Sensitization and Ex Vivo Autoradiography.
Adult male Sprague–Dawley rats, weighing 120 g at the start of the experiment, were used. They were housed one per cage with free access to food and water at a constant temperature of 20 °C on a 12:12 reverse light/dark cycle. Lights were off at 8:00 AM.
Each rat received an intraperitoneal injection of 1.5 mg/kg d-amphetamine sulfate (Sigma, St. Louis, MO) or 0.9% saline (1 mL/kg), daily for 10 days, followed by an additional 10 days without any injections.47,51
Rats were briefly anesthetized with 1.5–2% isoflurane, and tails were prewarmed for 5 min with a disposable heating pad to increase vasodilation. A catheter was placed into the lateral caudal tail vein. Subsequently, [3H]MCL-536 (6 μCi/300 g body weight in 200 μL of saline) was injected into the catheter using a 25-gauge needle, followed by 300 μL of saline to ensure that no ligand remained in the catheter. Immediately after injection, the catheter was removed from the tail vein, and the puncture site was sealed with a wound closure strip. At the end of the experiment (60 min), rats were sacrificed, and the brains were removed, cut into transverse sections, and exposed to BioMax MR Film (Carestream) as described above.
ACKNOWLEDGMENTS
This paper is dedicated to the memory of the late Philip Seeman MD, Ph.D., D.Sc., FRSC (1934–2021), an excellent, world renowned neuroscientist and an enthusiastic, kind-hearted, friendly collaborator for the last 40 years, who pioneered many developments in dopamine receptor research. Financial support of the National Institutes of Health (R44-OD024615 (J.L.N., S.S.), R43-OD020186 (J.L.N.), and R21-MH103718 (A.W.S.)) and the Branfman Family Foundation (J.L.N., A.W.S., S.S.) is gratefully acknowledged. MCL-565 was converted to [3H]MCL-536 by American Radiolabeled Chemicals, Inc. (St. Louis, MO), Dr. Surendra Gupta. We thank Dr. Joseph Pocher for tail vein catheterization training and support.
Footnotes
The authors declare no competing financial interest.
Contributor Information
Sivan Subburaju, Division of Basic Neuroscience, Medicinal Chemistry Laboratory, McLean Hospital, Belmont, Massachusetts 02478, United States; Department of Psychiatry, Harvard Medical School, Boston, Massachusetts 02115, United States.
Anna W. Sromek, Division of Basic Neuroscience, Medicinal Chemistry Laboratory, McLean Hospital, Belmont, Massachusetts 02478, United States; Department of Psychiatry, Harvard Medical School, Boston, Massachusetts 02115, United States
Philip Seeman, Departments of Pharmacology and Psychiatry, University of Toronto, Toronto, Ontario M5P 3L6, Canada.
John L. Neumeyer, Division of Basic Neuroscience, Medicinal Chemistry Laboratory, McLean Hospital, Belmont, Massachusetts 02478, United States; Department of Psychiatry, Harvard Medical School, Boston, Massachusetts 02115, United States.
REFERENCES
- (1).Bozzi Y, and Borrelli E (2006) Dopamine in neurotoxicity and neuroprotection: what do D2 receptors have to do with it? Trends Neurosci. 29 (3), 167–74. [DOI] [PubMed] [Google Scholar]
- (2).Marsden CA (2006) Dopamine: the rewarding years. Br. J. Pharmacol 147 (1), S136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Corripio I, Escarti MJ, Portella MJ, Perez V, Grasa E, Sauras RB, Alonso A, Safont G, Camacho MV, Duenas R, Arranz B, et al. (2011) Density of striatal D2 receptors in untreated first-episode psychosis: an I123-IBZM SPECT study. Eur. Neuro-psychopharmacol 21 (12), 861–6. [DOI] [PubMed] [Google Scholar]
- (4).Lieberman JA, Kane JM, and Alvir J (1987) Provocative tests with psychostimulant drugs in schizophrenia. Psychopharmacology (Berl) 91 (4), 415–33. [DOI] [PubMed] [Google Scholar]
- (5).Seeman P (2008) Dopamine D2(High) receptors on intact cells. Synapse 62 (4), 314–8. [DOI] [PubMed] [Google Scholar]
