Abstract
Introduction
Hyperbaric oxygen treatment (HBOT) may be complicated by oxygen toxicity seizures, which typically occur with hyperbaric partial pressures of oxygen exceeding 203 kPa (2 atmospheres absolute). All other hyperbaric units in Australia exclusively use a multiplace chamber when treating with United States Navy Treatment Table 6 (USN TT6) due to this perceived risk. The purpose of this study was to determine the safety of a monoplace chamber when treating decompression illness (DCI) with USN TT6.
Methods
A retrospective review of the medical records of all patients treated at Fiona Stanley Hospital Hyperbaric Medicine Unit with USN TT6 between November 2014 and June 2020 was undertaken. These data were combined with previous results from studies performed at our hyperbaric unit at Fremantle Hospital from 1989 to 2014, creating a data set covering a 30-year period.
Results
One thousand treatments with USN TT6 were performed between 1989 and 2020; 331 in a monoplace chamber and 669 in a multiplace chamber. Four seizures occurred: a rate of 0.59% (1/167) in a multiplace chamber; and none in a monoplace chamber, indicating no statistically significant difference between seizures in a monoplace versus multiplace chamber (P = 0.31).
Conclusions
The rate of oxygen toxicity seizures in a monoplace chamber is not significantly higher than for treatment in the multiplace chamber. We conclude that using the monoplace chamber for USN TT6 in selected patients poses an acceptably low seizure risk.
Keywords: Cerebral arterial gas embolism, Decompression illness, Diving medicine, Diving research, Hyperbaric oxygen treatment, Pressure chambers, Recompression
Introduction
Recompression using oxygen is the standard treatment for cases of decompression illness (DCI); a collective term for the dysbaric injuries cerebral arterial gas embolism (CAGE) and decompression sickness (DCS). Initial treatment usually involves recompression with United States Navy Treatment Table 6 (USN TT6).[ 1]
Oxygen toxicity seizures are a rare, but well-recognised and feared complication of hyperbaric oxygen treatment (HBOT), with a reported incidence in the region of 0.06%.[ 2] Evidence suggests that incidence is related to increased inhaled partial pressure of oxygen and duration of treatment.[ 2 - 7]
Hyperoxia creates free radicals that interact with membranes of neurological cells causing lipid peroxidation and alteration of electrical activity.[ 8 , 9] Added to this, increased levels of nitric oxide cause cerebral vasodilatation which counteracts the normal physiological vasoconstriction response to hyperoxia.[ 10]
The rate of seizures in the treatment of DCI is demonstrated to be higher at 0.28% to 1.11% likely reflecting the increased pressure and duration used in the treatment.[ 2 , 11 - 13] However, more seizures have been documented during initial USN TT6 treatments than with follow-up treatments to similar pressures (which are typically of shorter duration at 284 kPa and overall).[ 2] The role of monoplace chambers for the treatment of DCI has recently been reviewed by Clarke, who concluded that "today's monoplace chamber can successfully support a majority of DCI cases, when overseen by a knowledgeable physician".[ 14]
Monoplace chambers have been used to treat stable patients with DCI requiring USN TT6 in Western Australia since their introduction at Fremantle Hospital (FH) in 2001. More serious forms of DCI requiring inside attendant care and management continue to be treated in the multiplace chamber. The Hyperbaric Medicine Unit (HMU) at Fiona Stanley Hospital (FSH) has continued this practice since its transition from FH in November 2014. This is in contrast to all other HMUs in Australia with access to monoplace chambers, who view the risk of seizures during treatment with a USN TT6 as unacceptably high (personal communications, 2020).
The aim of this study was to determine if the treatment of DCI with USN TT6 in a monoplace chamber presents an unacceptable risk of seizures.
Methods
Written approval was obtained for data review and extraction (Governance, Evidence, Knowledge, Outcomes [GEKO] Quality Activity 35028).
A database of all treatments and complications for both units has been maintained since the opening of FH HMU in November 1989. We reviewed the records of all patients treated for DCI with USN TT6 between November 2014 and June 2020 at FSH. This involved review of patients' electronic medical records using the hospital records system Bossnet®, and accessing the HMU database to review the treatment profile and chamber used for each session. We determined the number of patients who received a USN TT6 and whether the treatment took place in a monoplace or multiplace chamber. We reviewed the notes for each session to determine if there were any complications with treatment, primarily central nervous system toxicity (CNS-OT). In addition, the log book containing comprehensive details of all seizure episodes since 1989 was cross referenced.
