Abstract
Objectives
Antibody response to the first dose of BNT162b2 SARS‐CoV‐2 is greater in COVID‐19‐convalescent than in infection‐naïve individuals. However, there are no data about T‐cell response in individuals with pre‐existing cellular immunity.
Methods
We evaluated T‐cell responses in parallel with SARS‐CoV‐2 antibody level after first dose of BNT162b2 vaccine in 23 infection‐naïve and 27 convalescent healthcare workers (HCWs) previously included in a study about humoral and T‐cell immunity.
Results
Overall, the antibody response was lower in the infection‐naïve group than in convalescent individuals (18 895 vs 662.7 AU mL−1, P < 0.001), and intermediate but significantly lower in convalescent HCWs with previous negative serology (25 174 vs 1793 AU mL−1; P = 0.015). Indeed, anti‐spike IgG titres after the first dose correlated with baseline anti‐nucleocapsid IgG titres (rho = 0.689; P < 0.001). Pre‐existing T‐cell immunity was observed in 78% of convalescent and 65% of the infection‐naïve HCWs. T‐cell response after the first dose of the vaccine was observed in nearly all the cases with pre‐existing T‐cell immunity, reaching 94% in convalescent HCWs and 93% in those with cross‐reactive T cells. It was lower in the infection‐naïve group (50%; P = 0.087) and in convalescent HCWs with negative serology (56%; P = 0.085). Notably, systemic reactogenicity after vaccination was mainly observed in those with pre‐existing T‐cell immunity (P = 0.051).
Conclusion
Here, we report that the first dose of BTN162b2 elicits a similar S‐specific T‐cell response in cases of either past infection or cross‐reactive T cells, but lower in the rest of infection‐naïve individuals and in convalescent HCWs who have lost detectable specific antibodies during follow‐up.
Keywords: antibodies, cellular immunity, COVID‐19, cross‐reactivity, mRNA vaccine, SARS‐CoV‐2
In this study, we found that a single dose of BNT162b2 vaccine generates a strong humoral (100%) and cellular response in convalescent COVID‐19 individuals (94%), and also in infection‐naïve healthcare workers with cross‐reactive immunity because of other coronaviruses (93%). However, this response was not observed in convalescent individuals with previous loss of specific antibodies after the disease, who had a T‐cell response similar to that of other infection‐naïve individuals. Thus, different factors could influence the rate of humoral and cellular responses to the vaccine.
Introduction
Successful robust immune responses have been reported for the BNT162b2 vaccine against the SARS‐CoV‐2 spike (S) protein.1, 2 Thus, the vaccine effectiveness for the prevention of hospitalisation because of COVID‐19 was found to be 87% after the second dose in an early case–control study,3 and 96% in a later comparison of person‐time incidence rates from a national registry.4
Moreover, it has been described a 80–90% of effectiveness for prevention of hospitalisation,5 after a single dose of BNT162b2 vaccine, with an excellent humoral and cellular response in patients with previous infection.6, 7 This fact led to changes in different national vaccine schedules, recommending a single dose of vaccine in those with history of previous COVID‐19.
However, there is a paucity of data about vaccine T‐cell response and the associated factors. Considering the important role of T cells in response to natural SARS‐CoV‐2 infection, a combination of strong humoral and cellular response to vaccination is likely to be the key factor in their clinical success. Specifically, the T‐cell response to the first dose in the presence of cross‐reactive immunity has not been previously evaluated, an important issue since pre‐existing T‐cell response to SARS‐CoV‐2 has been observed in 30–60% of unexposed individuals.8, 9
Therefore, we have investigated the full immunological responses to the first dose of BNT162b2 vaccine, including T‐cell response, according to the history of previous infection, persistence of specific antibodies or pre‐existing cross‐reactive T‐cell immunity.
Results
Fifty HCWs evaluated 3 months before vaccination (median, 103 days; IQR, 90–112) in a cross‐sectional study about humoral and T‐cell response to SARS‐CoV‐210 underwent blood analysis at least 17 days after the first dose of BNT162b2 vaccine. Characteristics of enrolled individuals are shown in Table 1. Briefly, we distinguished between two groups: a group of 27 convalescent HCWs (54%) with clinical and serological evidence of previous SARS‐CoV‐2 infection (18 of them continued with detectable antibodies at inclusion, but 9 who recovered from SARS‐CoV‐2 infection had lost detectable anti‐N‐specific antibodies against SARS‐CoV‐2 at the time of inclusion into the study), and a homogenous group of 23 infection‐naïve HCWs (46%), who had confirmed negative serology at inclusion, and did not refer previous suggestive symptoms (fever, cough, anosmia, ageusia, headache and diarrhoea) or a positive RT‐PCR. Just before vaccination, IgG against protein N continued to be positive in 12 out of 18 (67%) convalescent HCWs, and it remains negative in the 9 convalescent participants with previous negative serology and in the 23 infection‐naïve HCWs. Thus, to highlight, all the included HCWs had 3 consecutive serological determinations for a correct categorisation (diagnosis, inclusion and pre‐vaccination).