- (6).Seeman P (2010) Dopamine D2 receptors as treatment targets in schizophrenia. Clin Schizophr Relat Psychoses 4 (1), 56–73. [DOI] [PubMed] [Google Scholar]
- (7).Stoof JC, and Kebabian JW (1981) Opposing roles for D-1 and D-2 dopamine receptors in efflux of cyclic AMP from rat neostriatum. Nature 294 (5839), 366–8. [DOI] [PubMed] [Google Scholar]
- (8).Strange PG (1993) New insights into dopamine receptors in the central nervous system. Neurochem. Int 22 (3), 223–36. [DOI] [PubMed] [Google Scholar]
- (9).Jackson DM, and Westlind-Danielsson A (1994) Dopamine receptors: molecular biology, biochemistry and behavioural aspects. Pharmacol. Ther 64 (2), 291–370. [DOI] [PubMed] [Google Scholar]
- (10).Sibley DR, De Lean A, and Creese I (1982) Anterior pituitary dopamine receptors. Demonstration of interconvertible high and low affinity states of the D-2 dopamine receptor. J. Biol. Chem 257 (11), 6351–61. [PubMed] [Google Scholar]
- (11).Chio CL, Lajiness ME, and Huff RM (1994) Activation of heterologously expressed D3 dopamine receptors: comparison with D2 dopamine receptors. Mol. Pharmacol 45 (1), 51–60. [PubMed] [Google Scholar]
- (12).Seeman P, Weinshenker D, Quirion R, Srivastava LK, Bhardwaj SK, Grandy DK, Premont RT, Sotnikova TD, Boksa P, El-Ghundi M, et al. (2005) Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proc. Natl. Acad. Sci. U. S. A 102 (9), 3513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Usiello A, Baik JH, Rouge-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, and Borrelli E (2000) Distinct functions of the two isoforms of dopamine D2 receptors. Nature 408 (6809), 199–203. [DOI] [PubMed] [Google Scholar]
- (14).Gao YG, Baldessarini RJ, Kula NS, and Neumeyer JL (1990) Synthesis and dopamine receptor affinities of enantiomers of 2-substituted apomorphines and their N-n-propyl analogues. J. Med. Chem 33 (6), 1800–5. [DOI] [PubMed] [Google Scholar]
- (15).Neumeyer JL, Gao YG, Kula NS, and Baldessarini RJ (1990) Synthesis and dopamine receptor affinity of (R)-(−)-2-fluoro-N-n-propylnorapomorphine: a highly potent and selective dopamine D2 agonist. J. Med. Chem 33 (12), 3122–4. [DOI] [PubMed] [Google Scholar]
- (16).Baldessarini RJ, Kula NS, Gao Y, Campbell A, and Neumeyer JL (1991) R(−)2-fluoro-N-n-propylnorapomorphine: a very potent and D2-selective dopamine agonist. Neuropharmacology 30 (1), 97–9. [DOI] [PubMed] [Google Scholar]
- (17).Skinbjerg M, Namkung Y, Halldin C, Innis RB, and Sibley DR (2009) Pharmacological characterization of 2-methoxy-N-propylnorapomorphine’s interactions with D2 and D3 dopamine receptors. Synapse 63 (6), 462–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Finnema SJ, Seneca N, Farde L, Shchukin E, Sovago J, Gulyas B, Wikstrom HV, Innis RB, Neumeyer JL, and Halldin C (2005) A preliminary PET evaluation of the new dopamine D2 receptor agonist [11C]MNPA in cynomolgus monkey. Nucl. Med. Biol 32 (4), 353–60. [DOI] [PubMed] [Google Scholar]
- (19).Seneca N, Zoghbi SS, Skinbjerg M, Liow JS, Hong J, Sibley DR, Pike VW, Halldin C, and Innis RB (2008) Occupancy of dopamine D2/3 receptors in rat brain by endogenous dopamine measured with the agonist positron emission tomography radioligand [11C]MNPA. Synapse 62 (10), 756–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Hwang DR, Narendran R, Huang Y, Slifstein M, Talbot PS, Sudo Y, Van Berckel BN, Kegeles LS, Martinez D, and Laruelle M (2004) Quantitative analysis of (−)-N-(11)C-propyl-norapomorphine in vivo binding in nonhuman primates. J. Nucl. Med 45 (2), 338–46. [PubMed] [Google Scholar]
- (21).Willeit M, Ginovart N, Kapur S, Houle S, Hussey D, Seeman P, and Wilson AA (2006) High-affinity states of human brain dopamine D2/3 receptors imaged by the agonist [11C]-(+)-PHNO. Biol. Psychiatry 59 (5), 389–94. [DOI] [PubMed] [Google Scholar]