All patients who underwent recompression for suspected DCI using USN TT6 in a monoplace chamber were included in the study. Monoplace treatments were performed in either a Sechrist 3200 or Sechrist 3600 (Sechrist Industries, Anaheim, CA, USA) chamber.
Previous studies had been conducted at FH HMU covering two separate periods, totalling 25 years in the following periods: November 1989-November 2009;[ 2] and November 2009-November 2014 (previously unpublished audit). The results of these studies at FH were combined with the results from those at FSH to create a data set spanning 30 years. Statistical analysis was performed using a Fisher's exact test; a P-value of < 0.05 was considered to be statistically significant.
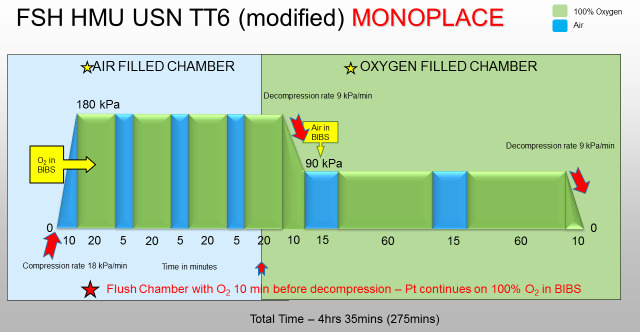
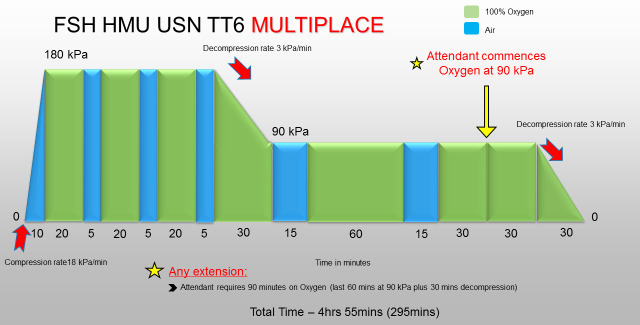
A USN TT6 in our unit consists of the tables shown in Figures 1 and 2.
Figure 1.
USN TT6 modified for use in the monoplace chamber. The pressure shown is gauge pressure. Total length of treatment is 4 h 35 min. BIBS − Built-in breathing system; FSH − Fiona Stanley Hospital; kPa − kilopascals; O2 − oxygen; Pt − patient; min – minutes; USN TT6 − United States Navy Treatment Table 6
Figure 2.
USN TT6 for use in the multiplace chamber. The pressure shown is gauge pressure. Total length of treatment is 4 h 55 min. FSH − Fiona Stanley Hospital; kPa − kilopascals; min – minutes; USN TT6 − United States Navy Treatment Table 6
The monoplace TT6 utilised was developed by Dr Robert Wong, a previous Medical Director of FH HMU, being modified from the multiplace and standard US Navy version by the addition of an extra 20 minute oxygen (O2) breathing period at 284 kPa (2.8 atmospheres absolute [ATA]) and then decompression to 190 kPa (1.9 ATA) over 10 minutes to compensate for the limitation of the Sechrist 3200 chamber which has a slowest decompression rate for this pressure differential of 10 minutes, and to allow the change of the in-chamber atmosphere from air to 100% O2. The advantage of this is that it avoids the use of a mask to breathe O2 for the 2 hours at 190 kPa and the further 30 minutes for decompression to sea level pressure.
When using the monoplace chamber for a USN TT6 treatment the patient initially uses a built-in breathing system (BIBS) oro-nasal mask secured with head straps to breathe 100% O2, with the surrounding chamber environment containing air. The air source is the hospital medical air supply which meets the Australian/New Zealand Standard 2568 for purity and absence of contamination. The exhaled breaths are into the surrounding chamber air, which is continually flushed at approximately 300 L·min-1, preventing O2 and carbon dioxide (CO2) accumulation. Scheduled air breaks are achieved by removal of the mask for 5 minutes. During decompression to 190 kPa the chamber air is replaced by 100% O2 while the patient continues to breathe O2 via the BIBS. At 190 kPa the BIBS is utilised to provide the required air break.