Table 1.
Characteristics of the 50 convalescent healthcare workers and differences according to immune status at baseline and after response
| Overall (50) | Convalescent (27) | Infection‐naïve (23) | |
|---|---|---|---|
| Age (years) | 48 (39–59) | 45 (34–59) | 52 (44–60) |
| Sex (female) | 32 (64%) | 17 (63%) | 15 (65%) |
| Body mass index (kg m−2) | 23.3 (21.5–24.7) | 23.4 (21.8–24.6) | 23.1 (21.3–25.3) |
| Comorbidities (n, %) | 15 (30%) | 5 (19%) | 10 (43%) |
| Hypertension | 5 (10%) | 2 (7%) | 3 (13%) |
| Diabetes | 2 (4%) | – | 2 (9%) |
| SARS‐CoV‐2 RT‐PCR positive at diagnosis | 23 (46%) | 23 (85%) | – |
| Positive serology after diagnosis | 27 (54%) | 27 (100%) | |
| Positive serology at inclusiona | 18 (36%) | 18 (67%) | 0 |
| Median value (RU mL−1, IQR) | 6.3 (3–13.1) | 13.3 (7–33) | 4.5 (3–6.2) |
| Positive serology pre‐vaccinea | 12 (24%) | 12 (44%) | 0 |
| Median value (RU mL−1, IQR) | 5.5 (4–11.3) | 10.3 (4.7–30) | 4.3 (3.7–5.8) |
| Time from infection to vaccination (months) | 10.2 (10–10.4) | 10.2 (10–10.4) | – |
| Time from inclusion to vaccination (days) | 103 (90–112) | 104 (92–114) | 99 (83–109) |
| S‐specific serology (IgG) after 1st dose (AU mL−1) | 1569 (489–21468) | 18 895 (1793–40 000) | 663 (318–1335) |
| CD8+ T‐cell response to S (%) | |||
| Pre‐vaccination | 18 (36%) | 13 (48%) | 5 (22%) |
| After 1st dose | 40 (80%) | 22 (81%) | 18 (78%) |
| CD4+ T‐cell response to S (%) | |||
| Pre‐vaccination | 17 (34%) | 10 (37%) | 7 (30%) |
| After 1st dose | 36 (72%) | 21 (78%) | 15 (65%) |
Data are expressed as median and interquartile range (IQR), and percentage.
N‐specific serology.
Following the first dose of the vaccine, humoral response was observed in 100% of participants, but anti‐S titres were 28‐fold higher in the individuals with previous infection than in infection‐naïve individuals (median, 18 895 vs 663 AU mL−1; P < 0.001; Figure 1a). Notably, HCWs with previous infection but negative serology at inclusion developed anti‐S titres post‐vaccination that were intermediate between the infection‐naïve and convalescent groups (1793 AU mL−1; IQR, 387–16 562), but still significantly lower in comparison with convalescent HCWs with persistent antibodies (25 174 vs 1793 AU mL−1; P = 0.015) (Figure 1b). Moreover, among convalescent HCWs, titres of anti‐S correlated with baseline anti‐N IgG titres (rho = 0.689; P < 0.001).
Figure 1.
