Abstract
Mutagenized human 293 cells containing an interleukin-1 (IL-1)-regulated herpes thymidine kinase gene, selected in IL-1 and gancyclovir, have yielded many independent clones that are unresponsive to IL-1. The four clones analyzed here carry recessive mutations and represent three complementation groups. Mutant A in complementation group I1 lacks IL-1 receptor-associated kinase (IRAK), while the mutants in the other two groups are defective in unknown components that function upstream of IRAK. Expression of exogenous IRAK in I1A cells (I1A-IRAK) restores their responsiveness to IL-1. Neither NFκB nor Jun kinase is activated in IL-1-treated I1A cells, but these responses are restored in I1A-IRAK cells, indicating that IRAK is required for both. To address the role of the kinase activity of IRAK in IL-1 signaling, its ATP binding site was mutated (K239A), completely abolishing kinase activity. In transfected I1A cells, IRAK-K239A was still phosphorylated upon IL-1 stimulation and, surprisingly, still complemented all the defects in the mutant cells. Therefore, IRAK must be phosphorylated by a different kinase, and phospho-IRAK must play a role in IL-1-mediated signaling that does not require its kinase activity.
Interleukin-1 (IL-1), a proinflammatory cytokine produced mainly by macrophages and monocytes in response to inflammation, infection, and other challenges, stimulates a wide spectrum of responses, including fever, lymphocyte activation, and leukocyte infusion to the site of injury or infection (16). IL-1 stimulates the expression of several genes by activating the transcription factors NF-κB, ATF, and AP-1 (6, 51, 52).
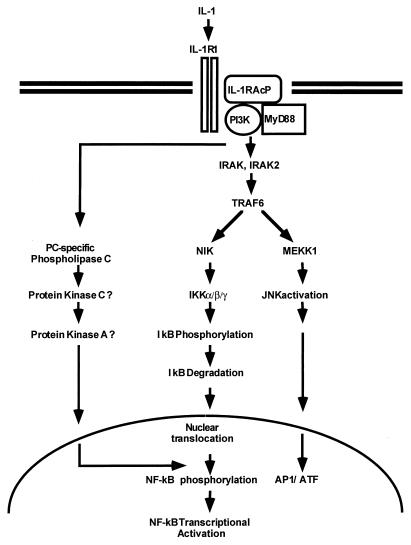
The activation of NF-κB has been studied extensively (4, 6, 16). NF-κB is kept in the cytoplasm through interaction with κB inhibitory proteins. Following stimulation with cytokines (e.g., IL-1 and tumor necrosis factor alpha [TNF-α]) or other agents (e.g., lipopolysaccharide, phorbol ester, and double-stranded RNA), IκB undergoes phosphorylation on specific serine residues and is rapidly ubiquitinated and degraded. The liberated NF-κB translocates to the nucleus, where it activates transcription (5, 63, 66, 69). Recent studies have provided a model for how NF-κB is activated in response to IL-1 (Fig. 1). First, a complex is formed between the type 1 receptor (IL-1R1) and the receptor accessory protein (IL-1RAcP) (21, 24, 29, 70). The cytosolic myeloid differentiation protein (MyD88) (36) is then recruited to the complex, where it functions as an adaptor, recruiting IL-1R-associated kinase (IRAK) in turn (10, 48, 71, 75). IRAK is phosphorylated and then leaves the receptor complex to interact with TRAF6 (11). IRAK2, an IRAK homolog, was shown to interact with the IL-1R complex, MyD88, and TRAF6 in transfected cells, but how IRAK and IRAK2 function in IL-1 signaling is not understood (48). Six TRAFs (TNF receptor-associated factors) have been described so far (2, 17, 22, 23, 25, 31, 49, 58). TRAF2 and TRAF5 have been implicated in activating NF-κB in response to the activation of members of the TNF-α receptor superfamily (2, 17, 22, 23, 25, 31, 49, 58). The TRAFs interact with NF-κB-inducing kinase (NIK), another serine-threonine kinase believed to be a common downstream component in activating NF-κB in response to IL-1, TNF-α, and other stimuli (41). TRAFs might also activate mitogen-activated protein kinase/ERK kinase kinase 1 (MEKK1) (30, 32, 35, 64, 76). Recently, two IκB kinases (IKKα and IKKβ) have been implicated in signal-induced phosphorylation of the IκB proteins (15, 44, 57, 73, 78). Both NIK and MEKK1 activate the IKKs by serine phosphorylation (34, 50). The activated IKKs then phosphorylate IκBs on specific serine residues, resulting in the degradation of IκB and activation of NF-κB. The IKKs are components of a large complex (15, 44, 78). Two additional components, NEMO (NF-κB essential modulator or IKKγ) and IKAP are also part of the IKK complex and are required for its formation (12, 59, 74).
FIG. 1.
A model for the IL-1 signaling pathway. See the text for details. PC, phosphatidylcholine.