- (22).Ginovart N, Galineau L, Willeit M, Mizrahi R, Bloomfield PM, Seeman P, Houle S, Kapur S, and Wilson AA (2006) Binding characteristics and sensitivity to endogenous dopamine of [11C]-(+)-PHNO, a new agonist radiotracer for imaging the high-affinity state of D2 receptors in vivo using positron emission tomography. J. Neurochem 97 (4), 1089–103. [DOI] [PubMed] [Google Scholar]
- (23).Sromek AW, Si YG, Zhang T, George SR, Seeman P, and Neumeyer JL (2011) Synthesis and Biological Evaluation of N-Fluoroalkyl and 2-Fluoroalkoxy Substituted Aporphines: Potential PET Ligands for Dopamine D(2) Receptors. ACS Med. Chem. Lett 2 (3), 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Sromek AW, Zhang S, Akurathi V, Packard AB, Li W, Alagille D, Morley TJ, Baldwin R, Tamagnan G, and Neumeyer JL (2014) Convenient synthesis of 18F-radiolabeled R-(−)-N-n-propyl-2-(3-fluoropropanoxy-11-hydroxynoraporphine. J. Labelled Compd. Radiopharm 57 (14), 725–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Subburaju S, Sromek AW, Seeman P, and Neumeyer JL (2018) New Dopamine D2 Receptor Agonist, [(3)H]MCL-536, for Detecting Dopamine D2high Receptors in Vivo. ACS Chem. Neurosci 9 (6), 1283–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Finnema SJ, Bang-Andersen B, Wikstrom HV, and Halldin C (2010) Current state of agonist radioligands for imaging of brain dopamine D2/D3 receptors in vivo with positron emission tomography. Curr. Top. Med. Chem 10 (15), 1477–98. [DOI] [PubMed] [Google Scholar]
- (27).Vasdev N, Seeman P, Garcia A, Stableford WT, Nobrega JN, Houle S, and Wilson AA (2007) Syntheses and in vitro evaluation of fluorinated naphthoxazines as dopamine D2/D3 receptor agonists: radiosynthesis, ex vivo biodistribution and auto-radiography of [(18)F]F-PHNO. Nucl. Med. Biol 34 (2), 195–203. [DOI] [PubMed] [Google Scholar]
- (28).Zijlstra S, Visser GM, Korf J, and Vaalburg W (1993) Synthesis and in vivo distribution in the rat of several fluorine-18 labeled N-fluoroalkylaporphines. Appl. Radiat. Isot 44 (4), 651–8. [DOI] [PubMed] [Google Scholar]
- (29).Bouthenet ML, Martres MP, Sales N, and Schwartz JC (1987) A detailed mapping of dopamine D-2 receptors in rat central nervous system by autoradiography with [125I]iodosulpride. Neuroscience 20 (1), 117–55. [DOI] [PubMed] [Google Scholar]
- (30).Fields JZ, Reisine TD, and Yamamura HI (1977) Biochemical demonstration of dopaminergic receptors in rat and human brain using [3H]spiroperidol. Brain Res. 136 (3), 578–84. [DOI] [PubMed] [Google Scholar]
- (31).Baudry M, Martres MP, and Schwartz JC (1979) 3H-Domperidone: a selective ligand for dopamine receptors. Naunyn-Schmiedeberg's Arch. Pharmacol 308 (3), 231–7. [DOI] [PubMed] [Google Scholar]
- (32).Kohler C, Hall H, Ogren SO, and Gawell L (1985) Specific in vitro and in vivo binding of 3H-raclopride. A potent substituted benzamide drug with high affinity for dopamine D-2 receptors in the rat brain. Biochem. Pharmacol 34 (13), 2251–9. [DOI] [PubMed] [Google Scholar]
- (33).Niznik HB, Grigoriadis DE, Pri-Bar I, Buchman O, and Seeman P (1985) Dopamine D2 receptors selectively labeled by a benzamide neuroleptic: [3H]-YM-09151–2. Naunyn-Schmiedebergs Arch. Pharmacol 329 (4), 333–43. [DOI] [PubMed] [Google Scholar]
- (34).Ross SB (1995) Heterogenous binding of 3H-remoxipride to membranes of rat liver and brain. Pharmacol. Toxicol 76 (1), 29–35. [DOI] [PubMed] [Google Scholar]
- (35).Seeman P, Chau-Wong M, Tedesco J, and Wong K (1976) Dopamine receptors in human and calf brains, using [3H]-apomorphine and an antipsychotic drug. Proc. Natl. Acad. Sci. U. S. A 73 (12), 4354–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Neumeyer JL, Reischig D, Arana GW, Campbell A, Baldessarini RJ, Kula NS, and Watling KJ (1983) Aporphines. 48. Enantioselectivity of (R)-(−)- and (S)-(+)-N-n-propylnorapo-morphine on dopamine receptors. J. Med. Chem 26 (4), 516–21. [DOI] [PubMed] [Google Scholar]
