Abstract
Many factors have been identified as having the ability to affect the sensitivity of rapid antigen detection (RAD) tests for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This study aimed to identify the impact of sample processing on the sensitivity of the RAD tests. We explored the effect of different inactivation methods, viral transport media (VTM) solutions, and sample preservation on the sensitivity of four RAD kits based on two SARS-CoV-2 strains. Compared with non-inactivation, heat inactivation significantly impacted the sensitivity of most RAD kits; however, β-propiolactone inactivation only had a minor effect. Some of the VTM solutions (VTM2, MANTACC) had a significant influence on the sensitivity of the RAD kits, especially for low viral-loads samples. The detection value of RAD kits was slightly decreased, while most of them were still in the detection range with the extension of preservation time and the increase of freeze–thaw cycles. Our results showed that selecting the appropriate inactivation methods and VTM solutions is necessary during reagent development, performance evaluation, and clinical application.
Keywords: SARS-CoV-2, Rapid antigen detection, Sensitivity, Sample process
1. Introduction
The global epidemic of coronavirus disease 2019 (COVID-19) has presented a major threat to public health worldwide. COVID-19 is an acute respiratory infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. According to the World Health Organization (WHO) report, as of 22 July, 2021, there have been 191,773,590 confirmed cases of COVID-19, including 4,127,963 deaths [2]. Consequently, there is an urgent need for rapid, simple, and accurate tests to diagnose SARS-CoV-2 infection.
At present, real-time reverse transcription-polymerase chain reaction (RT-PCR) assay is the gold standard for COVID-19 diagnosis. However, RT-PCR assay requires special equipment, trained personnel, and logistical planning for sample shipment. In addition, it results in communication, which is not conducive for timely prevention and control of the epidemic [3], [4], [5]. Conversely, rapid antigen detection (RAD) tests are simple and take only a few minutes to acquire results. The most important thing is the RAD tests for SARS-CoV-2 have been shown to have comparable sensitivity and specificity as the RT-PCR assay in high viral loads [6]. Hence, the demand for rapid SARS-CoV-2 antigen detection assay substantially increased. Still, the sensitivity of RAD tests, which is very important for epidemic prevention and control, considerably differs across studies [7], [8], [9], [10], [11]. For example, in a recent survey, the average sensitivity of RAD tests was 56.2% (95% CI: 29.5%–79.8%), with average specificity as 99.5% (95% CI: 98.1%–99.9%) [7]. According to the technical guidance of WHO, the effectiveness of the RAD tests depends on several factors, including the concentration of virus in the specimen, the time from onset of illness, the quality of the samples collected from a person, the way that the specimen is processed, and the precise formulation of the reagents in the test kits. At present, there is no systematic study on the effect of the samples' processing, including sample inactivation, VTM selection, and sample preservation on the RAD detection of SARS-CoV-2.
This study aimed to identify the impact of sample processing on the sensitivity of the RAD tests. A previous study showed that the volume of the used sample and the dilution factor in the assay diluent tube expressed marked differences in sensitivity on RAD tests [12]. Therefore, we wanted to investigate whether other sample processing conditions affect the sensitivity of RAD tests. Furthermore, we reported on the impact of different sample preservation conditions on RAD tests.
2. Materials and methods
2.1. SARS-CoV-2 cell culture
Experiments were performed in Biosafety Level-3 (BSL-3) laboratory using two SARS-CoV-2 strains, abbreviated as strain 1 (IPBCAMS-WH-01/2019 strain, no. EPI_ISL_402123), and strain 2 (IPBCAMS-AB061/2020 strain with D614G mutant site, https://bigd.big.ac.cn, Accession No. GWHAORV01000000). Both SARS-CoV-2 strains were cultured with Vero cells (ATCC, #CCL-81). Low-passage cells were used, and all cells were mycoplasma-free. Vero cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (HyClone, Logan, UT), 100 U/mL penicillin, and 100 U/mL streptomycins at 37 °C in a 5% CO2 humidified atmosphere. Cells were infected with SARS-CoV-2 at a multiplicity of infection (MOI) of 0.5. Unbound virus was washed away after one h, and cells were then cultured with fresh medium supplemented with 2% FBS at 37 °C for 48–72 h. The supernatant was then collected and clarified by spinning at 400 × g for 10 min. One aliquot was used for titration with 50% tissue culture infectivity dose (TCID50), and the other was used for virus inactivation and the RAD detection without freezing and thawing. The infectious titers of two strains used in the study, performed by the Reed and Muench method on Vero cells, were 6.3 × 105 and 3.2 × 105 TCID50/mL, respectively.
