Summary
Background
Post-mastectomy pain syndrome (PMPS) is a known debilitating surgical complication. While research on prevention, risk factors, and treatments have been conducted, there remains no cohesive treatment paradigm. The aim of our study is to synthesize the existing evidence on PMPS treatment, which may facilitate the implementation of standardized, effective management strategies.
Methods
Using Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines, a comprehensive search was developed and translated for MEDLINE, Cochrane Library, EMBASE, CINAHL, PsycINFO, Web of Science, and ClinicalTrials.gov. The databases were searched using a combination of free terms, phrase searching, and database-specific controlled vocabulary related to PMPS. All unique records were by two independent reviewers. Publications on chronic (>3 months duration) pain after breast cancer-related surgery were included. Limited case series, case reports, and editorials were not included.
Results
A total of 3402 articles from the years 1946–2019 resulted from the literature search after deduplication. Twenty-seven articles met final inclusion criteria for analysis, which revealed 10 major treatment modalities: fat grafting, neuroma surgery, lymphedema surgery, nerve blocks and neurolysis, laser, antidepressants, neuromodulators, physical therapy, mindfulness-based cognitive therapy, and capsaicin.
Conclusions
In this review, we present a comprehensive assessment of the treatments available for PMPS that may help guide breast surgeons and reconstructive surgeons to employ the most effective treatment strategies for these patients. This review supports the importance of multimodal, multidisciplinary care in improving the management of PMPS.
Key Words: Post-mastectomy pain syndrome, chronic pain after breast cancer surgery, neuropathic pain, treatment of PMPS, breast surgery pain management strategies, systematic review
Background
Over 1.7 million new cases of breast cancer are diagnosed every year in women worldwide.1 In the United States alone, an estimated 276,480 new cases of invasive breast cancer will be diagnosed in women in 2020.2 Approximately, 25–60% of these women will suffer from chronic pain after breast cancer-related surgery.3,4 Chronic pain after breast cancer surgery was originally reported by Wood in the 1978 paper “Intercostobrachial nerve entrapment syndrome.”5 Now, “Post-mastectomy pain syndrome (PMPS),” has become the main term to represent chronic pain persisting for at least 3 months after breast cancer-related surgery.6, 7, 8
Management of PMPS is often ineffective, and no gold standard treatment guideline exists. Interventions to treat neuropathic pain range from medical, surgical, to alternative medicine modalities. Identifying the most effective available treatments will guide pain management for breast cancer patients as well as reveal potential areas warranting further research and development. In 2017, a systematic review on treatment modalities for PMPS found 5 treatment modalities in the literature.9 However, the recent advances in peripheral neuroma surgery, nerve blocks, and alternative modalities to treat chronic neuropathic pain warrant further investigation of their reported roles in post breast cancer surgery-related pain syndromes. The primary aims of this review are to identify the reported treatment modalities for chronic pain after breast cancer-related surgery and to assess the outcomes of these available treatments. An updated comprehensive review of the available treatments for chronic pain after breast cancer procedures will help guide optimal care for these patients.
Materials and methods
This systematic review was designed using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.10 The protocol for the systematic review is registered with the National Institutes of Health Research database PROSPERO (ID=CRD42020153425).
Literature search
The review team worked with a research librarian (ABW) to design and conduct a formal systematic review of seven bibliographic databases to capture all pertinent articles specific to treatments for chronic pain after breast cancer-related surgery. The search was conducted using a combination of free terms and database-specific controlled vocabulary in MEDLINE (PubMed), the Cochrane Library databases (Wiley), EMBASE (Elsevier), CINAHL Plus with Full Text (Ebsco), PsycINFO (Ebsco), Web of Science (Clarivate), and ClinicalTrials.gov. The results were exported to EndNote and underwent a multi-pass deduplication process. All unique records were then uploaded into Rayyan for screening by the review authors. Publication sources include online bibliographic databases, clinical trials, key journals, and references of eligible studies and review articles were reviewed to include studies not previously captured by the original search terms. An example of the search strategy for PubMed is provided in Appendix A.
Selection criteria
Included in the data analysis were full text, original articles reporting outcomes for specific treatment modalities for chronic pain after breast cancer-related surgery. Definitions of the terms “chronic pain” and “breast cancer-related procedures” were critical in creating the selection criteria (Appendix B). Randomized controlled trials (RCTs), case–control, and case series studies with greater than 5 patients were included. Human studies and English language publications were included. There was no time period restriction.
Excluded from the data analysis were abstracts without full-text publication, book chapters, books, theses, unpublished/incomplete clinical trials, and non-scientific articles. Scientific articles with low quality of evidence, such as case reports, expert opinions, and small case series (<5) were excluded. Follow-up periods less than 1 month were excluded. Non-English language articles without available English translation were excluded. Animal studies were excluded.
Study Appraisal and Data Extraction
Articles captured by the search strategy were uploaded by the research librarian (ABW) to the Rayyan free online database.11 Two independent blinded reviewers screened the articles found in the literature search. Any conflicts were discussed between the reviewers, and when could not be resolved between the two reviewers, were settled by the senior author (MFE). After the initial screen was finished, the same two independent blinded viewers performed a screen of the full texts (included from screen 1) using EndNote.12 The articles that met inclusion criteria from the second screen were included in the final analysis.
Data extraction included treatment modality type, study design, total patients, follow-up time, outcome metrics used, and major outcome conclusions from the studies. Primary outcomes were limited to patient-reported pain characteristics, both quantitative and qualitative, shoulder range of motion (when indicated), and quality of life (QOL) scores. Treatment-specific details, such as technique and protocols, were also reported.