Recent studies provide evidence for a second signaling pathway parallel to the cascade leading to IκB degradation and specifically required for NF-κB-dependent transcriptional competency (Fig. 1). Protein kinase C, protein kinase A, and phosphatidylinositol-3 kinase (PI3K) have been implicated in this pathway, possibly through the phosphorylation of the p65 subunit (7, 14, 19, 20, 26, 38, 55, 56, 62, 63a, 80, 81). In addition to the activation of NF-κB, IL-1 and TNF-α also activate the transcription factors ATF and AP-1, through the activation of Jun kinase (8, 45, 51, 52). Signal-induced activation of Jun kinase (Fig. 1) may diverge from NF-κB activation at the level of the TRAF proteins (64). The activated TRAFs activate MEKK1, which in turn activates Jun kinase (32, 35, 50, 64).
Although much progress has been made in understanding signaling in response to IL-1, many questions remain and the proposed roles of many components in the pathway need to be confirmed genetically. For example, we do not know how NIK is activated in response to the activation of IRAK and TRAF6, how NIK activates IKK, or how MEKK1 and Jun kinase are activated. To help resolve these and other issues, we have taken a genetic approach to analyze IL-1 signaling, generating mutant cell lines lacking specific components of the pathway. In the human embryonic kidney cell line 293, an upstream region of the human IL-1-responsive gene E-selectin (also called ELAM-1 [61]) was used to drive the expression of thymidine kinase (TK) and a protein providing resistance to zeocin (Zeo). Negative selection against the expression of TK (39) and positive selection for the expression of Zeo provide a powerful dual system for isolating mutants unresponsive to IL-1 signaling and for complementing them. With this new selection scheme, we now have isolated four independent mutant cell lines that fail to respond to IL-1, in three complementation groups. While mutants in two of the complementation groups are defective in unknown components that lie upstream of IRAK, mutant cell line I1A (I denotes IL-1 unresponsive; 1 denotes complementation group 1; A denotes independent isolate A) lacks IRAK mRNA and protein. Using I1A cells, we show that IRAK is required for the activation of both NF-κB and Jun kinase by IL-1 and that IRAK functions between MyD88 and TRAF6 in the pathway. Furthermore, we find that the kinase activity of IRAK is not required for IL-1-mediated signaling.
MATERIALS AND METHODS
Biological reagents and cell culture.
Recombinant human IL-1β was provided by the National Cancer Institute. Recombinant human TNF-α was from Becton Dickinson (Paramus, N.J.). Antibodies recognizing IL-1R, IL-RAcP, MyD88, IRAK, IRAK2, TRAF6, NIK, IKK1, and IKK2 were described elsewhere (11, 24, 34, 57, 71). Anti-Jun was from Santa Cruz Biotechnology (Santa Cruz, Calif.). Human embryonic kidney 293 cells transfected with human IL-1R (10) were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, penicillin G (100 μg/ml), and streptomycin (100 μg/ml).
Recombinant plasmids.
The NF-κB-dependent E-selectin–luciferase (Luc) reporter gene plasmid pE-selectin-luc was described by Schindler and Baichwal (61). E-selectin–TK and E-selectin–Zeo were constructed by cloning the E-selectin promoter (−730 to +53) in front of the TK cDNA (3) or the Zeo resistance gene (Invitrogen). Mammalian expression vectors encoding IRAK (driven by the TK promoter), IRAK-K293A (driven by the TK promoter), MyD88 (driven by the cytomegalovirus [CMV] promoter), TRAF6 (driven by the CMV promoter), and the control expression plasmid pRK5 were described elsewhere (11).
Mutagenesis and selection.
The mutagenesis protocol was modified slightly from the one used by Pellegrini et al. (54). 293-TK/Zeo cells were expanded into pools of 107 cells each. One day after the cells in each pool had been split onto two 150-mm-diameter plates, they were treated with ICR191 (54) for 3 h, rinsed twice in serum-free medium, and cultured in complete medium. The concentration of ICR191 used was determined empirically to achieve 50 to 70% killing after each round of mutagenesis and was varied between 1 and 5 μg/ml. After recovery, the cells were subjected to a total of five rounds of mutagenesis, and cells in each mutagenized pool were split onto six 150-mm-diameter plates for selection. To isolate IL-1-unresponsive mutants, the cells were selected in gancyclovir (5 μg/ml) (Hoffmann-La Roche, Inc.) plus IL-1 (100 U/ml). Fresh gancyclovir and IL-1 were added every 3 days for 23 weeks. Clones were picked, expanded in nonselective medium, and analyzed by selection with gancyclovir, either alone, with IL-1 and Zeo (100 μg/ml; Invitrogen) or with IL-1.
Transfection and reporter assay.
For stable transfections, 2 × 105 cells were seeded onto a 10-cm-diameter plate and cotransfected the following day by the calcium phosphate method (60) with 10 μg of each expression construct plus 1 μg of pBabePURO. After 48 h, the cells were selected with 1 μg of puromycin per ml until clones appeared. For reporter gene assays, 2 × 105 cells were transfected by the same procedure with 1 μg of pE-selectin-luc, 1 μg of pSV2-β-gal, and 2,150 ng of each expression construct. After 48 h, the cells were split into three 35-mm-diameter plates and, the next day, stimulated with IL-1 and TNF-α for 4 h before harvest. Luciferase and β-galactosidase activities were determined by using the Promega luciferase assay system and chemiluminescence reagents (Promega), respectively.
Gel shift assay.