- (37).Levant B, Grigoriadis DE, and DeSouza EB (1992) Characterization of [3H]quinpirole binding to D2-like dopamine receptors in rat brain. J. Pharmacol Exp Ther 262 (3), 929–35. [PubMed] [Google Scholar]
- (38).Sromek AW, Provencher BA, Russell S, Chartoff E, Knapp BI, Bidlack JM, and Neumeyer JL (2014) Preliminary pharmacological evaluation of enantiomeric morphinans. ACS Chem. Neurosci 5 (2), 93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Richfield EK, Young AB, and Penney JB (1986) Properties of D2 dopamine receptor autoradiography: high percentage of high-affinity agonist sites and increased nucleotide sensitivity in tissue sections. Brain Res. 383 (1–2), 121–8. [DOI] [PubMed] [Google Scholar]
- (40).Wreggett KA, and Seeman P (1984) Agonist high- and low-affinity states of the D2-dopamine receptor in calf brain. Partial conversion by guanine nucleotide. Mol. Pharmacol 25 (1), 10–7. [PubMed] [Google Scholar]
- (41).De Lean A, Kilpatrick BF, and Caron MG (1982) Dopamine receptor of the porcine anterior pituitary gland. Evidence for two affinity states discriminated by both agonists and antagonists. Mol. Pharmacol 22 (2), 290–7. [PubMed] [Google Scholar]
- (42).Grigoriadis D, and Seeman P (1986) [3H]-domperidone labels only a single population of receptors which convert from high to low affinity for dopamine in rat brain. Naunyn-Schmiedeberg's Arch. Pharmacol 332 (1), 21–5. [DOI] [PubMed] [Google Scholar]
- (43).Cumming P, Wong DF, Gillings N, Hilton J, Scheffel U, and Gjedde A (2002) Specific binding of [(11)C]raclopride and N-[(3)H]propyl-norapomorphine to dopamine receptors in living mouse striatum: occupancy by endogenous dopamine and guanosine triphosphate-free G protein. J. Cereb. Blood Flow Metab 22 (5), 596–604. [DOI] [PubMed] [Google Scholar]
- (44).Narendran R, Hwang DR, Slifstein M, Hwang Y, Huang Y, Ekelund J, Guillin O, Scher E, Martinez D, and Laruelle M (2005) Measurement of the proportion of D2 receptors configured in state of high affinity for agonists in vivo: a positron emission tomography study using [11C]N-propyl-norapomorphine and [11C]-raclopride in baboons. J. Pharmacol. Exp. Ther 315 (1), 80–90. [DOI] [PubMed] [Google Scholar]
- (45).Cumming P (2011) Absolute abundances and affinity states of dopamine receptors in mammalian brain: A review. Synapse 65 (9), 892–909. [DOI] [PubMed] [Google Scholar]
- (46).Minuzzi L, and Cumming P (2010) Agonist binding fraction of dopamine D2/3 receptors in rat brain: a quantitative autoradiographic study. Neurochem. Int 56 (6–7), 747–52. [DOI] [PubMed] [Google Scholar]
- (47).Seeman P, Tallerico T, and Ko F (2003) Dopamine displaces [3H]domperidone from high-affinity sites of the dopamine D2 receptor, but not [3H]raclopride or [3H]spiperone in isotonic medium: Implications for human positron emission tomography. Synapse 49 (4), 209–15. [DOI] [PubMed] [Google Scholar]
- (48).Boyson SJ, McGonigle P, and Molinoff PB (1986) Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J. Neurosci 6 (11), 3177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Seeman P (2009) Dopamine D2High receptors measured ex vivo are elevated in amphetamine-sensitized animals. Synapse 63 (3), 186–92. [DOI] [PubMed] [Google Scholar]
- (50).Weidenauer A, Bauer M, Sauerzopf U, Bartova L, Nics L, Pfaff S, Philippe C, Berroteran-Infante N, Pichler V, Meyer BM, et al. (2020) On the relationship of first-episode psychosis to the amphetamine-sensitized state: a dopamine D2/3 receptor agonist radioligand study. Transl. Psychiatry 10 (1), 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Pierre PJ, and Vezina P (1998) D1 dopamine receptor blockade prevents the facilitation of amphetamine self-administration induced by prior exposure to the drug. Psychopharmacology (Berl) 138 (2), 159–66. [DOI] [PubMed] [Google Scholar]