Data Analysis
Due to heterogeneity of studies included, a thematic analysis of each treatment modality was conducted. Data points from the articles were organized on Microsoft Excel spreadsheets.13
Results
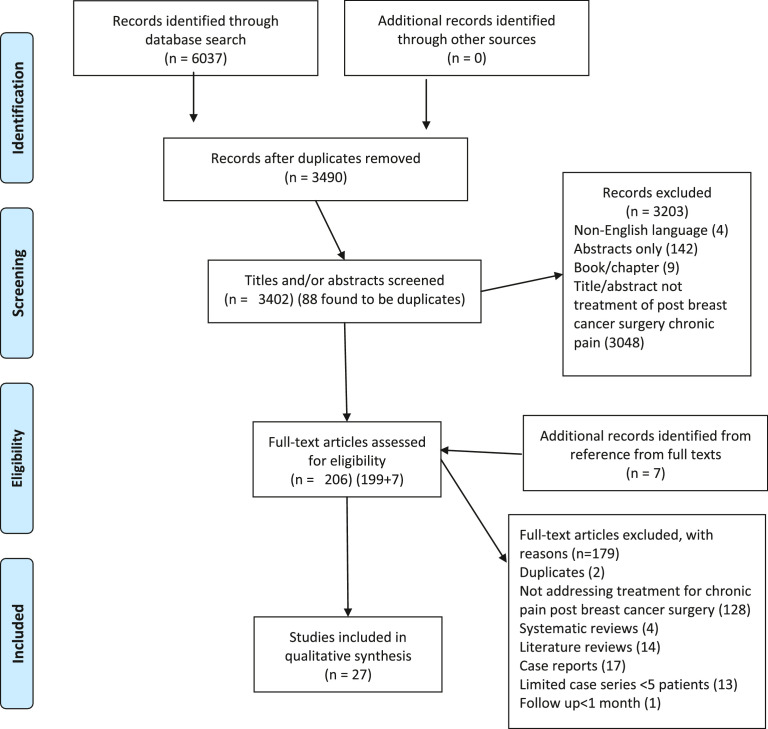
A total of 3490 articles from the years 1946–2019 resulted from the literature search after deduplication. After two rounds of screening, 27 articles were included and were categorized by treatment modality (Figure 1). Ten unique treatment modality groups were identified with a range of reported outcomes (Table 1, list of included articles).
Fig. 1.
PRISMA-P flow diagram demonstrating the method of identifying articles for the systematic review
Table 1.
Included articles identified from our search strategy with main data points extracted for the systematic review
| Treatment Modality | Reference | Level of Evidence, Design | N (# patients) | Follow-up (mo.), Avg. (Range) | Findings |
|---|---|---|---|---|---|
| Fat Grafting | Caviggioli et al., 2011 14 | II, nonblinded RCT | 98 | 13 (12–15) | Fat graft significantly reduced VAS score compared with control (p = 0.0005). 28/34 treated patients stopped analgesic therapy. |
| Maione et al., 2014 15 | II, nonblinded RCT | 92 | 10 (9–12) | Fat graft significantly reduced VAS score compared with control (p < 0.005). | |
| Juhl et al., 2016 16 | II, nonblinded RCT | 15 | 6 | Fat graft significantly reduced VAS score (p < 0.001), improved QOL, improved quality of scar, and reduced NPSI score compared with control. | |
| Peripheral Nerve Surgery | Broyles et al., 2016 17 | IV, retrospective case series | 7 | 16.5 (1–48) | 4 patients—“excellent,” 1 patient—good,” 2 patients—“poor” self-reported pain reduction |
| Ducic et al., 2006 19 | IV, retrospective case series | 5 | 8.7 (2–20) | 3 patients—total resolution of pain, 1 patient—50% resolution, 1 patient—no relief | |
| Wong, 2001 18 | IV, retrospective case series | 5 | 48 | 4 patients—total resolution of pain, 1 patient—continued pain in distribution of intercostobrachial nerve | |
| Lymphedema Surgery | Becker et al., 2008 20 | IV, case series | 6 | 21 (13–38) | Neuropathic pain disappeared immediately after surgery. Upper limb lymphedema resolved in five patients. All patients stopped pain medication. |
| Nerve Blocks and Radiofrequency Neurolysis | Hoseinzade et al., 2008 *21 | II, prospective randomized comparative study | 60 | 3 | Gabapentin group had significantly lower pain scores (p < 0.001), but lower QOL scores compared with SGB + bupivacaine group. |
| Abbas et al., 2011 22 | II, prospective randomized comparative study | 50 | 3 | Anterior paratracheal fluoroscopic SGB group and oblique fluoroscopic SGB group both had significantly decreased VAS, daily morphine consumption (p < 0.05), and areas of allodynia. Oblique group had fewer side effects. | |
| Abbas et al., 2018 25 | II, prospective randomized controlled trial | 80 | 6 | TRF significantly reduced VAS score (p < 0.001), improved functionality, and decreased rescue analgesia use compared with PRF. | |
| Fam et al., 2018 24 | IV, prospective case series | 100 | 6 | PRF + steroid injection significantly decreased VAS scores (p = 0.0057) and improved QOL. | |
| Kirvela et al., 1992 23 | IV, retrospective case series | 10 | 5 | Pain relief after 88% of PVBs lasted <1 month, pain relief after 6% of PVBs lasted >5 months | |
| Cutaneous Laser Therapy | Ebid et al., 2015 26 | I, double-blinded RCT | 61 | 3 | Laser therapy significantly decreased VAS scores (p = 0.0021), increased shoulder ROM, and improved QOL compared with placebo. |
| SNRIs and TCAs | Kalso et al., 1996 27 | I, double-blinded randomized cross-over study | 15 | 4 | Amitriptyline significantly reduced pain in arm (p < 0.01) and around breast scar (p < 0.05) compared with placebo. Patients experienced significant adverse effects. |
| Tasmuth et al., 2002 28 | I, double-blinded randomized cross-over study | 13 | 10 | Venlafaxine significantly reduced average pain (p < 0.05) and maximum pain intensity compared with placebo. | |
| Neuromodulators | Vilholm et al., 2008 29 | I, double-blinded randomized cross-over study | 25 | 4 | Levetiracetam had no effect on pain. |
| Patarica-Huber et al., 2011 31 | II, prospective randomized comparative study | 75 | 1.5 | Multimodal therapy group (gabapentin + NSAID + morphine) had better initial pain control than gabapentin or gabapentin + NSAID groups (p = 0.000). No intergroup difference in pain control at 6 weeks. | |
| Hoseinzade et al., 2008*21 | II, prospective randomized comparative study | 60 | 3 | Gabapentin group had significantly lower pain scores (p < 0.001), but lower QOL scores compared with SGB + bupivacaine group. | |