An NF-κB binding site (5′-GAGCAGAGGGAAATTCCGTAACTT-3′) from the IP-10 gene (40) was used as a probe. Complementary oligonucleotides, end labeled with polynucleotide kinase (Boehringer Mannheim) and [γ-32P]ATP, were annealed by slow cooling. Approximately 20,000 cpm of probe were used per assay. Cytoplasmic extracts were prepared as described by Kessler et al. (27) and Levy et al. (33). The binding reaction was carried out at room temperature for 20 min in a total volume of 20 μl containing 20 mM HEPES buffer (pH 7.0), 10 mM KCl, 0.1% Nonidet P-40, 0.5 mM dithiothreitol, 0.25 mM phenylmethanesulfonyl fluoride, and 10% glycerol.
Immunoblotting, immunoprecipitation, in vitro kinase, and Northern assays.
For immunoprecipitation and immunoblotting, cells at 80% confluency were harvested from 10-cm-diameter dishes, washed once with phosphate-buffered saline, and lysed for 10 min at 4°C in 0.5 ml of 0.5% Nonidet P-40 lysis buffer containing 50 mM Tris-Cl (pH 8.0), 100 mM NaCl, 10% glycerol, 0.1 mM EDTA, 20 mM sodium fluoride, 1 mM sodium orthovanadate, 1 mM dithiothreitol, 0.4 mM phenylmethanesulfonyl fluoride, aprotinin (3 μg/ml), pepstatin (2 μg/ml), and leupeptin (1 μg/ml). Cellular debris was removed by centrifugation at 10,000 × g for 5 min. For immunoblotting, cell extracts were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane. Immunoblot analysis was performed with rabbit polyclonal antibodies, visualized with horseradish peroxidase-coupled goat anti-rabbit immunoglobulin, using the Amersham ECL (enhanced chemiluminescence Western blotting detection system. For immunoprecipitation and in vitro kinase assays, cell extracts were incubated with 1 μl of the polyclonal antibody for 2 h and then incubated with 50 μl of protein A-Sepharose beads for 1 h. The beads were washed three times with lysis buffer and once with kinase reaction buffer (20 mM HEPES [pH 7.0], 20 mM MgCl2). In vitro kinase reactions were performed in 50 μl of buffer containing 20 mM HEPES (pH 7.0), 20 mM MgCl2, 1 mM ATP, and 10 μCi of [γ-32P]ATP at 30°C for 30 min. For the Jun kinase assay, 2 μg of glutathione S-transferase–Jun (Alexis Corporation) was included in the reaction. Samples were analyzed by SDS-PAGE (10% gel) followed by autoradiography.
For Northern analysis, total RNA was isolated by using the TRIzol reagent (GIBCO BRL). Appropriate gene-specific probes were made with a random priming kit (Amersham). Transfers to the positively charged nylon membrane Hybond-N were performed according to the procedure provided by Amersham.
RESULTS
Attempts to isolate mutants by using established strategies and development of a novel approach.
Mutant clones defective in the induction of cell surface marker CD2 or CD4, or both, driven by an IL-1-responsive promoter, were obtained by cell sorting but were too unstable to work with. They reverted to wild type after being cultured for three to four passages. To allow lethal selection with promoters that drive significant basal expression of marker genes, a new double drug selection was set up, with the Zeo and herpesvirus TK genes. Cells die in gancyclovir when TK is expressed, and cells that express the Zeo gene survive exposure to zeocin. The previously used negative selection with 6-thioguanine in cells carrying a signal-regulated gpt gene (13, 65, 68) works well only when the promoter gives very low basal expression (for example, the interferon-responsive 6-16 promoter). A major advantage of gancyclovir-TK selection over 6-thioguanine–gpt selection is that the concentration of gancyclovir can be manipulated to allow cells with a low level of constitutive TK expression to survive but still to kill cells with an induced level of expression. Also, since gancyclovir is a poor substrate for mammalian TK, the selection does not require a TK-null cell line.
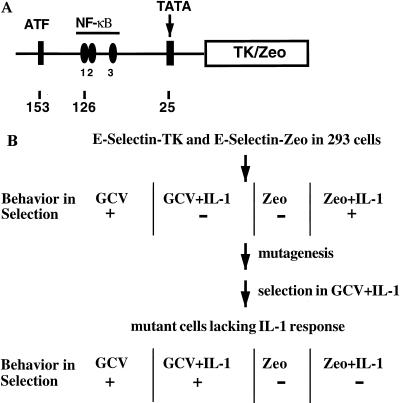
To construct plasmids in which TK and Zeo can be induced by IL-1 or TNF-α, an upstream fragment (−730 to +52) of the E-selectin gene (Fig. 2A) was cloned upstream of TK and Zeo. The E-selectin promoter contains binding sites for both NF-κB and ATF, and mutation of either site abolishes the IL-1-induced promoter activity (72). The E-selectin–TK and E-selectin–Zeo constructs were cotransfected into 293 cells, in which the E-selectin promoter has a low basal activity and a high induced activity (Fig. 3B). The transfected cells were selected for clones that survive in gancyclovir, die completely in gancyclovir plus IL-1, die in Zeo, and survive in Zeo plus IL-1 (Fig. 2B). The clone used for mutagenesis is called 293-TK/Zeo.
FIG. 2.