In the event of features of oxygen toxicity developing whilst breathing 100% O2 via the BIBS, the gas supply to the mask can be switched immediately to air. If prodromal features of CNS-OT develop while the chamber is filled with O2, the patient is instructed to breathe air via the BIBS and the chamber can also be purged with air at 400 L·min-1, although this will take many minutes to achieve an air atmosphere.
Results
There were 1,000 treatments with USN TT6 performed between 1989 and 2020, 331 in a monoplace chamber and 669 in a multiplace chamber. There were four recorded oxygen toxicity seizures within this 30-year study period. All seizures occurred in the multiplace chamber; a rate of 0.59% (1/167 treatments), and none occurred in a monoplace chamber. These data are summarised in Table 1. There was no statistically significant difference between seizure rate in a monoplace versus multiplace chamber (P = 0.31).
Table 1. Oxygen toxicity seizure rate during 1,000 USN TT6 (United States Navy Treatment Table 6) treatments. USN TT6 used for decompression illness (DCI) including iatrogenic embolism. FH – Fremantle Hospital; FSH − Fiona Stanley Hospital; Mono − monoplace chamber; Multi − multiplace chamber .
| Time Period | Chamber | USN TT6 numbers | Seizure number | Seizure % | Seizure rate |
| 2014−2020 FSH data | Mono | 90 | 0 | 0 | 0 |
| Multi | 33 | 0 | 0 | 0 | |
| 2009−2014 FH data Unpublished | Mono | 120 | 0 | 0 | 0 |
| Multi | 43 | 0 | 0 | 0 | |
| 1989−2009 FH data Published[2] | Mono | 121 | 0 | 0 | 0 |
| Multi | 593 | 4 | 0.67 | 1/148 | |
| Totals | Mono | 331 | 0 | 0 | 0 |
| Multi | 669 | 4 | 0.59 | 1/167 | |
| Both | 1000 | 4 | 0.40 | 1/250 |
One seizure occurred in the first O2 period, two in the third and two during the 190 kPa (1.9 ATA) phase of TT6. Details of individual cases experiencing seizures are summarised in Table 2.
Table 2. Details of four patients experiencing oxygen toxicity seizures during USN TT6 treatments for decompression sickness (DCS) or cerebral arterial gas embolism (CAGE). All seizures occurred between 1989 and 2009 at Fremantle Hospital. CT − computerised tomography; EEG – electroencephalogram; F − female; M – male .
| Case | Age (y) | Sex | Indication | Risk factor | HBOT sessions | O2 period | Pre-seizure time on O2 (min) | Comments |
| 1 | 31 | M | CAGE | Salt water aspiration | 11 | 5th (at 193 kPa) | 125 | PaCO2 48 mmHg prior |
| 2 | 24 | M | CAGE | Nil | 2 | End of 3rd | 59 | Nil |
| 3 | 36 | F | DCS | Nil | 2 | End of 1st | 16 | CT brain normal |
| EEG epileptiform | ||||||||
| 4 | 36 | F | DCS | Nil | 2 | End of 3rd | 55 | Same patient as 3 |
One seizure that occurred during the study period was in a complex patient with previous subarachnoid haemorrhage (SAH) following basilar artery tip rupture which had been coiled twice. His SAH previously presented with seizures and he was known to have three other aneurysms under surveillance. His history revealed a head strike before diving, and a moment of decreased consciousness in the water followed by a tonic-clonic seizure during descent at 3 metres depth. His brain computerised tomography scan (CT) was difficult to interpret due to previous coiling, but did not reveal any bleeding nor intravascular gas. He was treated for presumed CAGE given a normal brain CT, and suffered a further two-minute seizure at 190 kPa in the final hour of USN TT6 in the multiplace chamber. The subsequent collateral history revealed a likely seizure disorder rather than oxygen toxicity. For this reason the episode was excluded from analysis but is reported elsewhere.[ 15]
Discussion
This study presents a data set spanning 30 years, including 20 years of using monoplace chambers to treat selected patients for DCI with USN TT6. During the period studied there were four seizures presumed secondary to CNS-OT recorded, all of which occurred in the multiplace chamber. This study showed no significant difference between seizure occurrence in a multiplace versus monoplace chamber (P = 0.31), however, it is likely that it was underpowered to demonstrate such a difference.