2.2. Virus inactivation
Beta-propiolactone (BPL, Shanghai Macklin Biochemical Co., Ltd.) was used at a dilution of 1:4,000, and SARS-CoV-2 stocks were incubated with BPL for 48 h at 4 °C, after which samples were transferred to 37 °C for two hours to hydrolyze all residual BPL.
Microcentrifuge polypropylene tubes (1.5 mL) containing the virus were exposed to direct heat in a heat block at 56 °C for 30 min. After being heated, all samples were left to cool down to room temperature and centrifuged to collect condensation within the tube.
2.3. RAD kits and VTMs
The four included RAD tests were supplied by: Guangzhou Wondfo Biotech Co., Ltd. (Colloidal Gold), ACON Biotech (Hangzhou) Co., Ltd. (Colloidal Gold), Shenzhen Bioeasy Biotechnology Co., Ltd. (Immunofluorescence), and Beijing Kinghawk Pharmaceutical Co., Ltd. (Colloidal Gold). We refer to the different test kits using Kit A∼D hereafter. The VTM solutions were synthesized by Tryptose Phosphate Broth, MANTACC, Ardent BioMed, and YOCON, as represented by VTM 1, VTM2, VTM3, and VTM4, respectively.
2.4. SARS-CoV-2 testing
For investigating the effect of different virus inactivation methods on the sensitivity of RAD kits, we used the 2-fold serial dilutions of two viral stocks and sample matrixes of RAD kits as diluents that included 20 replicates for each dilution and were applied to LoD determination for 4 RAD kits. The LoD was reported as the level of virus that gives a 95% or higher detection rate. During the evaluation of LoD, results were independently scored by two authors in the form of a band on an immunochromatography paper. In case of discrepant assessments, a third person was consulted to reach a final decision (Wondfo, ACON, and Kinghawk).
For investigating the influence of different VTM solutions, viral stocks with high (50 × LoD), medium (10 × LoD), and low viral loads (2.5 × LoD) were treated with four VTM solutions or sample matrixes of RAD kits based on the determined LoD titer of RAD kits. Then the testing was performed using three RAD kits within 30 min.
For investigating the effect of different virus preservation conditions, viral stocks with high (50 × LoD), medium (10 × LoD), and low viral loads (2.5 × LoD) were treated with sample matrixes of RAD kits under different temperatures and times (including room temperature for 1 h, 4 h and 8 h, 2∼8 °C for 1 h, 8 h and 24 h, −20 °C for one day, three days and seven days, −20 °C freezing and thawing once, twice and three times) based on the determined LoD titer of RAD kits, after which the testing was performed using three RAD kits.
2.5. Standard color card of the colloidal gold platform and immunofluorescence platform
The results of colloidal gold RAD test kits were divided into C1-C9 according to the color development intensity from high to low, where B represented negative and C1-C9 represented positively. From C9-C1, the smaller the number, the more antigens bound to the RAD test kits.
The results of immunofluorescence RAD test kits were obtained as the T/C ratio of fluorescence value. We divided them into “<0.039” ”0.039∼0.1” “0.1∼0.5” ”0.5∼1.2” “1.2∼2.0” and ”2.0∼3.0” according to the intensity from low to high, where “<0.039” representing negative and ”0.039∼3.0” representing positive values. With the increase of the ratio, more antigens tend to bind to the RAD test kits.
3. Results
3.1. Influence of virus inactivation on the sensitivity of the RAD kits
To investigate the effect of different virus inactivation methods on the sensitivity of RAD kits, two cultures of SARS-CoV-2 strains with varying dilution ratios were selected and divided into three sample inactivation groups: non-inactivation, β-propiolactone inactivation, and heat inactivation [13]. The effect of different inactivation methods on the limit of detection (LoD) of RAD kits was evaluated. The LoD ratio of other inactivation assays revealed that heat inactivation significantly impacted the sensitivity of most RAD kits compared with non-inactivation (8.0 for Kit A and B, 4.0 for Kit D, 2.0 for Kit C). However, β-propiolactone inactivation had only a minor effect on the two tested RAD kits (Table 1 ).
Table 1.