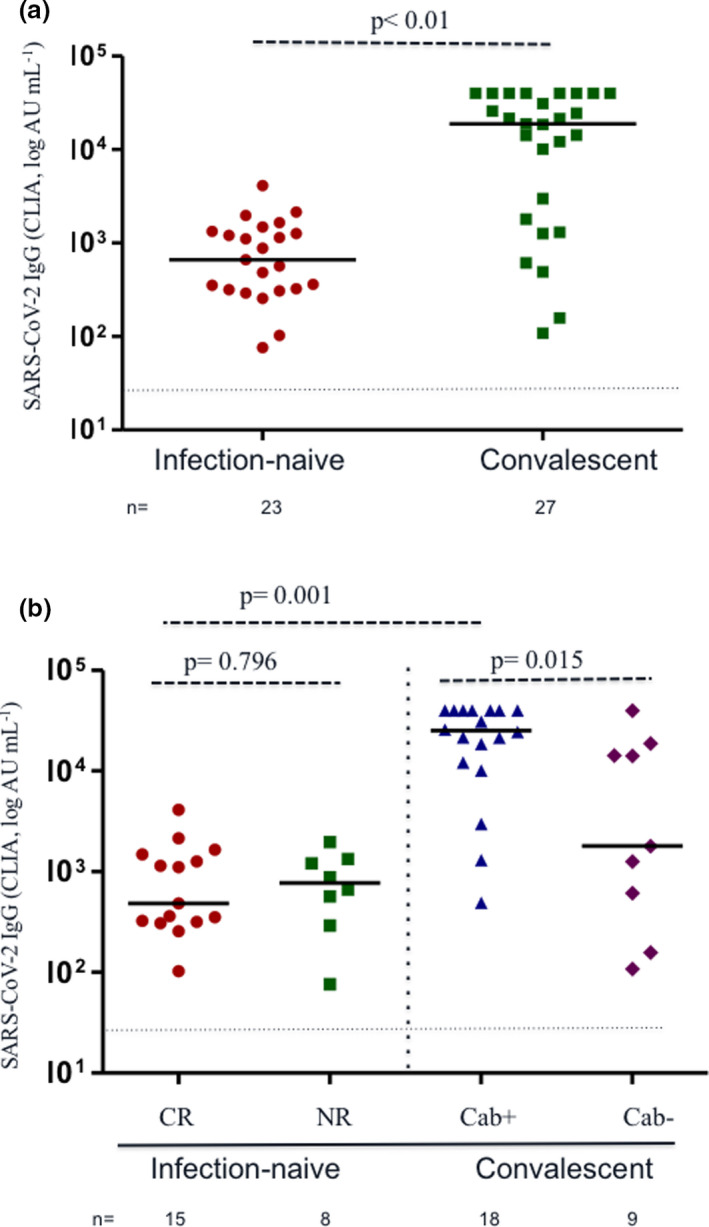
(a) S‐specific antibody responses after the first dose of the BTN162b2 mRNA vaccine against SARS‐CoV‐2. Comparison between infection‐naïve (n = 23, red circles) and convalescent healthcare workers (HCWs) (n = 27, green squares). The latter group included nine individuals without a positive N‐specific serology at inclusion. (b) S‐specific antibody responses after the first dose of the BTN162b2 mRNA vaccine against SARS‐CoV‐2. Comparisons between HCWs with cross‐reactive immunity (CR, red circles), non‐reactive (NR, green squares), convalescent with persistence of antibodies (Cab+, blue triangles) and recovered HCWs with loss of antibodies during follow‐up (Cab−, purple diamonds). Each dot represents an individual after the first dose of vaccine. Lines represent group medians. The dashed line indicates limit of detection (50 AU mL−1). The number of individuals in each group is shown below the graphs.
Pre‐existing CD4+ and CD8+ T‐cell response to peptides of the spike (S), membrane (M) and/or nucleocapsid (N) was found in 78% (21/27) of convalescent HCWs (5/9, 56%, among those without antibodies at inclusion), and in 65% (15/23) of the infection‐naïve participants. Specifically, cellular response to S peptides was observed in 36% (18/50), 22% (5/23) in the infection‐naïve group and 48% (13/27) in the convalescent group. Notably, although the breadth and magnitude of cellular response were slightly higher in the convalescent group (Supplementary figure 1a and b), T‐cell cross‐reactivity to M or N peptides was observed in 52% and in 39% of the infection‐naïve HCWs, respectively. For convalescent HCWs, a significant correlation was found between T‐cell CD8+ against protein S at inclusion and antibody titres before vaccination (rho = 0.435; P = 0.038).
After first dose of vaccination, there was a strong T‐cell response to S peptides in almost all the HCWs included (Figure 2a and b), which correlated with the humoral response in those with past infection, albeit it was not significant (rho = 0.365; P = 0.061). Strikingly, among the infection‐naïve individuals, CD8+ and CD4+ T‐cell responses against S peptides were increased in frequency and magnitude in those having cross‐reactive immunity (CR: from 5/15, 33%, to 14/15, 93%), in comparison with those without pre‐existing T‐cell reactivity (non‐reactive, NR, from none to 4/8, 50%), albeit it was not significant probably because of the small sample size (Figure 2c and d). Additionally, responses to S increased from 48% (13/27) to 81% (22/27; P < 0.01) among convalescent HCWs, a change predominantly observed among individuals with past infections and antibody response at inclusion (17/18, 94%) compared with those with antibody loss (56%, 5/9).