Although CNS-OT is a rare occurrence, the treatment of DCI presents a higher risk of seizure when compared to treatment of other conditions. The reasons behind this are likely multifactorial relating to the pressure and duration of treatment, inter-individual variability and the physiology behind the injury itself.[ 13]
It is for this reason that all other Australian HMUs with monoplace chamber capability in addition to a multiplace chamber, use a multiplace chamber for USN TT6 treatment, as it allows an inside attendant to be available in the event of a seizure.
PRESSURE
Treatment with USN TT6 involves pressurisation to 284 kPa. Since Donald made the association between increased oxygen pressure and duration of exposure with seizure risk,[ 3] research has been aimed at quantifying this relationship. Multiple studies have shown this association, with any treatment above 203 kPa showing significantly increased risk.2,4 Although one study reported a threefold increase in seizure incidence with increased chamber pressure to 284 kPa, this did not reach statistical significance.4 Other research, however, demonstrated a significant relationship between increasing pressures above 203 kPa and seizure risk.[ 2]
Early data reported a significant increase in CNS symptoms at 340 kPa when compared to treatment at 284 kPa for the same duration.[ 16] Although this does not delineate seizures from other CNS symptoms, logic would suggest that increasing pressure would increase seizure risk.
DURATION
Whether the duration of hyperbaric oxygen exposure infers an increased seizure risk is contentious. Donald described the association between duration of exposure to hyperbaric oxygen and risk of seizures.[ 3] However, later research found no relationship between length of treatment and seizure occurrence when treating carbon monoxide poisoning at 284 kPa.[ 17] This could be due to the addition of regular air breaks which is postulated to reduce the risk[ 18] although the addition of air breaks was not demonstrated to influence seizure rate in a review of CNS-OT at 243 kPa in Australian HMUs.[ 19] A previous study reported that receiving air breaks was actually a risk factor for having a seizure.[ 4]
As the risk of oxygen toxicity seizure increases with pressure and duration of O2 exposure,[ 2 - 7] it would follow that USN TT6 infers increased risk for CNS toxicity given the initial pressure of 284 kPa and total treatment duration of more than four hours. This being said, a large study showed no significant increased risk of seizure when treating with USN TT6 compared to subsequent treatments with USN TT5, citing the small patient numbers as a possibility for this result.[ 2] These two treatment tables have identical initial treatment profiles for the first two 20-minute oxygen breathing periods at 284 kPa, but the USN TT5 is shorter overall because of one less 20-minute oxygen period at 284 kPa and a reduced treatment time at 190 kPa. The study reported no seizures during 731 USN TT5 vs. four reported cases in 721 USN TT6. Despite the absolute numbers, this did not reach statistical significance.[ 2] We were unable to find any reported cases in the literature of seizure during USN TT5.
PATHOLOGY OF DCI
The higher reported rate of seizures when treating DCI indicates a risk specifically related to the injury process. The 'first treatment effect' was described by Wilkinson after reporting that the incidence of seizures in the first treatment for DCS was higher than subsequent treatments at 1.8%.[ 13] This could be due to the postulated neurological injury and that the first treatment is the longest and most provocative exposure. This has also been reported with HBOT for treatment of carbon monoxide poisoning.[ 2 , 17 , 20] Our study findings were consistent with this result, as all seizures reported were during the first treatment for DCI.
Wilkinson reported an incidence of seizures of 0.5% when treating DCS at 284 kPa compared to medical indications (including carbon monoxide poisoning and treatment of acute infections),[ 13] and although this did not achieve statistical significance, it is a trend reported by others.[ 2 , 12] Interestingly, he reported that the occurrence of seizures when treating diving-related CAGE was not elevated similarly to DCS, but it was for iatrogenic CAGE (2/53). He commented that the small numbers complicate interpretation. This higher rate of oxygen toxicity has also been reported when treating DCI at pressures between 240 kPa and 290 kPa, with an incidence of 0.6% recorded.[ 12] One hypothesis behind this increased risk with DCI is that nitrogen bubbles create a neurological injury which increases the susceptibility to CNS-OT.[ 13]
INDIVIDUAL RESPONSE
As Donald observed, despite knowing possible risk factors, the susceptibility to oxygen toxicity varies between individuals and within the same person on different days.[ 3 , 21] This has been substantiated by reports that some people appear resistant to the side effects of hyperoxia.