| Kaur et al., 2019 30 | IV, prospective open-label single-arm study | 35 | 7.6 | Pregabalin significantly reduced VAS score (p = 0.001) and improved QOL at end of 2 months; 83% of patients had pain relief. | |
| Topical Medication Therapy | Watson et al., 1992 72 | II, RCT | 23 | (10–36) | Topical capsaicin significantly reduced VAS score for jabbing pain and overall pain relief scales compared with placebo (p < 0.05). No difference in VAS for steady pain; 62% of treated group had 50% or better improvement in pain. |
| Fallon et al,, 2015 73 | IV, prospective case series (uncontrolled proof-of-concept open-label trial) | 12 | 1.5 | 1% menthol cream improved total pain scores in 82% of patients (p < 0.001) and reduced pain scores by 30% in 50% of patients. | |
| Watson et al., 1989 74 | IV, prospective case series | 14 | 6 | 86% of patients had reduced pain 1 mo. after topical capsaicin treatment; 57% of patients had good or excellent response; 50% of patients had good pain relief at 6 mo. | |
| Dini et al., 1993 75 | IV, uncontrolled open-label trial | 19 | 3 | 85% of patients had good pain relief 3 months after topical capsaicin treatment. | |
| Physical Therapy | De Groef et al., 2018 32 | I, double-blinded RCT | 50 | 12 | Myofascial techniques significantly reduced VAS scores in arm at 3 months compared with control group (p = 0.046). |
| Cantarero-Villaneuva et al., 2012 34 | I, double-blinded RCT | 65 | 2 | Water physical therapy group had significantly reduced shoulder/axillary pain (p = 0.046) and active trigger points compared with control group. No changes in widespread pressure pain hyperalgesia found. | |
| Ammitzboll et al., 2019 33 | I, double-blinded RCT | 158 | 12 | Progressive resistance training did not reduce pain. | |
| Rangon et al., 2018 35 | I, double-blinded RCT | 20 | 2 | Ischemic compression + kinesiotherapy group had increased pressure pain threshold over myofascial trigger points compared with kinesiotherapy group (p < 0.05). | |
| Cognitive Therapy | Johannsen et al., 2016 49 | II, RCT with waitlist control | 129 | 6 | Cognitive therapy group had significantly reduced pain intensity (p = 0.002), improved QOL, and reduced nonprescription pain medication use compared with control group. |
RCT = Randomized controlled trial
VAS = Visual Analog Scale
QOL = Quality of life
NPSI = Neuropathic pain symptom inventory
SGB = Stellate ganglion block
TRF = Thermal radiofrequency
PRF = Pulsed radiofrequency
PVB = Paravertebral block
ROM = Range of motion
Included in two categories
Fat Grafting
Three articles evaluating the effectiveness of autologous fat grafting in PMPS patients met the inclusion criteria, with a combined total of 205 patients and a mean follow-up time of 10 months across the three studies.14, 15, 16 One study was an RCT, while the remaining two studies were non-randomized trials. Among the treatment modalities reviewed, autologous fat grafting had among the highest level evidence supporting its effectiveness in treating PMPS.
Peripheral Nerve Surgery
Somatic pain from entrapment or irritation of intercostal nerves is a common cause of chronic pain in post-mastectomy patients. Three separate, case series devoted to surgical treatment met the inclusion criteria.17, 18, 19 A total of 14 patients were followed for an average of 24 months after intervention. In all the articles, the intercostal nerve(s) causing pain was noted and confirmed using a diagnostic nerve block. Neuroma excision was performed with(out) muscle implantation. In total, more than 80% of patients treated described partial or complete relief of their pain.
Lymphedema Surgery
One article met inclusion criteria that discussed the outcomes of microsurgical lymph node transplantation on chronic pain after breast cancer-related surgery.20 The study presented is a case series of 6 women with average follow-up of 21 months. The surgical technique involved scar release, followed by identification of the posterior circumflex lymphatics. Lymph nodes were harvested in the inguinal region and transplanted in the axillary region. They found that neuropathic pain resolved acutely after surgery and pain resolution persisted at long-term follow-up. Lymphedema symptoms also improved, specifically breast edema resolved in 2/3 patients and upper limb lymphedema resolved in 5/6 patients.
Nerve Blocks and Radiofrequency Neurolysis
Five studies on nerve blocks and neurolysis techniques met inclusion criteria.21, 22, 23, 24, 25 Areas of intervention focused on the sympathetic nerves (stellate ganglia) and dorsal spinal nerves.21, 22, 23 Three studies investigated blocks; the remaining two studies investigated radiofrequency ablation.24,25 These studies were grouped under one category as they involve perineural treatment techniques, as opposed to direct surgical management.