Double drug selection with TK and Zeo under control of the E-selectin promoter. (A) E-selectin–TK and E-selectin–Zeo. An upstream fragment of the E-selectin gene (−730 to +52), containing one ATF and three NF-κB binding sites and a TATA box, was cloned in front of the TK cDNA or the Zeo gene. (B) Drug selection. E-selectin–TK and E-selectin–Zeo were cotransfected into 293 cells, and the transfected cells were selected in Zeo plus IL-1. Individual clones were assayed for survival in gancyclovir (GCV), death in gancyclovir plus IL-1, death in Zeo, and survival in Zeo plus IL-1. One such clone was expanded and subjected to five rounds of mutagenesis. IL-1-unresponsive mutants were isolated by selecting the mutagenized pools in gancyclovir plus IL-1. Putative mutants were then tested for survival in gancyclovir and gancyclovir plus IL-1 and for death in Zeo and Zeo plus IL-1.
FIG. 3.
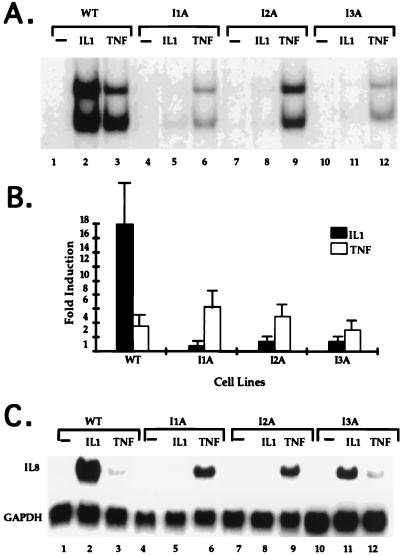
Analyses of IL-1-unresponsive mutants. (A) NF-κB gel shift assay. Cell extracts were made from 293-TK/Zeo cells (WT [wild type]) and the IL-1-unresponsive mutants treated for 15 min with IL-1 (100 U/ml) or TNF-α (20 ng/ml) or untreat. The NF-κB binding site from the IP-10 gene was used as a probe. The two bands in the gel shift assay are due mainly to p50-p65 heterodimers (bottom) and p65-p65 homodimers (top) (63a). (B) Luciferase reporter assay. E-selectin–Luc (1 μg/10-cm-diameter plate) was transiently transfected into 293-TK/Zeo cells (WT) and the IL-1-unresponsive mutants. Thirty-six hours later, the cells were either left untreated or stimulated for 4 h more with IL-1 (100 U/ml; closed bars) or TNF-α (20 ng/ml; hatched bars). Luciferase activities were normalized to β-galactosidase. Data are presented as the fold induction of luciferase activity in the treated cells. Shown are the averages and standard deviations from three independent experiments. (C) Northern analysis of IL-8 gene expression. Total RNAs were made from 293-TK/Zeo cells (WT) and the IL-1-unresponsive mutants treated for 6 h with IL-1 (100 U/ml) or TNF-α (20 ng/ml) or untreated. Human IL-8 cDNA was used as a probe, and the signals were normalized after reprobing with a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA.
Isolation of IL-1-unresponsive mutants.
For mutagenesis, 293-TK/Zeo cells were expanded to pools of 107 cells each, subjected to five rounds of mutagenesis with ICR191 (an intercalating agent that induces frameshift mutations), and selected in gancyclovir plus IL-1. Clones picked from each of six separately mutagenized pools (Table 1) were expanded in nonselective medium and analyzed by drug selection. Over 90% of the clones survived in gancyclovir plus IL-1 and died in Zeo plus IL-1. Ten to thirty percent of the clones from each of four pools had lost the ability to activate NF-κB in a gel shift assay after IL-1 treatment (Table 1, pools 1 to 4). Clones from the other two pools still retained IL-1-induced NF-κB activation and thus either are defective in IL-1-mediated pathways that do not affect the liberation of NF-κB from IκB or are cis mutants in which the TK construct is inactivated (Table 1, pools 5 and 6). Since clones from the same mutagenized pool may be siblings, only one mutant clone from each pool was used for further study. The mutant clones were named according to their complementation groups as described below. IL-1 failed to activate NF-κB substantially in all four mutant clones (Fig. 3A and data not shown for clone I2B). An E-selectin-driven luciferase plasmid was transfected transiently into each mutant cell line. Both IL-1 and TNF-α induced luciferase in wild-type (293-TK/Zeo) cells (Fig. 3B). The response to IL-1 was absent in all four mutant clones, while their TNF-α response was intact, revealing that these four clones are specifically defective in IL-1 signaling. We also studied the endogenous IL-1-responsive IL-8 gene, which is induced by both IL-1 and TNF-α in wild-type 293-TK/Zeo cells. In the mutant cells, IL-1-induced IL-8 gene expression was reduced greatly, while the response to TNF-α was intact (Fig. 3C). In Fig. 3C, clones I1A and I2A show an enhanced TNF response compared to wild-type or I3A cells. However, this difference was not observed consistently.
TABLE 1.
Clones that do not respond to IL-1
| Pool | No. of clones
|
|||
|---|---|---|---|---|
| Picked | That live in gancyclovir + IL-1 and die in Zeo + IL-1 | Assayed by κB binding | Lacking IL-1-induced κB binding | |
| 1 | 40 | 38 | 20 | 3 |
| 2 | 38 | 35 | 10 | 3 |
| 3 | 42 | 41 | 10 | 1 |
| 4 | 28 | 27 | 10 | 2 |
| 5 | 36 | 34 | 34 | 0 |
| 6 | 31 | 29 | 29 | 0 |
Dominance and complementation.