The use of monoplace chambers presents an opportunity to treat patients without the need for an inside attendant. This eliminates the risk of DCI to the attendant staff member,[ 21] and reduces costs involved by limiting the number of staff required to be present during treatment. It also allows rapid removal of a patient in the case of an emergency, something that is more difficult to achieve in a multiplace chamber given the potential risk of DCI to the attendant - an outside attendant may have to pressurise to remove the patient and then allow safe decompression of the inside attendant. Another benefit of monoplace chambers is availability. At least in the USA, there are many more monoplace than multiplace chambers. Utilisation of the monoplace chamber for treatment of DCI could avoid potentially long transport times to a suitable multiplace facility.
OTHER RISK FACTORS
Hypercapnia is a recognised risk factor for increased seizure rate, as are numerous medications which are recognised to lower the seizure threshold. One of the patients who seized during a TT6 treatment of CAGE had aspirated and had a mildly elevated PaCO2 of 48 mmHg (normal 35-45) documented in the emergency department prior to commencing HBOT. He was otherwise treated conservatively and did not require intubation.
During treatment in a monoplace chamber, an outside attendant can closely monitor the patient for prodromal symptoms of CNS-OT and the BIBS rapidly switched to deliver air when appropriate, although it has been reported that not all oxygen toxicity seizures have prodromal symptoms, and signs are notoriously "unpredictable" with "large variation".[ 2
The incidence of DCI in hyperbaric attendants ranges from 0 to 37 per 100,000 sessions (0.037%) and, although this presents a small risk, it is a risk that can be reduced by using a monoplace chamber where appropriate.[ 22]
Monoplace chambers have been used safely to treat with USN TT6 in centres without access to a multiplace chamber.11 The use of monoplace chambers in the treatment of DCI was first advocated in 1974.[ 23] However, shorter treatment tables using no air breaks were used (unlike a USN TT6) as was the case in a 2006 report.[ 24] Results from the present study concur with those of these previous studies despite the different treatment tables used.
It should be noted that patients in this unit are screened for appropriateness for USN TT6 treatment in a monoplace chamber. Unstable patients requiring one-to-one nursing care or medical intervention, analgesia, repeated neurological examination or ongoing haemodynamic support would be treated in a multiplace chamber. Information on managing intensive care-level patients in monoplace chambers can be found elsewhere.[ 25] Neither benzodiazepines nor other anticonvulsant medications were administered prophylactically to patients undergoing HBOT.
Conclusion
Treatment of appropriately selected DCI cases using USN TT6 in a monoplace chamber appears to be an acceptable, safe and cost-effective option.
Footnotes
Conflict of interest and funding: nil
Contributor Information
Samantha Bonnington, Department of Hyperbaric Medicine, Fiona Stanley Hospital, Murdoch, Western Australia.
Neil Banham, Department of Hyperbaric Medicine, Fiona Stanley Hospital, Murdoch, Western Australia.
Kevin Foley, Department of Hyperbaric Medicine, Fiona Stanley Hospital, Murdoch, Western Australia.
Ian Gawthrope, Department of Hyperbaric Medicine, Fiona Stanley Hospital, Murdoch, Western Australia.