The LoD value of each RAD test kit.
| Kits | Strains | The limit of detection |
||||
|---|---|---|---|---|---|---|
| Un-inactivated1 | BPL-inactivated1 | LoD ratio2 | Heat-inactivated1 | LoD ratio3 | ||
| Kit A | Strain 1 | 39.4 | 39.4 | 1.0 | 315.0 | 8.0 |
| Strain 2 | 20.0 | 20.0 | 1.0 | 160.0 | 8.0 | |
| Kit B | Strain 1 | 4.9 | 4.9 | 1.0 | 39.4 | 8.0 |
| Strain 2 | 2.5 | 2.5 | 1.0 | 20.0 | 8.0 | |
| Kit C | Strain 1 | 19.7 | 19.7 | 1.0 | 39.4 | 2.0 |
| Strain 2 | 10.0 | 5.0 | 0.5 | 20.0 | 2.0 | |
| Kit D | Strain 1 | 7.0 | 14.0 | 2.0 | 28.0 | 4.0 |
| Strain 2 | 12.7 | 25.4 | 2.0 | 50.9 | 4.0 | |
Notes:1The results are expressed as TCID50/mL; 2the LoD ratio of BPL-inactivated to un-inactivated is calculated; 3the LoD ratio of heat-inactivated to un-inactivated is calculated.
3.2. Influence of VTMs on the sensitivity of the RAD kits
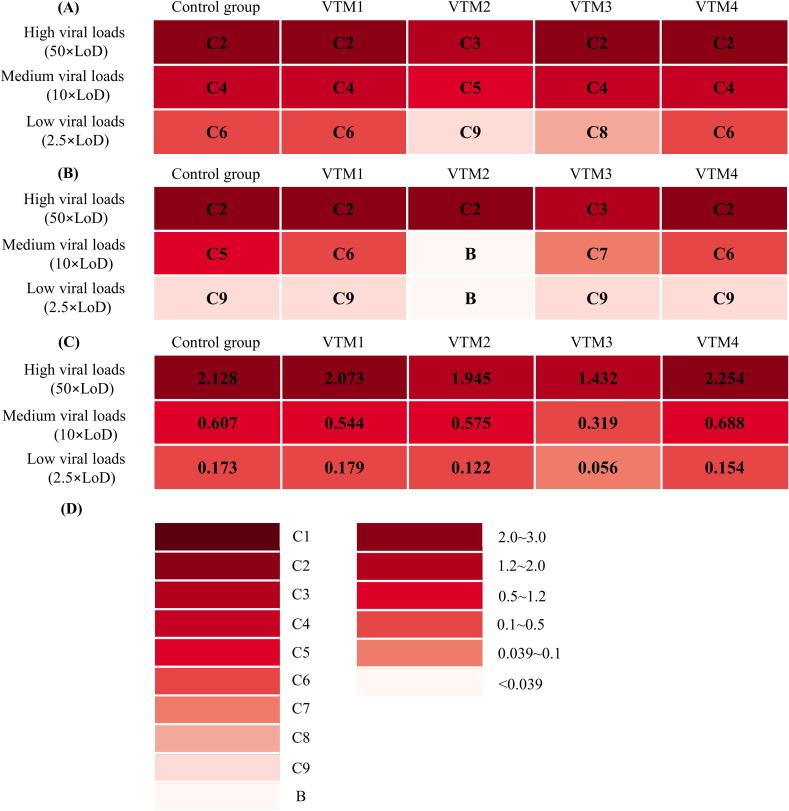
Wondfo and Beijing Kinghawk determined the band color intensity of the virus solutions, and BIOEASY determined the fluorescence values of the virus solutions. According to the assay, VTM 2 and VTM3 significantly influence the sensitivity of all RAD kits, especially for low viral loads (2.5 × LoD) samples, while VTM 1 and VTM 4 had little impact (Fig. 1 ).
Fig. 1.
The effect of different VTM solutions (virus sampling tubes) on RAD kits. (A) Wondfo Biotech, (B) Beijing Kinghawk, (C) BIOEASY, (D) Standard color card of the colloidal gold platform, and immunofluorescence platform.