Combining the studies yielded a total of 300 post-mastectomy patients with an average follow-up of 4.6 months after the study intervention. Three studies were prospective randomized comparative, and two were case series with 10 and 100 patients. Thoracic paravertebral blocks showed immediate but short-term (<1 month) pain relief in 99% of patients.23 The case series of 100 patients who underwent pulsed radiofrequency (PRF) and steroid injection (targeting T2 and T3 dorsal root ganglia) for intercostal brachial neuralgia post-mastectomy demonstrated that 6-month mean visual analog scale (VAS) for pain significantly decreased and QOL increased significantly (p < .001).24
Cutaneous Laser Therapy
A single article on the long-term effect of pulsed high-intensity laser therapy (HILT) in the treatment of PMPS met inclusion criteria.26 This study is a double-blind RCT of 61 subjects, 30 treated with HILT and a routine physical therapy (PT) program, and 31 treated with placebo HILT and a routine PT program. HILT consisted of 15-minute treatments with an Nd:YAG laser three times per week for 4 weeks. The areas scanned with the laser were in the affected breast, axilla, and upper arm. After 3 months, the treatment group had significantly (p < 0.05) increased shoulder range of motion, decreased VAS scores, and improved QOL scores.
SSNRIs and TCAs
Two studies evaluating the efficacy of antidepressants met the inclusion criteria, with a total of 28 patients and mean follow-up time of 10 weeks.27,28 Both studies were randomized double-blind cross-over studies with a 2-week washout period between placebo and treatment. In the first, Kalso et al. evaluated the effectiveness of amitriptyline, a tricyclic antidepressant (TCA) in 15 patients.27 Patients were evaluated at 2 and 4 weeks from the beginning of treatment with either drug. Amitriptyline was shown to significantly relieve neuropathic pain in the arm and around the breast scar, with 8/15 patients showing more than a 50% decrease in pain intensity.
Tasmuth et al. investigated the efficacy of venlafaxine, a selective serotonin norepinephrine reuptake inhibitor (SSNRI) on neuropathic pain following treatment of breast cancer in 13 patients.28 Pain relief was significantly better during venlafaxine treatment (p < 0.05), with 11/15 patients having at least 50% pain relief. Further, maximum pain intensity was significantly lower on the maximum tolerated dose of venlafaxine compared with placebo (p < 0.05).
Neuromodulators
Four articles on the efficacy of neuromodulator medications for chronic post breast cancer surgery pain met inclusion criteria.21,29, 30, 31 The mean follow-up period across all four studies was 3.3 months and a total of 195 subjects were enrolled. One study was a randomized cross-over study, two were prospective comparative studies, and one was a prospective case series with 35 patients.21,29, 30, 31. The two prospective comparative studies compared the effects of gabapentin with other treatments for chronic post-mastectomy pain. In one study, gabapentin in combination with a non-steroidal anti-inflammatory drugs (NSAID) and morphine was shown to have better initial pain control than gabapentin alone or gabapentin + NSAID regimens.31 After 2 weeks of treatment until the 6-week final follow-up, all three drug regimen groups had >50% pain relief (p < 0.0001), and there was no significant intergroup difference (p > 0.05).
The single-arm, open-label prospective study of 35 patients examined the effect of pregabalin in the treatment of chronic post-mastectomy pain.30 The results demonstrated that pregabalin significantly reduced VAS scores compared with baseline at 1 and 2 months of follow-up, and QOL significantly improved.
Topical Medication Therapy
Four studies evaluating the impact of topical agents on PMPS. The combined total number of participants was 74 and the mean follow-up time was 10 months (range <1 month–30 months). Three of these articles focused on topical capsaicin, one of which was an RCT and the remaining two were case series. Watson et al. demonstrated that use of 0.075% capsaicin cream three times daily led to a significant reduction in jabbing/sharp pain sensations (p = 0.001) and overall pain levels (p = 0.03).9
Physical Therapy
Four blinded RCTs testing the benefits of various forms of PT on PMPS met the inclusion criteria.32, 33, 34, 35 Combining the studies yielded a total of 294 patients, with a mean follow-up time of 28 weeks. PT in addition to myofascial therapy, and 8-week water PT, both demonstrated significant improvements in regional pain scores.
Cognitive Therapy
One randomized clinical trial studying the effect of mindfulness-based cognitive therapy (MBCT) on PMPS was included in this analysis.36 The study followed 129 women with PMPS for 6 months. Patients were randomized to either 8 weeks of MBCT or a waitlist-control arm. Patients undergoing therapy showed significant and sustained reductions in pain intensity (p = 0.002), including within the neuropathic pain subscale (p = 0.036).
Discussion
The results of this systematic review of seven major databases for original articles on the treatment of PMPS yielded 27 articles, which presented 10 unique treatment modalities. From this list, we categorized treatment groups based on their pathophysiologic targets. These targets are both centrally and peripherally mediated. Central targets alter the faulty neurotransmission and secondary learned behaviors. Peripheral targets include the distal sensory nerves and their altered course in the axilla and chest wall. These nerves are susceptible to iatrogenic injury and external, scar-mediated compression.
Medical, psychiatric management (gabapentin, pregabalin, amitriptyline, venlafaxine, capsaicin, and CBT)
Peripheral nerve injury during surgery can lead to cortical changes, known as central sensitization, involved in persistent post-surgical neuropathic pain.37 Gabapentin, amitriptyline, and venlafaxine were found to offer pain relief for breast cancer patients with chronic pain, by targeting centralized pain pathways. Gabapentin inhibits neuropathic pain through enhancement of GABA activity and decreasing excitable synaptic transmission targets.38, 39, 40 Key mechanisms of TCAs and SSNRIs in neuropathic pain inhibition are 1) reuptake inhibition of noradrenaline, 2) noradrenaline inhibition of neuropathic pain via α2-adrenergic receptors in the dorsal horn of the spinal cord, and 3) altering noradrenaline levels in the brainstem.41, 42, 43, 44, 45, 46
Of the topical treatments studied, capsaicin appears to be the most promising. Capsaicin's mechanism of action is reported to be through the attenuation of cutaneous hypersensitivity through a process described as “defunctionalization.”47, 48, 49 Adverse side effects from the ointment, such as new burning sensations were commonly reported, which may explain its apparent lack of popularity.