Figure 2.
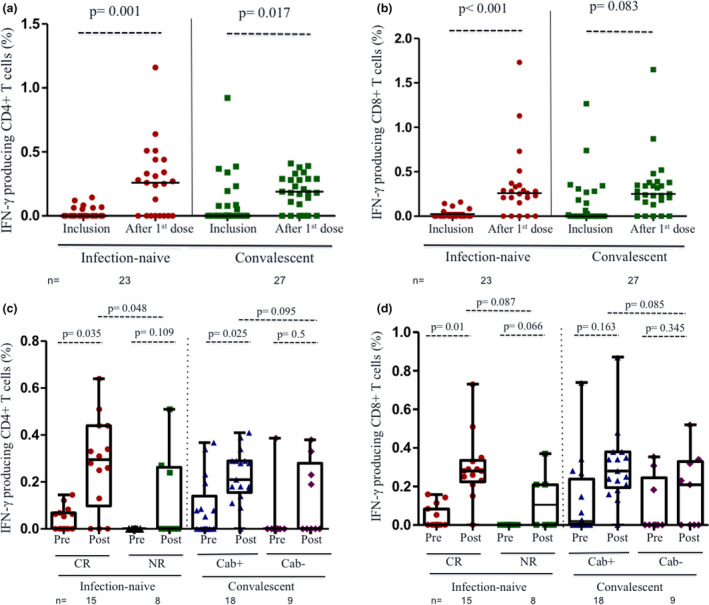
T‐cell response to first dose of BNT162b2 vaccine in infection‐naïve and convalescent healthcare workers (HCWs). T‐cell response was evaluated after stimulation with peptides of protein S. (a) IFN‐γ‐producing CD4+ T cells against S peptides in infection‐naïve (red circles) and convalescent participants (green squares) at inclusion and after vaccination. (b) IFN‐γ‐producing CD8+ T cells against S peptides in the same groups at inclusion and after vaccination. (c) Comparison of the proportion of IFN‐γ‐producing CD4+ T cells against S peptides at inclusion (pre) and after vaccination (post) according to four categories: cross‐reactive immunity (CR, red circles), non‐reactive (NR, green squares), convalescent with persistence of antibodies (Cab+, blue triangles) and recovered HCWs with loss of antibodies at inclusion (Cab−, purple diamonds). (d) Comparison of the proportion of IFN‐γ‐producing CD8+ T cells against S peptides at inclusion (pre) and after vaccination (post) in each of the four groups above categorised. Each dot represents an individual. Lines represent group medians. The number of individuals in each group is shown below the graphs.
Of note, among the 18 participants with pre‐existing T‐cell responses to S (13 convalescent and 5 cross‐reactive), the magnitude of increase was lower in comparison with that observed in those without previous response to S (Figure 3a and b), and even there were an inverse correlation between baseline values and an increase in CD8+ (rho = −0.427; P = 0.002) and CD4+ (rho = −0.381; P = 0.007) T cells.
Figure 3.
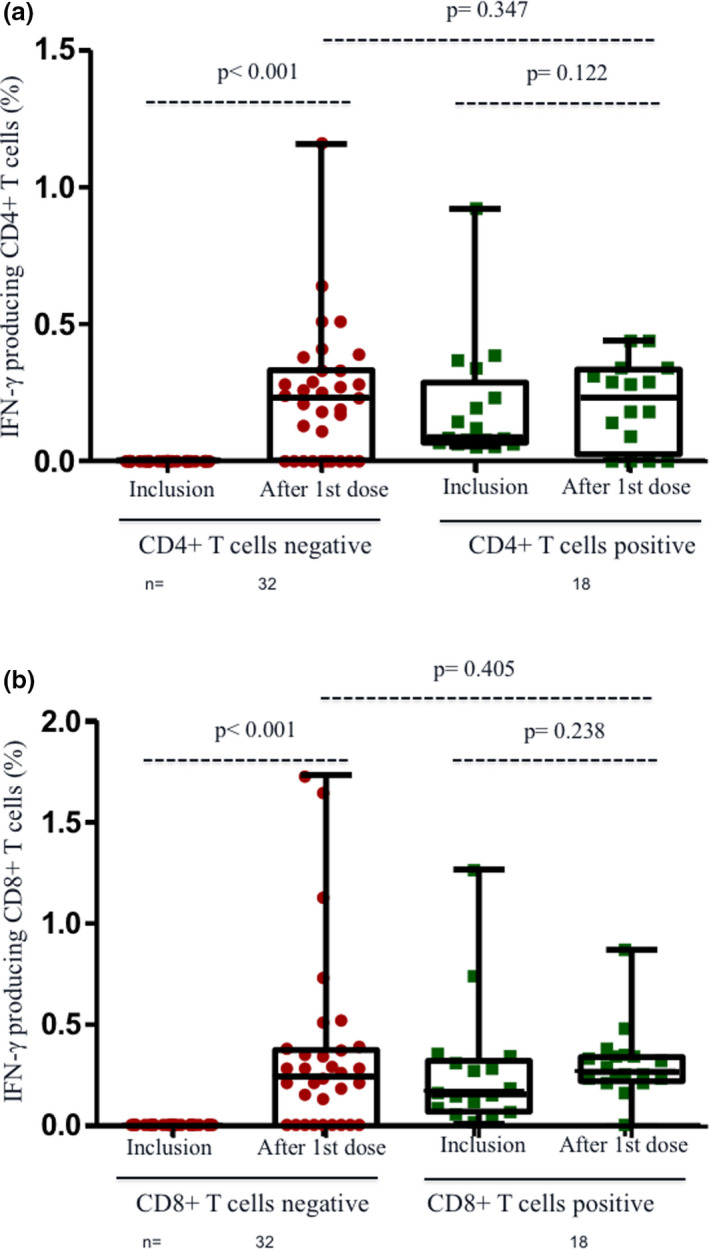
T‐cell response to first dose of BNT162b2 vaccine in infection‐naïve and convalescent healthcare workers (HCWs) according to pre‐existing T‐cell immunity against S peptides. (a) IFN‐γ‐producing CD4+ T cells against S peptides at inclusion and after 1st dose of vaccination according to previous CD4+ reactivity to protein S. (b) IFN‐γ‐producing CD8+ T cells against S peptides at inclusion and after 1st dose of vaccination according to previous CD8+ T‐cell reactivity to protein S. Lines represent group medians. The number of individuals in each group is shown below the graphs.
All the participants were asked about any reaction to the vaccine, divided into local (pain, swelling and erythema at injection site) and systemic effects (fatigue, malaise, headache, insomnia, fever or chills, muscle or joint pain, enlargement of lymph nodes, nausea or rash), and graded in duration and intensity. Any reaction to the vaccine was observed in 33 HCWs (66%), predominantly mild‐to‐moderate pain at the injection site, but 20 HCWs referred systemic events, mainly fatigue, myalgias and headache (Supplementary figure 2), more frequently observed in those with pre‐existing cellular immunity in comparison with non‐reactive, infection‐naïve HCWs (P = 0.051). Indeed, no systemic side effects were observed in this subgroup of participants.