3.3. Influence of virus preservation on the sensitivity of the RAD kits
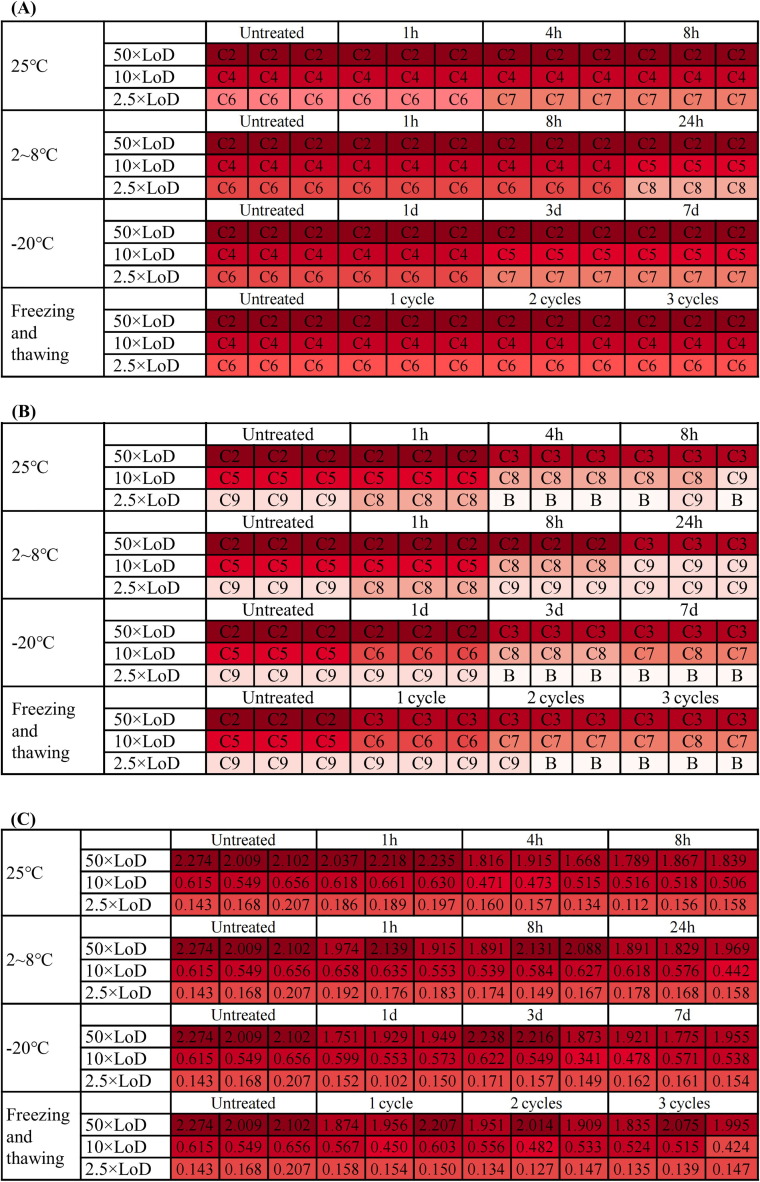
Finally, based on the determined LoD of RAD kits, the influence of different virus preservation temperatures and time on the sensitivity of RAD kits were measured. Three virus solutions with high (50 × LoD), medium (10 × LoD), and low viral loads (2.5 × LoD) were used to detect the sensitivity of RAD kits under different storage conditions. The assay showed that with the extension of preservation time and the increase in freeze–thaw cycles, the detection value of RAD kits slightly decreased. Nevertheless, most of them (such as Wondfo Biotech and BIOEASY) were still in the range of detection even for low viral load (2.5 × LoD) samples (Fig. 2 ).
Fig. 2.
The influence of different virus preservation methods on the LoD of RAD kits. (A) Wondfo Biotech, (B) Beijing Kinghawk, (C) BIOEASY.
4. Discussion
This study explored the effect of different inactivation methods, VTM solutions, and sample preservation on the sensitivity of four RAD kits. According to our results, heat inactivation may not be a suitable inactivation method for SARS-CoV-2 testing, especially for clinical testing and performance evaluation. This finding was consistent with a previous study reporting that heat inactivation harmed the efficiency of RT-PCR assay for SARS-CoV-2. Heat inactivation could be one of the possible factors of false-negative results in the RT-PCR assay of SARS-CoV-2 detection [14], [15]. Heat inactivation destroys the structure of the RNA and protein of SARS-CoV-2. RT-PCR assay can detect the nucleotide of SARS-CoV-2, while the RAD test can detect the antigen of SARS-CoV-2. Thus, heat inactivation could substantially affect the sensitivity of RAD kits.
Chemical inactivators might contribute a lot in protecting the laboratory personnel in charge of detecting SARS-CoV-2, especially nucleic acid detection because chemical inactivation can inactivate clinical samples containing SARS-CoV-2 [16]. Similarly, there might be some chemicals existing in VTMs that can denature and inactivate protein, thus affecting the performance of RAD kits. The VTM solution's impact on the RAD kits might be mainly conferred by the detection antibodies used in the kits, which utilize different epitopes to recognize the antigens in other kits. During sample inactivation, the VTM solution might change the tertiary structure of the antigen in the sample and cause it to degrade. Thus, the recognition between antigen and detection antibodies in the following detection process might greatly vary. Therefore, the reaction of different reagents to the same VTM was different, indicating that choosing the appropriate VTM solutions for RAD testing is necessary.