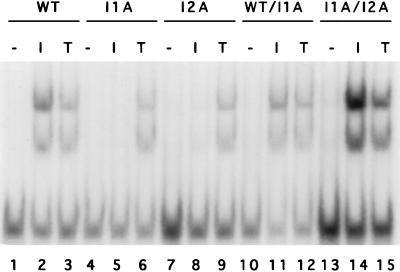
Puromycin-resistant populations were made from each of the four mutant clones and fused with hygromycin-resistant 293-TK/Zeo cells. After selection with both drugs, the IL-1-induced activation of NF-κB was restored in all of the heterokaryons (Fig. 4, lanes 1 to 6 and 10 to 12, and data not shown), indicating that the mutations are all recessive. To assign complementation groups, puromycin-resistant and hygromycin-resistant populations from each mutant clone were fused pairwise, and IL-1-induced NF-κB activation was examined. Clones I2A and I2B are in the same complementation group since IL-1-induced NFκB activation was not restored in the heterokaryons (data not shown). I1A and I3A are in different complementation groups (Fig. 4, lanes 4 to 9 and 13 to 15, and data not shown). The isolation of mutants in three different complementation groups strongly suggests that these cell lines are defective in different signaling components.
FIG. 4.
NF-κB gel shift assay for dominance and complementation. Extracts were made from 293-TK/Zeo cells (WT [wild type]), clones I1A and I2A, and heterokaryons WT/I1A and I1A/I2A, treated for 15 min with IL-1 (100 U/ml) or TNF-α (20 ng/ml) or untreated. The NF-κB binding site from the IP-10 gene was used as a probe.
Loss of IRAK accounts for the phenotype of I1A cells.
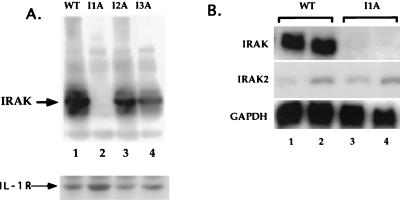
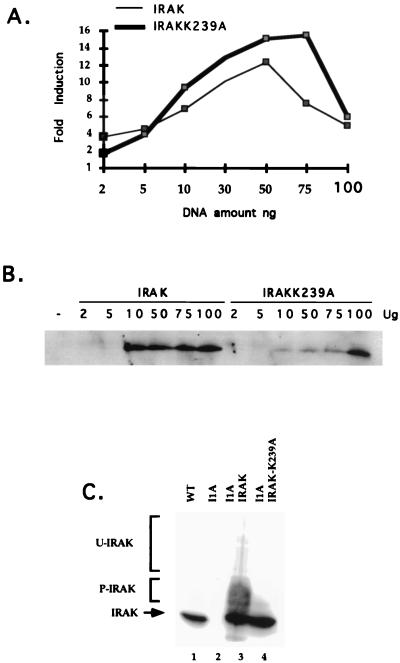
All four mutant clones were assayed with antibodies against the known signaling components IL-1R1, IL-1RAcP, MyD88, IRAK, IRAK2, TRAF6, NIK, IKK1, and IKK2. I1A cells lack IRAK (Fig. 5A, lane 2), a serine-threonine kinase recruited to the IL-1R complex upon IL-1 stimulation. This result was confirmed by Northern analysis, showing that IRAK mRNA is also absent in I1A cells (Fig. 5B, lanes 3 and 4). No other known component was missing in I1A cells, and no known component was missing in the other three mutant clones (data not shown). To determine whether IRAK can complement the defect in I1A cells, increasing amounts of TK-driven IRAK cDNA were cotransfected transiently with E-selectin–Luc into I1A cells. With an optimal amount of DNA (50 to 100 ng), IL-1-induced expression of luciferase was restored in the IRAK-transfected I1A cells (Fig. 6A and B), whereas expression was not observed in I1A cells transfected with vector DNA (data not shown). TK-IRAK was also transfected stably into I1A cells (Fig. 6C). Although constitutive activation of NF-κB was observed in these I1A-IRAK cells, IL-1 induces the activation of NF-κB further (data not shown). Taken together, the results show that IRAK can complement the defect in I1A cells, indicating that their failure to respond to IL-1 is likely due solely to the lack of this protein.
FIG. 5.
Clone I1A lacks IRAK. (A) Analysis of the IRAK protein. Extracts were made from 293-TK/Zeo (WT [wild type]) cells and from the IL-1-unresponsive mutants. Aliquots were analyzed with anti-IRAK after Western transfer. The same blot was probed with anti-IL-1R1. (B) Analysis of IRAK and IRAK2 mRNAs. Total RNA made from 293-TK/Zeo cells (WT) and mutant I1A was analyzed by the Northern procedure with IRAK or IRAK2 cDNA as the probe.
FIG. 6.