Behavioral cognitive therapy is theorized to target centralized pain centers, as one RCT included found a significant decrease in pain intensity that persisted over time.36 The mechanism of cortical neuromodulation through mindfulness-based therapies has been well studied. Leading explanations for its effect are through reducing expectations of pain and attenuating psychological and neural processes.50,51
Paravertebral blockade, neurolysis
Non-surgical methods to address peripheral nerve pain include blockade or neurolysis of C7–T4 stellate ganglions, or PRF of T2–T3 dorsal root ganglia.22,24,25 Thoracic paravertebral blocks achieve immediate but short-term pain relief (<1 month). PRF with steroid injection showed an initial slower response but longer lasting (6 months) relief. The timing and duration of PRF effects are likely neuromodulatory, due to thermal damage altering synaptic transmission.52, 53, 54 Blocks may be useful in cases where immediate pain relief is needed, whereas PRF may offer less profound, but longer term relief. Neither are shown to be curative.
Painful Neuroma
Peripheral nerve injury can lead to aberrant growth and painful neuroma formation. Nerves at high risk of traction and/or laceration injury at the time of mastectomy are the intercostal brachial cutaneous nerve (ICBN), long thoracic, thoracodorsal, lateral branches of the intercostals, lateral, and medial pectoral nerves. The results from our review indicate that the surgical technique of neuroma excision and implantation can provide long-term relief from chronic neuropathic pain.17, 18, 19
Scar Management (fat transfer, laser, lymphatic surgery)
Abnormal scarring, such as scar contracture and subcutaneous fibrous adhesions, are recognized as adverse outcomes may be treated. This phenomenon can be exacerbated by axillary node dissection and adjuvant radiation. Not only does contracture lead to stiffness and decreased mobility, but also may compress local peripheral nerves. The promising results from Level I/II fat grafting studies support its use for neuropathic pain relief in breast cancer patients at high risk for scar contracture.14, 15, 16 However, it remains uncertain if the mechanism of pain relief from fat grafting is scar release which increases suppleness of tissue and decompresses peripheral nerves, and/or stem cell modification. A recent meta-analysis revealed that breast reconstruction does not increase the incidence of post-mastectomy chronic pain, however, the study did not account for reconstructive type.55 Another study demonstrated that mastectomy alone versus immediate reconstruction (implant or autologous), did not have a difference in chronic pain outcomes.56 High-powered comparative studies on chronic pain outcomes from patients with autologous reconstruction versus no reconstruction and implant-based reconstruction would further elucidate this question.
The successful pain relief outcomes from one laser study can be regarded similarly to the results from fat grafting.26 Treating the skin with a non-ablative laser may release scar contractures through stimulation of the local inflammatory response which increases blood flow, vascular permeability and cell metabolism.57,58 Laser therapy may also directly affect local peripheral nerves through a laser induced neural block that causes change in nociception.59, 60, 61 While the only study included was an RCT, definite conclusions are unable to be reached with one study.
The case series that demonstrated the potential use of lymph node transplantation in treatment of chronic pain and lymphedema after breast cancer surgery also highlights the importance of scar release, nerve decompression, and increased perfusion to the region of pain.20 Nerve decompression in this setting is twofold, from local fluid accumulation and from scar contracture. Node transplantation treats both of these factors, and likely explains the neuropathic pain relief. However, questions remain regarding transplantation in patients with chronic lymphedema and pain.62
Musculoskeletal dysfunction
While PMPS is widely regarded to be a neuropathic disorder, it has been suggested that the musculoskeletal system and myofascial pain may contribute to chronic post-operative pain syndromes.63 Muscle fibrosis and increased motor nerve excitability, secondary to inflammation, can create myofascial trigger points (TrPs).64, 65, 66 Active TrPs have been found in multiple muscles in patients with PMPS, but not in healthy controls.72
PT programs utilizing strengthening exercises and massage are effective for improving shoulder pain by reducing presence of TrPs. The results in our review support the role of PT in treating PMPS patients experiencing neck and shoulder/axillary pain, and highlight the benefits of water exercises that allow patients to perform exercises they would be unable to perform on land.32, 33, 34, 35
Involvement of the neuromuscular junction in chronic myofascial pain is also the basis of studies testing the clinical benefit of botulinum toxin on persistent post-mastectomy pain. Botulinum toxin A inhibits presynaptic release of acetylcholine and substance P, causing functional denervation of the damaged muscle and blocking transmission of pain to the central nervous system (CNS).67,68 However, BTX-A infiltration in the pectoralis major muscle did not show a clinical benefit in the only RCT.69
A limitation of this systematic review is reviewer bias. To minimize this bias, two independent reviewers screened all articles in the method supported by the PRISMA-P systematic review protocol.70 Another limitation is that there are few studies of high-level evidence for each modality, which risks publication bias. This study aimed to present the existing evidence to guide treatment recommendations, but cannot establish guidelines.
Finally, subgroup analysis was not feasible in our study population. Future studies should address treatments tailored to a specific patient profile.
Conclusion
Our comprehensive assessment of the treatments available for chronic pain after breast cancer-related surgery demonstrates diverse modalities that may reliably provide pain relief. No one solution is fail proof or without its side effect profile. The data support the role of progressive, multidisciplinary management for this select patient population. Future studies are warranted to investigate an algorithm-based, multimodal therapy protocol for chronic pain after breast cancer-related surgery.
Acknowledgments
Financial Disclosure Statement
Dr. Ellis, Dr. Chappell, Ms. Yuksel, Mr. Sasson, Ms. Wescott, and Dr. Connor have nothing to disclose.
Funding
No funding was received for this article.
Ethical Approval
N/A
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jpra.2021.07.006.