Discussion
For the first time, we showed that infection‐naïve individuals with cross‐reactive T‐cell immunity elicit a lower humoral response, but similar cellular response to that observed in convalescent individuals. This fact has been observed after natural infection in individuals with cross‐reactivity,10 since CD8+ T cells are effector and central memory with functional potential on antigen re‐exposure.11 In addition, we confirmed the robust cellular and humoral response observed in convalescent HCWs,12 and therefore the high level of protection after a single dose of BTN162b2 vaccine in this subgroup of patients.
In previous works, a close correlation between humoral response, measured as anti‐S titres, and neutralising antibodies has been described.13 However, we described a weak correlation between humoral and cellular responses, only observed in those with past infection. Thus, whereas 100% of HCWs had positive serology after vaccination, the rate of T‐cell response ranged from 50% in infection‐naïve HCWs without cross‐reactivity to 94% of response observed in convalescent individuals with previous positive serology.
In line with this, convalescent participants with previous loss of specific antibodies, as determined 3 months before vaccination, generated lower humoral and T‐cell responses in frequency (56%) and magnitude after the first dose of vaccine, in a similar rate of response to infection‐naïve participant. Indeed, a weak cellular response after natural infection has already been described in patients with early loss of antibodies,14 attributed to the lack of an initial adaptive immune response in individuals asymptomatic or with mild disease.15, 16 Thus, infection history, time from infection and presence of antibodies should be cautiously considered when recommending the administration of only a single dose to those with past infection, especially because of the crucial role of cellular immunity for the defences against SARS‐CoV‐2 variants of concern.17
Notably, we observed a slightly lower cellular response to protein S in those already reactive to this protein, as previously described,13, 18 even after influenza vaccine.19 Several possibilities could explain this muted response. The presence of a previous immune response could be high enough to bind and prevent presentation of S epitopes, thereby limiting further stimulation of the immune response.20 Other possibilities include hyperstimulation‐induced T‐cell anergy, expansion/deletion of T‐cell clones and trafficking to mucosal surfaces.20, 21 In any case, the clinical significance of a lower quantitative T‐cell response, if any, has not been established.