Analysis of I1A cells complemented with IRAK or IRAK-K239A. (A) Cells were cotransfected transiently with E-selectin–Luc and increasing amounts of a TK promoter-driven IRAK expression vector or TK promoter-driven IRAK-K239A expression vector. Thirty-six hours later, the cells were either left untreated or stimulated for 4 h more with IL-1 (100 U/ml) before harvest. Luciferase activities were normalized to β-galactosidase. Data are presented as fold induction of luciferase activity in the treated cells. The experiments were repeated four times. Shown are the data from a typical experiment. (B) Western analysis with anti-IRAK of extracts from I1A cells transiently transfected with increasing amounts of IRAK or IRAK-K239A. (C) Western analysis of extracts from 293-TK/Zeo (WT [wild type]), I1A, or I1A cells stably transfected with IRAK or IRAK-K239A.
Functions of IRAK in IL-1 signaling.
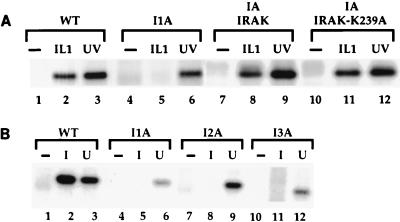
IL-1 stimulation also leads to the activation of Jun kinase, and previous studies have suggested that IRAK may be involved (57). Both IL-1 and UV treatment activated Jun kinase in wild-type 293-TK/Zeo cells (Fig. 7A, lanes 1 to 3), and the activation of Jun kinase induced by IL-1 but not by UV treatment was abolished in I1A cells (Fig. 7A, lanes 4 and 5). The IL-1-induced activation of Jun kinase was restored in I1A-IRAK cells (Fig. 7A, lanes 7 to 9). Taken together, these results show that IRAK is required for IL-1-induced but not UV-induced activation of Jun kinase.
FIG. 7.
IL-1-induced activation of Jun kinase in mutant cells. Immunoprecipitates were prepared from cell extracts with anti-Jun kinase, followed by an in vitro kinase assay. (A) Analysis of extracts from 293-TK/Zeo (WT [wild type]) cells and I1A cells, untransfected or stably transfected with IRAK or IRAK-K239A, untreated, stimulated with IL-1, or treated with UV (40 J/m2). (B) Analysis of extracts from clones I1A, I2A, and I3A untreated, stimulated with IL-1, or treated with UV (40 J/m2).
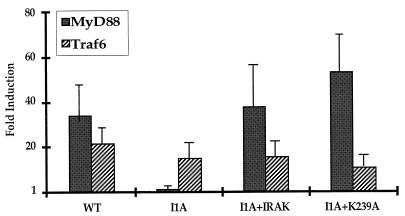
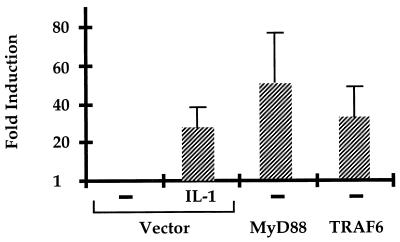
Previous studies have shown that ectopic expression of MyD88 induces NF-κB activation strongly even in the absence of IL-1 (48, 71). When MyD88 was cotransfected with E-selectin–Luc into wild-type 293-TK/Zeo cells, promoter activity was dramatically increased compared to cells cotransfected with the vector control, but constitutive activation of the E-selectin promoter in response to overexpression of MyD88 was not observed in I1A cells, which lack IRAK (Fig. 8). In I1A cells stably transfected with IRAK, the effect of MyD88 was restored (Fig. 8). We conclude that MyD88 cannot interact with downstream components in the pathway in the absence of IRAK. Overexpression of TRAF6 can also constitutively induce NF-κB activation (11). When TRAF6 was cotransfected with E-selectin–Luc into either wild-type 293-TK/Zeo cells or I1A cells, promoter activity was increased in the absence of IL-1 (Fig. 8). Therefore, TRAF6 interacts with components of the signaling pathway downstream of IRAK, as previously proposed by Cao et al. (11). Taken together, our results confirm that IRAK functions between MyD88 and TRAF6.
FIG. 8.
Constitutive stimulation of signaling by MyD88 or TRAF6 in I1A cells. 293-TK/Zeo (WT [wild type]) cells and I1A cells stably transfected with IRAK or IRAK-K239A were cotransfected transiently with E-selectin–Luc, with a vector control, or with a MyD88 or TRAF6 expression vector. Luciferase activities were normalized to β-galactosidase. Data are presented as fold induction of luciferase in cells transfected with MyD88 (solid bars) or TRAF6. Shown are the averages and standard deviations from three independent experiments.
The kinase activity of IRAK is not required for its function in IL-1 signaling.
IRAK, a serine-threonine kinase, is recruited to the receptor complex upon IL-1 stimulation, where it becomes highly phosphorylated (10). Phosphorylated IRAK then leaves the receptor to interact with TRAF6 and propagate the signal (11). The phosphorylation sites of IRAK have not yet been identified, and it is also not clear whether IRAK phosphorylates itself or is phosphorylated by another kinase. To examine whether the kinase activity of IRAK is required for signaling, its ATP binding site was inactivated by changing the lysine at amino acid 239 to alanine (K239A mutation). IRAK-K239A, driven by the TK promoter, was transfected into I1A cells (I1A-IRAK-K239A). As expected, the K239A mutation inactivates the kinase activity of IRAK (Fig. 9). Surprisingly, however, IRAK-K239A functions about as well as wild-type IRAK in vivo. The luciferase reporter assay showed that activation of the E-selectin promoter by IL-1 was restored in I1A-IRAK-K239A cells as well as in I1A-IRAK cells (Fig. 6A and B). The K239A mutation in IRAK does not affect its ability to restore the IL-1-induced activation of NF-κB (data not shown) or the activation of Jun kinase in I1A cells (Fig. 7A, lanes 10 to 12). Finally, the constitutive activation of E-selectin–Luc in response to overexpression of MyD88 was also restored in I1A-IRAK-K239A cells (Fig. 8), revealing that the kinase activity of IRAK is not required for its interaction with MyD88 either.