Appendix. Supplementary materials
References
- 1.The American Institute for Cancer Research . World Cancer Research Fund International; 2020. How diet, nutrition and physical activity affect breast cancer risk.https://www.wcrf.org/dietandcancer/breast-cancer Accessed March 31. [Google Scholar]
- 2.American Cancer Society . American Cancer Society; Atlanta: 2020. Cancer Facts & Figures 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gärtner R, Jensen M-B, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302(18):1985–1992. doi: 10.1001/jama.2009.1568. [DOI] [PubMed] [Google Scholar]
- 4.Elzohry AAM, Abd Elhamed MF, Mahran MH. Post Mastectomy Pain is No Longer Nightmare. J Fam Med. 2018;1(1):1–11. [Google Scholar]
- 5.Wood KM. Intercostobrachial nerve entrapment syndrome. South Med J. 1978;71(6):662–663. doi: 10.1097/00007611-197806000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Waltho D, Rockwell G. Post-breast surgery pain syndrome: establishing a consensus for the definition of post-mastectomy pain syndrome to provide a standardized clinical and research approach - a review of the literature and discussion. Can J Surg. 2016;59(5):342–350. doi: 10.1503/cjs.000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1–226. [PubMed] [Google Scholar]
- 8.Granek I, Ashikari R, Foley K. The post-mastectomy pain syndrome: clinical and anatomical correlates. In: Vol 3. American Society of Clinical Oncology; 1984:122.
- 9.Larsson IM, Ahm Sørensen J, Bille C. The Post-mastectomy Pain Syndrome-A Systematic Review of the Treatment Modalities. Breast J. 2017;23(3):338–343. doi: 10.1111/tbj.12739. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomson Reuters . Clarivate Analytics; 2019. EndNote. [Google Scholar]
- 13.Neyeloff JL, Fuchs SC, Moreira LB. Meta-analyses and Forest plots using a microsoft excel spreadsheet: step-by-step guide focusing on descriptive data analysis. BMC Res Notes. 2012;5:52. doi: 10.1186/1756-0500-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caviggioli F, Maione L, Forcellini D, Klinger F, Klinger M. Autologous fat graft in postmastectomy pain syndrome. Plast Reconstr Surg. 2011;128(2):349–352. doi: 10.1097/PRS.0b013e31821e70e7. [DOI] [PubMed] [Google Scholar]
- 15.Maione L, Vinci V, Caviggioli F. Autologous fat graft in postmastectomy pain syndrome following breast conservative surgery and radiotherapy. Aesthetic Plast Surg. 2014;38(3):528–532. doi: 10.1007/s00266-014-0311-9. [DOI] [PubMed] [Google Scholar]
- 16.Juhl AA, Karlsson P, Damsgaard TE. Fat grafting for alleviating persistent pain after breast cancer treatment: A randomized controlled trial. J Plast Reconstr Aesthet Surg. 2016;69(9):1192–1202. doi: 10.1016/j.bjps.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Broyles JM, Tuffaha SH, Williams EH, Glickman L, George TA. Lee Dellon A. Pain after breast surgery: Etiology, diagnosis, and definitive management. Microsurgery. 2016;36(7):535–538. doi: 10.1002/micr.30055. [DOI] [PubMed] [Google Scholar]
- 18.Wong L. Intercostal neuromas: a treatable cause of postoperative breast surgery pain. Ann Plast Surg. 2001;46(5):481–484. doi: 10.1097/00000637-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Ducic I, Larson EE. Outcomes of surgical treatment for chronic postoperative breast and abdominal pain attributed to the intercostal nerve. J Am Coll Surg. 2006;203(3):304–310. doi: 10.1016/j.jamcollsurg.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Becker C, Pham DNM, Assouad J, Badia A, Foucault C, Riquet M. Postmastectomy neuropathic pain: results of microsurgical lymph nodes transplantation. Breast. 2008;17(5):472–476. doi: 10.1016/j.breast.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Hosseinzadeh H, Mohammad Pour A, Agha Mohammadi D, Sanaei S. Comparing the Effect of Stellate Ganglion Block and Gabapentin on the Post Mastectomy Pain Syndrome. Shiraz E Medical Journal. 2008;9(2):88. -86. [Google Scholar]
- 22.Nabil Abbas D, Abd El Ghafar EM, Ibrahim WA, Omran AF. Fluoroscopic stellate ganglion block for postmastectomy pain: a comparison of the classic anterior approach and the oblique approach. Clin J Pain. 2011;27(3):207–213. doi: 10.1097/AJP.0b013e3181fb1ef1. [DOI] [PubMed] [Google Scholar]
- 23.Kirvelä O, Antila H. Thoracic paravertebral block in chronic postoperative pain. Reg Anesth. 1992;17(6):348–350. [PubMed] [Google Scholar]
- 24.Fam BN, El-Sayed GGE-D, Reyad RM, Mansour I. Efficacy and safety of pulsed radiofrequency and steroid injection for intercostobrachial neuralgia in postmastectomy pain syndrome - A clinical trial. Saudi J Anaesth. 2018;12(2):227–234. doi: 10.4103/sja.SJA_576_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbas DN, Reyad RM. Thermal Versus Super Voltage Pulsed Radiofrequency of Stellate Ganglion in Post-Mastectomy Neuropathic Pain Syndrome: A Prospective Randomized Trial. Pain Physician. 2018;21(4):351–362. [PubMed] [Google Scholar]
- 26.Ebid AA, El-Sodany AM. Long-term effect of pulsed high-intensity laser therapy in the treatment of post-mastectomy pain syndrome: a double blind, placebo-control, randomized study. Lasers Med Sci. 2015;30(6):1747–1755. doi: 10.1007/s10103-015-1780-z. [DOI] [PubMed] [Google Scholar]