References
- Vann RD, Butler FK, Mitchell SJ, Moon RE. Decompression illness. Lancet. 2011;377(9760):153–64. doi: 10.1016/S0140-6736(10)61085-9. [DOI] [PubMed] [Google Scholar]
- Banham ND. Oxygen toxicity seizures: 20 years’ experience from a single hyperbaric unit. Diving Hyperb Med. 2011;41:202–10. [PubMed] [Google Scholar]
- Donald KW. Oxygen poisoning in man. Br Med J. 1947;1(4506):667. [PMC free article] [PubMed] [Google Scholar]
- Heyboer M 3rd, Jennings S, Grant WD, Ojevwe C, Byrne J, Wojcik SM. Seizure incidence by treatment pressure in patients undergoing hyperbaric oxygen therapy. Undersea Hyperb Med. 2014;41:379–85. [PubMed] [Google Scholar]
- Smerz R. Incidence of oxygen toxicity during the treatment of dysbarism. Undersea Hyperb Med. 2004;31:199–202. [PubMed] [Google Scholar]
- Hampson N, Atik D. Central nervous system oxygen toxicity during routine hyperbaric oxygen therapy. Undersea Hyperb Med. 2003;30:147–53. [PubMed] [Google Scholar]
- Manning EP. Central nervous system oxygen toxicity and hyperbaric oxygen seizures. Aerosp Med Hum Perform. 2016;87:477–86. doi: 10.3357/AMHP.4463.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman TS, Thom SR. Physiology and medicine of hyperbaric oxygen therapy. Philadelphia: Saunders Elsevier; 2008. p. 529- 30. [Google Scholar]
- Torbati D, Church DF, Keller JM, Pryor WA. Free radical generation in the brain precedes hyperbaric oxygen-induced convulsions. Free Radic Biol Med. 1992;13:101–6. doi: 10.1016/0891-5849(92)90070-w. [DOI] [PubMed] [Google Scholar]
- Chavko M, Auker CR, McCarron RM. Relationship between protein nitration and oxidation and development of hyperoxic seizures. Nitric Oxide. 2003;9:18–23. doi: 10.1016/s1089-8603(03)00045-4. [DOI] [PubMed] [Google Scholar]
- Weaver LK. Monoplace hyperbaric chamber use of US Navy Table 6: A 20-year experience. Undersea Hyperb Med. 2006;33:85–8. [PubMed] [Google Scholar]
- Smerz RW. Incidence of oxygen toxicity during the treatment of dysbarism. Undersea Hyperb Med. 2004;31:199–202. [PubMed] [Google Scholar]
- Wilkinson D, Wright S, Goble S. The clinical incidence of central nervous system toxicity at 284 kPa (2.8 ATA) SPUMS Journal. 2005;35:120–4. [Google Scholar]
- Clarke R. Monoplace chamber treatment of decompression illness: Review and commentary. Diving Hyperb Med. 2020;50:264–72. doi: 10.28920/dhm50.3.264-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley K, Banham N, Bonnington S, Gawthrope I. Oxygen toxicity seizure mimics. Diving Hyperb Med. 2021;51:161–4. doi: 10.28920/dhm51.2.161-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarborough OD Symptoms of oxygen poisoning and limits of tolerance at rest and at work. US Navy Experimental Diving Unit, Proj. X-337, Sub No. 62, Report No:. 1, 1947.
- Hampson NB, Simonson SG, Kramer CC, Piantadosi CA. Central nervous system oxygen toxicity during hyperbaric treatment of patients with carbon monoxide poisoning. Undersea Hyperb Med. 1996;23:215–9. [PubMed] [Google Scholar]
- Costa DA, Ganilha JS, Barata PC, Guerreiro FG. Seizure frequency in more than 180,000 treatment sessions with hyperbaric oxygen therapy – a single centre 20-year analysis. Diving Hyperb Med. 2019;49:167–74. doi: 10.28920/dhm49.3.167-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherlock S, Way M, Tabah A. Audit of practice in Australasian hyperbaric units on the incidence of central nervous system oxygen toxicity. Diving Hyperb Med. 2018;48:73–8. doi: 10.28920/dhm48.2.73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders RW, Katz KD, Suyama J, Akhtar J, O’Toole KS, Corll D, et al. Seizure during hyperbaric oxygen therapy for carbon monoxide toxicity: A case series and five-year experience. J Emerg Med. 2012;42:e69–72. doi: 10.1016/j.jemermed.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Donald K. Oxygen and the diver. Hayley-Sawn: The SPA Ltd: 1995. [Google Scholar]
- Pougnet R, Pougnet L, Lucas D, Henckes A, Loddé B, Dewitte JD. Health effects of hyperbaric exposure on chamber attendants: a literature review. Int Marit Health. 2018;69:58–62. doi: 10.5603/IMH.2018.0009. [DOI] [PubMed] [Google Scholar]
- Hart GB. Treatment of decompression illness and air embolism with hyperbaric oxygen. Aerosp Med. 1974;45:1190–3. [PubMed] [Google Scholar]
- Cianci P, Slade JB Jr. Delayed treatment of decompression sickness with short, no-air-break tables: Review of 140 cases. Aviat Space Environ Med. 2006;77:1003–8. [PubMed] [Google Scholar]
- Weaver LK. Hyperbaric oxygen in the critically ill. Crit Care Med. 2011;39:1784–91. doi: 10.1097/CCM.0b013e31821858d1. [DOI] [PubMed] [Google Scholar]