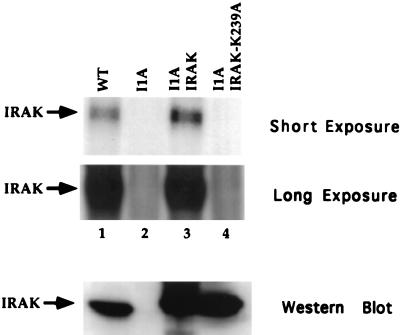
FIG. 9.
IRAK kinase assay. Cell extracts were made from 293-TK/Zeo (WT [wild type]), I1A, and I1A stably transfected with IRAK or IRAK-K239A. Immunoprecipitates were prepared with anti-IRAK, followed by an in vitro kinase assay. Short and long exposures are presented. The immunoprecipitated samples were also analyzed with anti-IRAK (bottom panel).
Phosphorylation of IRAK by another kinase.
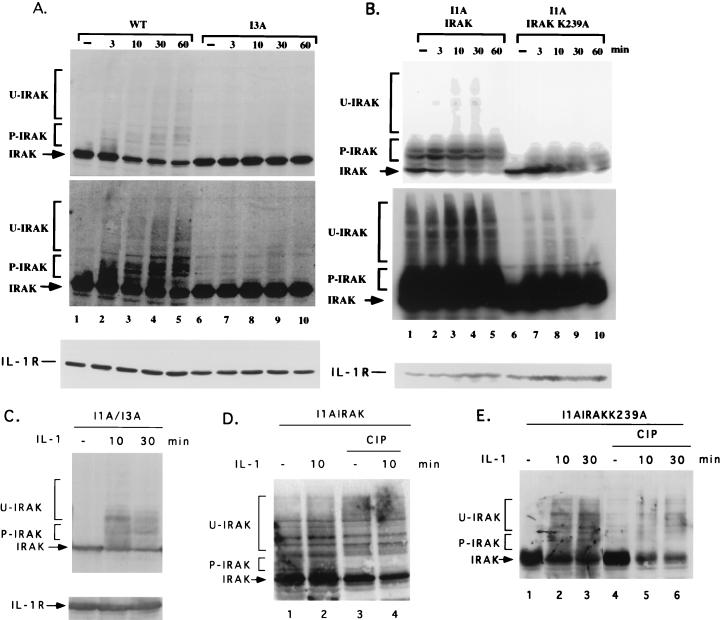
Previous work has shown that the majority of IRAK translocates to the IL-1R complex following IL-1 stimulation, where it becomes multiply phosphorylated and subsequently degraded by proteosomes (75). We observed the same phenomenon in 293-TK/Zeo cells. IRAK was phosphorylated and degraded upon IL-1 treatment (Fig. 10A, lanes 2 to 5). Some of the upper bands appearing after IL-1 stimulation may represent ubiquitinated forms of IRAK (9a). IL-1R was not degraded upon IL-1 stimulation in 293-TK/Zeo cells (Fig. 10A, bottom). In stably transfected I1A cells, IRAK was phosphorylated and ubiquitinated before IL-1 stimulation, probably due to its overexpression (Fig. 10B, lane 1, and data not shown). However, IRAK was still degraded after IL-1 treatment, possibly due to further phosphorylation after stimulation (Fig. 10B, lanes 2 to 5). The loss of several shifted IRAK bands following treatment with calf intestinal phosphatase confirmed that they are phosphorylated forms (Fig. 10D, lanes 3 and 4). IRAK-K239A was not phosphorylated or ubiquitinated before stimulation (Fig. 10B, lane 6) but was still phosphorylated, ubiquitinated, and degraded after IL-1 treatment (Fig. 10B, lanes 7 to 10). The phosphorylation of IRAK-K239A following IL-1 stimulation was also confirmed by the loss of several phosphorylated bands of IRAK-K239A following phosphatase treatment (Fig. 10E, lanes 4 to 6). Since IRAK-K239A cannot phosphorylate itself (Fig. 9), its phosphorylation in response to IL-1 must be due to another kinase. It is possible that IRAK is phosphorylated both by itself and by a different kinase upon IL-1 treatment. Since IRAK-K239A complements I1A cells just as well as wild-type IRAK (Fig. 6, 7A, and 8 and data not shown), it is likely that the phosphorylation of IRAK by a different kinase plays a more important role in signaling than does IRAK autophosphorylation. Further work is needed to determine the residues modified in each situation.
FIG. 10.