- 27.Kalso E, Tasmuth T, Neuvonen PJ. Amitriptyline effectively relieves neuropathic pain following treatment of breast cancer. Pain. 1996;64(2):293–302. doi: 10.1016/0304-3959(95)00138-7. [DOI] [PubMed] [Google Scholar]
- 28.Tasmuth T, Härtel B, Kalso E. Venlafaxine in neuropathic pain following treatment of breast cancer. Eur J Pain. 2002;6(1):17–24. doi: 10.1053/eujp.2001.0266. [DOI] [PubMed] [Google Scholar]
- 29.Vilholm OJ, Cold S, Rasmussen L, Sindrup SH. Effect of levetiracetam on the postmastectomy pain syndrome. Eur J Neurol. 2008;15(8):851–857. doi: 10.1111/j.1468-1331.2008.02206.x. [DOI] [PubMed] [Google Scholar]
- 30.Kaur N, Kumar A, Saxena AK, Grover RK. Pregabalin in the treatment of postmastectomy chronic pain: Results of an open label, single-arm clinical study. Breast J. 2019;25(3):465–468. doi: 10.1111/tbj.13242. [DOI] [PubMed] [Google Scholar]
- 31.Patarica-Huber E, Boskov N, Pjevic M. Multimodal approach to therapy-related neuropathic pain in breast cancer. Journal of BU ON: official journal of the Balkan Union of Oncology. 2011;16(1):40–45. [PubMed] [Google Scholar]
- 32.De Groef A, Van Kampen M, Vervloesem N. Effect of myofascial techniques for treatment of persistent arm pain after breast cancer treatment: randomized controlled trial. Clin Rehabil. 2018;32(4):451–461. doi: 10.1177/0269215517730863. [DOI] [PubMed] [Google Scholar]
- 33.Ammitzbøll G, Andersen KG, Bidstrup PE. Effect of progressive resistance training on persistent pain after axillary dissection in breast cancer: a randomized controlled trial. Breast Cancer Res Treat. 2020;179(1):173–183. doi: 10.1007/s10549-019-05461-z. [DOI] [PubMed] [Google Scholar]
- 34.Cantarero-Villanueva I, Fernández-Lao C, Fernández-de-Las-Peñas C. Effectiveness of water physical therapy on pain, pressure pain sensitivity, and myofascial trigger points in breast cancer survivors: a randomized, controlled clinical trial. Pain Med. 2012;13(11):1509–1519. doi: 10.1111/j.1526-4637.2012.01481.x. [DOI] [PubMed] [Google Scholar]
- 35.Rangon FB, Koga Ferreira VT, Rezende MS, Apolinário A, Ferro AP, Guirro EC de O. Ischemic compression and kinesiotherapy on chronic myofascial pain in breast cancer survivors. J Bodyw Mov Ther. 2018;22(1):69–75. doi: 10.1016/j.jbmt.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Johannsen M, O'Connor M, O'Toole MS, Jensen AB, Højris I, Zachariae R. Efficacy of Mindfulness-Based Cognitive Therapy on Late Post-Treatment Pain in Women Treated for Primary Breast Cancer: A Randomized Controlled Trial. J Clin Oncol. 2016;34(28):3390–3399. doi: 10.1200/JCO.2015.65.0770. [DOI] [PubMed] [Google Scholar]
- 37.Borsook D, Kussman BD, George E, Becerra LR, Burke DW. Surgically induced neuropathic pain: understanding the perioperative process. Ann Surg. 2013;257(3):403–412. doi: 10.1097/SLA.0b013e3182701a7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gottrup H, Juhl G, Kristensen AD. Chronic oral gabapentin reduces elements of central sensitization in human experimental hyperalgesia. Anesthesiology. 2004;101(6):1400–1408. doi: 10.1097/00000542-200412000-00021. [DOI] [PubMed] [Google Scholar]
- 39.Moore RA, Wiffen PJ, Derry S, Toelle T, Rice ASC. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;(4) doi: 10.1002/14651858.CD007938.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kukkar A, Bali A, Singh N, Jaggi AS. Implications and mechanism of action of gabapentin in neuropathic pain. Arch Pharm Res. 2013;36(3):237–251. doi: 10.1007/s12272-013-0057-y. [DOI] [PubMed] [Google Scholar]
- 41.Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150(3):573–581. doi: 10.1016/j.pain.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 42.Pan H-L, Wu Z-Z, Zhou H-Y, Chen S-R, Zhang H-M, Li D-P. Modulation of pain transmission by G-protein-coupled receptors. Pharmacol Ther. 2008;117(1):141–161. doi: 10.1016/j.pharmthera.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimura M, Saito S, Obata H. Dexmedetomidine decreases hyperalgesia in neuropathic pain by increasing acetylcholine in the spinal cord. Neurosci Lett. 2012;529(1):70–74. doi: 10.1016/j.neulet.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 44.Hayashida K, Eisenach JC. Spinal alpha 2-adrenoceptor-mediated analgesia in neuropathic pain reflects brain-derived nerve growth factor and changes in spinal cholinergic neuronal function. Anesthesiology. 2010;113(2):406–412. doi: 10.1097/ALN.0b013e3181de6d2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Obata H. Analgesic mechanisms of antidepressants for neuropathic pain. Int J Mol Sci. 2017;18(11) doi: 10.3390/ijms18112483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brightwell JJ, Taylor BK. Noradrenergic neurons in the locus coeruleus contribute to neuropathic pain. Neuroscience. 2009;160(1):174–185. doi: 10.1016/j.neuroscience.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomtsyan A, Faltynek CR. Wiley; 2010. Vanilloid Receptor TRPV1 in Drug Discovery: Targeting Pain and Other Pathological Disorders. [Google Scholar]