Our results showed that, along with the extension of preservation time and the increase of freeze–thaw cycles, the detection value of RAD kits slightly decreased, but most of them were still in the range of detection. A previous study showed that the copy number of the DNA target decreased after ten freeze–thaw cycles, which affected the performance of the droplet digital PCR [17]. Also, there was no access to HIV viral load testing when the whole blood was stored in EDTA tubes or plasma preparation tubes for more than 6 h at 25 °C [18]. Therefore, reasonable storage temperature and preservation time of samples are essential measures to ensure the accuracy of test results.
Altogether, we identified the effect of different virus inactivation methods, VTM solution, and preservation conditions on the sensitivity of RAD kits. Nevertheless, there are other factors as well. Given that the concentration of virus in the samples, the time from onset of illness, and the quality of the specimen collected from a person can also affect the sensitivity of RAD kits, these should be further considered. Besides, previous studies have shown that SARS-CoV and SARS-CoV-2 all have cross-reaction in all assays [19]. Therefore, the question remains whether SARS-CoV interaction will also occur in the process of RAD tests. Clinical validation and studies are necessary to confirm the observed cross-detection to provide a clinical reference for RAD tests.
Our study revealed that different virus inactivation methods and VTM solutions significantly impact the sensitivity of RAD kits. Therefore, it is necessary to select the appropriate inactivation methods and VTM solutions. Furthermore, as most RAD kits remain stable under different storage conditions, it is not required to consider the samples' preservation conditions. According to our results, during reagent development, performance evaluation, and clinical application, samples should not be treated by heat inactivation or unqualified VTM solutions before evaluating the performance of clinical samples, such as the lowest detection limitation. Moreover, samples should not be treated by heat inactivation or unqualified VTM solutions in clinical trials. In addition, attention should also be given to the warnings of the approved reagents, such as sample collection and preservation. To sum up, selecting VTM solutions might be more convenient, as they can effectively crack and inactivate viruses without affecting antigen testing.
Acknowledgements
This work was supported by China's National Science and Technology Major Project (2018ZX10102001) and the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2019PT310029 2020PT310004).
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Author contributions
Haiwei Zhou: Conceptualization, Data Curation, Formal Analysis, Writing – Original Draft. Conghui Wang: Data Curation, Formal Analysis, Resources, Supervision. Jian Rao: Data Curation, Formal Analysis. Lan Chen: Data Curation, Formal Analysis. Tingting Ma: Formal Analysis. Donglai Liu: Formal Analysis. Lili Ren: Conceptualization, Project Administration, Writing - Review & Editing. Sihong Xu: Conceptualization, Project Administration, Writing - Review & Editing.
References
- 1.Shi Y., Wang G., Cai X.P., Deng J.W., Zheng L., Zhu H.H., Zheng M., Yang B., Chen Z. An overview of COVID-19. J. Zhejiang Univ. Sci. B. 2020;21:343–360. doi: 10.1631/jzus.B2000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization, WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/, 2021 (accessed 22 July 2021).