To date, real‐life studies observed variable rates of vaccine‐induced reactions mostly after the second dose and in individuals with prior COVID‐19.22 Also, Krammer et al.18 described that seropositive vaccine recipients had a higher rate of systemic events than seronegative individuals, suggesting a correlation between humoral immunity and reactogenicity. We observed a higher incidence of side effects in those HCWs with pre‐existing T‐cell immunity than in non‐reactive HCWs, establishing the role of cellular immunity on reactogenicity to the vaccine antigens and explaining differences in the rate of reactions.
Limitations of our clinical study include the small sample size. Also, time from T‐cell evaluation (inclusion) to vaccination was about 3 months, and we cannot preclude a progressive decrease in T‐cell immunity. Finally, asymptomatic infections and misclassification of cross‐reactivity in the infection‐naïve group were possible but unlikely because of the high sensitivity of the repeated serological test. Indeed, as indirect confirmation of cross‐reactivity, the humoral response was similar in all the infection‐naïve HCWs and lower to that observed in convalescent participants.
In conclusion, our study confirms that convalescent HCWs reach adequate humoral and cellular response after a single dose of a mRNA vaccine and that pre‐existing T‐cell immunity favors cellular response. It also suggests that there are differences in humoral and cellular response according to the presence of cross‐reactivity and to the evolution of antibodies in recovered individuals, and therefore, current recommendations about vaccine schedule should be re‐evaluated. Finally, the duration of antibody and T‐cell responses according to baseline immune status needs further investigation.
Methods
Ethical approval
The study and its amendments were approved by our IRB (EC162/20), and all the participants gave written informed consent.
Patients
Fifty healthcare workers (HCWs) previously included in a cross‐sectional study about humoral and T‐cell response to SARS‐CoV‐2 with a median of 101 days before vaccination (interquartile range, IQR, 90–112).
Serological determinations
At diagnosis, inclusion into the study and previous vaccination, the presence of specific antibodies against protein N was assessed by SARS‐CoV‐2 ELISA [COVID‐19‐SARS‐CoV‐2 IgG ELISA; Demeditec, Germany; positivity threshold 11 relative units (RU) mL−1]. After the first dose of vaccination, participants were tested again for antibodies to the SARS‐CoV‐2 nucleocapsid and, in parallel, to the S domain of the spike protein [SARS‐CoV‐2 IgG II Quant Alinity; Abbott, Maidenhead, UK; positivity threshold 50 arbitrary units (AU) mL−1].
Cellular response
Cellular immune response was assessed at inclusion and post‐vaccination first dose. Briefly, SARS‐CoV‐2‐specific CD4+ and CD8+ T cells were measured using in vitro stimulation with SARS‐CoV‐2 peptide pools of viral proteins encompassing the spike (S), membrane (M) and nucleocapsid (N), followed by quantitation of CD4+ and CD8+ T‐cell‐specific interferon (IFN)‐γ in live cell flow cytometry, using peripheral blood mononuclear cell (PBMC) samples from all subjects. It was considered reactive and therefore defined as the presence of cellular response, if the proportion of positive cells in stimulated wells was at least twofold higher in comparison with the negative control wells (unstimulated).
In detail, EDTA (ethylenediaminetetraacetic acid)–blood samples were collected from all individuals. After centrifugation at 200 g for 10 min, plasma fraction was collected and again centrifuged at 1200 g for 15 min, aliquoted and stored at −80°C. The cellular fraction was diluted with phosphate‐buffered saline (PBS) and subjected to Ficoll density gradient centrifugation at 500 g for 20 min. PBMCs were washed and frozen in foetal bovine serum (FBS) with 8% dimethyl sulphoxide (DMSO, Sigma, USA) in liquid nitrogen.