- 48.Holzer P. The pharmacological challenge to tame the transient receptor potential vanilloid-1 (TRPV1) nocisensor. Br J Pharmacol. 2008;155(8):1145–1162. doi: 10.1038/bjp.2008.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anand P, Bley K. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br J Anaesth. 2011;107(4):490–502. doi: 10.1093/bja/aer260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeidan F, Vago DR. Mindfulness meditation-based pain relief: a mechanistic account. Ann N Y Acad Sci. 2016;1373(1):114–127. doi: 10.1111/nyas.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gard T, Hölzel BK, Sack AT. Pain attenuation through mindfulness is associated with decreased cognitive control and increased sensory processing in the brain. Cereb Cortex. 2012;22(11):2692–2702. doi: 10.1093/cercor/bhr352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cahana A. Pulsed radiofrequency: a neurobiologic and clinical reality. Anesthesiology. 2005;103(6):1311. doi: 10.1097/00000542-200512000-00027. author reply 1313-4. [DOI] [PubMed] [Google Scholar]
- 53.Cahana A, Van Zundert J, Macrea L, van Kleef M, Sluijter M. Pulsed radiofrequency: current clinical and biological literature available. Pain Med. 2006;7(5):411–423. doi: 10.1111/j.1526-4637.2006.00148.x. [DOI] [PubMed] [Google Scholar]
- 54.Chua NHL, Vissers KC, Sluijter ME. Pulsed radiofrequency treatment in interventional pain management: mechanisms and potential indications-a review. Acta Neurochir (Wien) 2011;153(4):763–771. doi: 10.1007/s00701-010-0881-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reghunathan M, Rahgozar P, Sbitany H, Srinivasa DR. Breast Reconstruction Does Not Increase the Incidence of Postmastectomy Pain Syndrome: Results of a Meta-Analysis. Ann Plast Surg. November 2019 doi: 10.1097/SAP.0000000000002062. [DOI] [PubMed] [Google Scholar]
- 56.Henderson JR, Tao A, Kirwan CC, Barr L. Immediate breast reconstruction does not increase postmastectomy pain. Ann Surg Oncol. 2014;21(1):113–117. doi: 10.1245/s10434-013-3293-y. [DOI] [PubMed] [Google Scholar]
- 57.Kujawa J, Zavodnik L, Zavodnik I, Buko V, Lapshyna A, Bryszewska M. Effect of low-intensity (3.75-25 J/cm2) near-infrared (810 nm) laser radiation on red blood cell ATPase activities and membrane structure. J Clin Laser Med Surg. 2004;22(2):111–117. doi: 10.1089/104454704774076163. [DOI] [PubMed] [Google Scholar]
- 58.Monici M, Cialdai F, Fusi F, Romano G, Pratesi R. Effects of pulsed Nd: YAG laser at molecular and cellular level. A study on the basis of Hilterapia. Energy Health. 2008;3:27–33. [Google Scholar]
- 59.Gigo-Benato D, Geuna S, Rochkind S. Phototherapy for enhancing peripheral nerve repair: a review of the literature. Muscle Nerve. 2005;31(6):694–701. doi: 10.1002/mus.20305. [DOI] [PubMed] [Google Scholar]
- 60.Rochkind S, Drory V, Alon M, Nissan M, Ouaknine GE. Laser phototherapy (780 nm), a new modality in treatment of long-term incomplete peripheral nerve injury: a randomized double-blind placebo-controlled study. Photomed Laser Surg. 2007;25(5):436–442. doi: 10.1089/pho.2007.2093. [DOI] [PubMed] [Google Scholar]
- 61.Chow R, Armati P, Laakso E-L, Bjordal JM, Baxter GD. Inhibitory effects of laser irradiation on peripheral mammalian nerves and relevance to analgesic effects: a systematic review. Photomed Laser Surg. 2011;29(6):365–381. doi: 10.1089/pho.2010.2928. [DOI] [PubMed] [Google Scholar]
- 62.Schaverien MV, Coroneos CJ. Surgical treatment of lymphedema. Plast Reconstr Surg. 2019;144(3):738–758. doi: 10.1097/PRS.0000000000005993. [DOI] [PubMed] [Google Scholar]
- 63.Kudel I, Edwards RR, Kozachik S. Predictors and consequences of multiple persistent postmastectomy pains. J Pain Symptom Manage. 2007;34(6):619–627. doi: 10.1016/j.jpainsymman.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 64.Kuan T-S, Hsieh Y-L, Chen S-M, Chen J-T, Yen W-C, Hong C-Z. The myofascial trigger point region: correlation between the degree of irritability and the prevalence of endplate noise. Am J Phys Med Rehabil. 2007;86(3):183–189. doi: 10.1097/PHM.0b013e3180320ea7. [DOI] [PubMed] [Google Scholar]
- 65.Simons DG. 2nd ed. Williams & Wilkins; Baltimore: 1999. Travell & Simons’ Myofascial Pain and Dysfunction: The Trigger Point Manual; p. 1056. [Google Scholar]
- 66.Vas L, Pai R. Ultrasound-Guided Dry Needling As a Treatment For Postmastectomy Pain Syndrome - A Case Series of Twenty Patients. Indian J Palliat Care. 2019;25(1):93–102. doi: 10.4103/IJPC.IJPC_24_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nigam PK, Nigam A. Botulinum toxin. Indian J Dermatol. 2010;55(1):8–14. doi: 10.4103/0019-5154.60343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dutta SR, Passi D, Singh M, Singh P, Sharma S, Sharma A. Botulinum toxin the poison that heals: A brief review. Natl J Maxillofac Surg. 2016;7(1):10–16. doi: 10.4103/0975-5950.196133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Groef A, Devoogdt N, Van Kampen M. The effectiveness of Botulinum Toxin A for treatment of upper limb impairments and dysfunctions in breast cancer survivors: A randomised controlled trial. Eur J Cancer Care (Engl) 2020;29(1):e13175. doi: 10.1111/ecc.13175. [DOI] [PubMed] [Google Scholar]
- 70.Moher D, Shamseer L, Clarke M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.