- 3.Sethuraman N., Jeremiah S.S., Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020;323:2249–2251. doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- 4.Pillonel T., Scherz V., Jaton K., Greub G., Bertelli C. Letter to the editor: SARS-CoV-2 detection by real-time RT-PCR. Euro Surveill. 2020;25:2000880. doi: 10.2807/1560-7917.ES.2020.25.21.2000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaimayo C., Kaewnaphan B., Tanlieng N., Athipanyasilp N., Sirijatuphat R., Chayakulkeeree M., Angkasekwinai N., Sutthent R., Puangpunngam N., Tharmviboonsri T., et al. Rapid SARS-CoV-2 antigen detection assay in comparison with real-time RT-PCR assay for laboratory diagnosis of COVID-19 in Thailand. Virol. J. 2020;17:1–7. doi: 10.1186/s12985-020-01452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liotti F.M., Menchinelli G., Lalle E., Palucci I., Marchetti S., Colavita F., Sorda M.L., Sberna G., Bordi L., Sanguinetti M., et al. Performance of a novel diagnostic assay for rapid SARS-CoV-2 antigen detection in nasopharynx samples. Clin. Microbiol. Infect. 2021;27:487–488. doi: 10.1016/j.cmi.2020.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinnes J., Deeks J.J., Berhane S., Taylor M., Adriano A., Davenport C., Dittrich S., Emperador D., Takwoingi Y., Cunningham J., et al. Cochrane COVID-19 Diagnostic Test Accuracy Group, Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2021;8:CD013705. doi: 10.1002/14651858.CD013705.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niclot S.L., Cuffel A., Pape S.L., Fellous C.V., Joubert L.M., Afonso A.M.R., Goff J.L., Delaugerre C. Evaluation of a rapid diagnostic assay for detection of SARS CoV-2 antigen in nasopharyngeal swab. J. Clin. Microbiol. 2020;58:e00977-20. doi: 10.1128/JCM.00977-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krüttgen A., Cornelissen C.G., Dreher M., Hornef M.W., Imöhl M., Kleines M. Comparison of the SARS-CoV-2 rapid antigen test to the real star Sars-CoV-2 RT PCR kit. J. Virol. Methods. 2021;288:114024. doi: 10.1016/j.jviromet.2020.114024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porte L., Legarraga P., Vollrath V., Aguilera X., Munita J.M., Araos R., Pizarro G., Vial P., Iruretagoyena M., Dittrich S., Weitzel T. Evaluation of novel antigen-based rapid detection test for the diagnosis of SARS-CoV-2 in respiratory samples. Int. J. Infect. Dis. 2020;99:328–333. doi: 10.1016/j.ijid.2020.05.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cerutti F., Burdino E., Milia M.G., Allice T., Gregori G., Bruzzone B., Ghisetti V. Urgent need of rapid tests for SARS CoV-2 antigen detection: evaluation of the SD-Biosensor antigen test for SARS-CoV-2. J. Clin. Virol. 2020;132:104654. doi: 10.1016/j.jcv.2020.104654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mak G.C., Cheng P.K., Lau S.S., Wong K.K., Lau C.S., Lam E.T., Chan R.C., Tsang D.N. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J. Clin. Virol. 2020;129:104500. doi: 10.1016/j.jcv.2020.104500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren L.L., Wang Y.M., Wu Z.Q., Xiang Z.C., Guo L., Xu T., Jiang Y.Z., Xiong Y., Li Y.J., Li X.W., et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin. Med. J. (Engl). 2020;133:1015–1024. doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen P.S., He Y.T., Huang Y.L., Chen L.L., Huang H., Yu X.G., He X.H., Ou Y.J., Huang B., Liu M. Inactivation of new coronary virus before quantitative real-time PCR testing. Chin. J. Lab. Med. 2019;43(2020):364–367. doi: 10.3760/cma.j.cn114452-20200202-00046. [DOI] [Google Scholar]
- 15.Pan Y., Long L.Y., Zhang D.T., Yuan T.T., Cui S.J., Yang P., Wang Q.Y., Ren S.M. Potential false-negative nucleic acid testing results for Severe Acute Respiratory Syndrome Coronavirus 2 from thermal inactivation of samples with low viral loads. Clin. Chem. 2020;66:794–801. doi: 10.1093/clinchem/hvaa091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pastorino B., Touret F., Gilles M., Luciani L., Lamballerie X.D., Charrel R.N. Evaluation of chemical protocols for inactivating SARS-CoV-2 infectious samples. Viruses. 2020;12:624. doi: 10.3390/v12060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue P.P., Zhang J.Y. Effects of storage time and freeze-thawing on the stability of bacterial genomic DNA evaluated by droplet digital PCR. Lett. Biotechnol. 2020;31:571–575. doi: 10.3969/j.issn.1009-0002.2020.05.012. [DOI] [Google Scholar]
- 18.Bonner K., Siemieniuk R.A., Boozary A., Roberts T., Fajardo E., Cohn J. Expanding access to HIV viral load testing: a systematic review of RNA stability in EDTA tubes and PPT beyond current time and temperature thresholds. Plos One. 2014;9:e113813. doi: 10.1371/journal.pone.0113813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corman V.M., Haage V.C., Bleicker T., Schmidt M.L., Mühlemann B., Zuchowski M., Jo W.K., Tscheak P., Buchner E.M., Müller M.A., Krumbholz A., Drexler J.F., Drosten C. Comparison of seven commercial SARS-CoV-2 rapid point-of-care antigen tests: a single-centre laboratory evaluation study. Lancet Microbe. 2021;2:e311–e319. doi: 10.1016/S2666-5247(21)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]