PBMCs were thawed and plated in 96‐well flat‐bottom plates at 106 cells/well in RPMI 1640 culture medium (Gibco, USA) supplemented with 10% human serum (AB serum, Sigma), 100 IU of penicillin/streptomycin per mL (Gibco, USA) and 2 mm l‐glutamine, and after 24 h, cells were stimulated in five different conditions in the presence of 1 µg mL−1 purified anti‐CD28 antibody (Miltenyi, Germany). Three wells were stimulated with each of the SARS‐CoV‐2 peptide pools S, M and N at a concentration of 1 µg mL−1, the lower concentration with adequate response after testing peptide concentrations of 0.5, 1, 1.5 and 2 µg mL−1 in six samples to find the appropriate concentration (Supplementary figure 3a). Each peptide pool was composed of 15‐mer sequences with 11 amino acid overlap, covering the immunodominant sequence domains of the surface glycoprotein spike (S), the complete sequence of the membrane glycoprotein M and the complete nucleocapsid phosphoprotein N of SARS‐CoV‐2 (PepTivator SARS‐CoV‐2 Prot S, M and N; Miltenyi Biotec, Cologne, Germany). In addition, one well was stimulated with culture medium alone as a negative control (unstimulated), and other well was stimulated adding 1.5 mg SEB (staphylococcal enterotoxin B; Sigma, Germany) as the positive control. An unresponsive sample to SEB would be excluded from the analysis. Stimulated PBMCs were incubated for 2 h before adding brefeldin A (Rapid Cytokine Inspector CD4/CD8 T‐Cell Kit; Miltenyi, Germany) into the medium to stop cytokine release and kept in culture for other 14 h. After stimulation, staining of the cells was carried out with the following fluorochrome‐conjugated antibodies using a Rapid Cytokine Inspector CD4+/CD8+ T‐Cell Kit (Miltenyi, Germany): CD3‐VioBlue, CD4‐APC, CD8‐FITC, CD14‐PerCP, CD20‐PerCP, IFN‐γ‐PE and FcR blocking reagent. To exclude dead cells, viability 405/520 fixable dye staining (Miltenyi, Germany) was added for the last 10 min of incubation. Fixation and permeabilisation were performed according to the manufacturer's protocol. Samples were measured and analysed by flow cytometry on a MACSQuant Analyzer 10 using MACSQuantify software. At least 105 cells were analysed and gated with the following strategy. Single (FSC‐A/FSC‐H dot plot) and live cells were first selected. Cell debris, monocytes and B cells were excluded from the analysis with CD14‐ and CD20‐PerCP antibodies. Then, lymphocytes were selected with a FSC‐A/SSC‐A dot plot, and CD3+ T cells were gated (Supplementary figure 3b). IFN‐γ expression was finally analysed separately for CD4+ and CD8+ T cells, and it was considered significant if there was at least a twofold increase in reactive cells in comparison with unstimulated pools. Data are presented with background subtraction.
Statistical analysis
Comparisons between groups were performed using the two‐tailed statistical tests, chi‐square or Fisher exact tests for categorical variables, and the Mann–Whitney U‐test or one‐way analysis of variance (Kruskal–Wallis test) with Dunn's correction for multiple comparisons, as appropriate. Paired samples were compared using the Wilcoxon signed‐rank test. Correlation analysis was performed using the non‐parametric Spearman test. Statistical significance was defined as two‐sided P‐values < 0.05. Data were analysed using GraphPad Prism (version 8.4.3; GraphPad Software, San Diego, CA, USA).
Conflict of interest
The authors declare no conflict of interest.
Author contributions
Jose L Casado: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Validation; Writing‐original draft. Johannes Haemmerle: Data curation; Formal analysis; Investigation; Methodology; Project administration; Writing‐review & editing. Pilar Vizcarra: Data curation; Formal analysis; Investigation; Methodology; Writing‐review & editing. Mario Rodriguez‐Dominguez: Formal analysis; Investigation; Methodology; Writing‐review & editing. Tamara Velasco: Data curation; Investigation; Methodology; Writing‐review & editing. Hector Velasco: Data curation; Investigation; Methodology; Writing‐review & editing. Elena Centenera: Data curation; Investigation; Methodology; Writing‐review & editing. Beatriz Romero‐Hernandez: Formal analysis; Investigation; Methodology; Writing‐review & editing. Marina Fernandez‐Escribano: Formal analysis; Investigation; Methodology; Writing‐review & editing. Alejandro Vallejo: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Writing‐review & editing.
Supporting information
Acknowledgments
We thank Ana Abad for database management. The study was funded, in part, by a grant from Instituto de Salud Carlos III (ISCIII COV‐20‐01304). This funded source was not involved in the collection, analysis and interpretation of the data. Additionally, this study was supported by departmental discretionary funds available to the first corresponding author.
Contributor Information
Jose L Casado, Email: jose.casado@salud.madrid.org, @JoseLCasa.
Alejandro Vallejo, Email: alejandro.vallejo@salud.madrid.org.
References
- 1.Baden LR, El Sahly HM, Essink Bet al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med 2021; 384: 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack FP, Thomas SJ, Kitchin Net al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med 2020; 383: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dagan N, Barda N, Kepten Eet al. BNT162b2 mRNA Covid‐19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021; 384: 1412–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haas EJ, Angulo FJ, McLaughlin JMet al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS‐CoV‐2 infections and COVID‐19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021; 397: 1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vasileiou E, Simpson CR, Shi Tet al. Interim findings from first‐dose mass COVID‐19 vaccination roll‐out and COVID‐19 hospital admissions in Scotland: a national prospective cohort study. Lancet 2021; 397: 1646–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prendecki M, Clarke C, Brown Jet al. Effect of previous SARS‐CoV‐2 infection on humoral and T‐cell responses to single‐dose BNT162b2 vaccine. Lancet 2021; 397: 1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazzoni A, Di Lauria N, Maggi Let al. First‐dose mRNA vaccination is sufficient to reactivate immunological memory to SARS‐CoV‐2 in subjects who have recovered from COVID‐19. J Clin Invest 2021; 131: e149150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grifoni A, Weiskopf D, Ramirez SIet al. Targets of T cell responses to SARS‐CoV‐2 coronavirus in humans with COVID‐19 disease and unexposed individuals. Cell 2020; 181: 1489–1501 e1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Bert N, Tan AT, Kunasegaran Ket al. SARS‐CoV‐2‐specific T cell immunity in cases of COVID‐19 and SARS, and uninfected controls. Nature 2020; 584: 457–462. [DOI] [PubMed] [Google Scholar]
- 10.Casado JL, Häemmerle J, Vizcarra Pet al. SARS CoV‐2 infections in healthcare workers with a pre‐existing T‐cell response: a prospective cohort study. Clin Microbiol Infect 2021; 27: 916.e1–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng Y, Mentzer AJ, Liu Get al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS‐CoV‐2 in UK convalescent individuals following COVID‐19. Nat Immunol 2020; 21: 1336–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tauzin A, Nayrac M, Benlarbi Met al. A single dose of the SARS‐CoV‐2 vaccine BNT162b2 elicits Fc‐mediated antibody effector functions and T cell responses. Cell Host Microbe 2021; 29: 1137–1150 e1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahin U, Muik A, Derhovanessian Eet al. COVID‐19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020; 586: 594–599. [DOI] [PubMed] [Google Scholar]
- 14.Sekine T, Perez‐Potti A, Rivera‐Ballesteros Oet al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID‐19. Cell 2020; 183: 158–168 e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carsetti R, Zaffina S, Piano Mortari Eet al. Different innate and adaptive immune responses to SARS‐CoV‐2 infection of asymptomatic, mild, and severe cases. Front Immunol 2020; 11: 610300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casado JL, Vizcarra P, Velasco Het al. Progressive and parallel decline of humoral and T‐cell immunity in convalescent healthcare workers with asymptomatic or mild‐to‐moderate severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis 2021; 224: 241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stankov MV, Cossmann A, Bonifacius Aet al. Humoral and cellular immune responses against SARS‐CoV‐2 variants and human coronaviruses after single BNT162b2 vaccination. Clin Infect Dis 2021. 10.1093/cid/ciab555. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krammer F, Srivastava K, Alshammary Het al. Antibody responses in seropositive persons after a single dose of SARS‐CoV‐2 mRNA vaccine. N Engl J Med 2021; 384: 1372–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrati C, Gioia C, Castilletti Cet al. Cellular and humoral immune responses to pandemic influenza vaccine in healthy and in highly active antiretroviral therapy‐treated HIV patients. AIDS Res Hum Retroviruses 2012; 28: 1606–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sauer K, Harris T. An effective COVID‐19 vaccine needs to engage T cells. Front Immunol 2020; 11: 581807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keynan Y, Card CM, Ball BT, Li Y, Plummer FA, Fowke KR. Cellular immune responses to recurring influenza strains have limited boosting ability and limited cross‐reactivity to other strains. Clin Microbiol Infect 2010; 16: 1179–1186. [DOI] [PubMed] [Google Scholar]
- 22.Menni C, Klaser K, May Aet al. Vaccine side‐effects and SARS‐CoV‐2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis 2021; 21: 939–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