Western analysis of IRAK as a function of time after stimulation with IL-1. Shown are results for wild-type 293TK/Zeo (WT) cells and I3A cells (A), I1A cells transfected with IRAK or IRAK-K239A (B), and I1A/I3A heterokaryons (C), either untreated or treated with IL-1. Cell extracts were analyzed by the Western procedure with anti-IRAK. P-IRAK, phosphorylated IRAK; U-IRAK, ubiquitinated IRAK. The top portions of panels A and B are short exposures, and the bottom portions are long exposures. The same transfers were probed with anti-IL-1R1 to control for loading. (D and E) Extracts of I1A cells transfected with IRAK or IRAK-K239A, with or without IL-1 stimulation, were either untreated or treated with calf intestinal phosphatase (CIP).
IRAK2 can also complement mutant I1A cells.
IRAK2, identified as a homologue of IRAK, has also been implicated in IL-1 signaling (48). Although IRAK2 interacts with the IL-1R complex and forms complexes with MyD88 and TRAF6 (48), its exact role in signaling is not clear. IRAK2 was expressed in both wild-type and IRAK-deficient I1A cells, but its mRNA was at a much lower level than the mRNA for IRAK (Fig. 5B). Western analysis indicated that the level of IRAK2 protein is also relatively low in 293-TK/Zeo cells (data not shown). IRAK2 restored responsiveness to IL-1 when overexpressed in IRAK-deficient I1A cells (Fig. 11). Constitutive activation of the pathway in response to overexpression of MyD88 was also restored in I1A cells transfected with IRAK2 (Fig. 11), showing that MyD88 can also signal through IRAK2.
FIG. 11.
Complementation of I1A cells with IRAK2. E-selectin–Luc was cotransfected transiently with control vector, MyD88, or TRAF6 into I1A cells stably transfected with CMV-IRAK2 (I1A-IRAK2 cells). The I1A-IRAK2 cells transfected with E-selectin–Luc and the control vector were treated with IL-1 (100 U/ml, 4 h). Data are presented as the fold induction of luciferase activity in IL-1-treated or untreated cells transfected with MyD88 or TRAF6 compared to cells transfected with the control vector. Shown are the averages and standard deviations from three independent experiments.
Mutants in complementation groups I2 and I3 are defective in components upstream of IRAK.
The IL-1-induced activation of NF-κB is greatly reduced in mutant clones I2A and I3A (Fig. 3A). Western analyses of IL-1R, IL-1RAcP, MyD88, IRAK, IRAK2, TRAF6, NIK, IKK1, and IKK2 revealed that none of these components is missing in any of the three mutant clones (data not shown). IL-1-induced Jun kinase activation was completely absent in all three clones, whereas UV-induced Jun kinase activation was normal (Fig. 7B). Therefore, all three clones are defective in components required for the activation of both NF-κB and Jun kinase. In response to IL-1, IRAK is phosphorylated, ubiquitinated, and degraded in wild-type cells (Fig. 10A, lanes 1 to 5). Interestingly, IRAK was not phosphorylated, ubiquitinated, or degraded upon IL-1 stimulation in any of the three mutants. (Data for clone I3A are presented in Fig. 10A, lanes 6 to 10; data for clones I2A are not shown.) However, IRAK was phosphorylated and degraded in the I1A/I3A heterokaryons, suggesting that the IRAK in I3A cells is intact (Fig. 10C). These results suggest that the defects in these mutant clones are upstream of IRAK, a conclusion further supported by the results of an experiment in which MyD88 was cotransfected with E-selectin–Luc into the mutant clones. The E-selectin promoter was activated constitutively in all three mutants (data not shown). Therefore, the defects in these mutants are upstream of both IRAK and MyD88.
DISCUSSION
Roles of IRAK and IRAK2 in IL-1 signaling.
Although biochemical studies have yielded important information concerning the IL-1 signaling pathway (10, 11, 48, 70, 71, 75), genetic information is still largely lacking. We have now obtained the IRAK-null cell line I1A, allowing a detailed evaluation of the role of this protein in the IL-1 response. IRAK was cloned originally through its association with IL-1R (10). Upon IL-1 stimulation, IRAK associates rapidly with the receptor complex and becomes highly phosphorylated (10). Our work now shows that the IL-1-induced activation of both NF-κB and Jun kinase is abolished in IRAK-deficient I1A cells, providing strong genetic evidence that IRAK indeed is essential for these two pathways. How IRAK is activated upon IL-1 stimulation and how it functions remain to be determined. Recently, IRAK has also been implicated in the IL-18 and Toll-dependent signaling pathways (1, 28, 43, 47). How IRAK is activated in these pathways and the role played by IRAK also need to be elucidated.
MyD88 coprecipitates with IL-1R1, IL-1RAcP, and IRAK and has a high affinity for hypophosphorylated IRAK, suggesting that IRAK might be recruited to the receptor complex through an interaction involving MyD88 (71). The ectopic expression of MyD88 strongly induces NF-κB activation in wild-type cells (48, 71). We now find that the constitutive activity of MyD88 is lost in the absence of IRAK, suggesting that MyD88 cannot signal to downstream components of the pathway without IRAK. MyD88 may interact with IRAK directly and may indeed function as an adaptor to recruit IRAK to the receptor complex.
IRAK leaves the receptor after activation and forms a complex with TRAF6 (11). Overexpression of TRAF6 can also lead to constitutive activation of NF-κB (11). We find that the constitutive activation of NF-κB by TRAF6 is the same in IRAK-deficient I1A cells as in wild-type controls, confirming that TRAF6 can interact with downstream components of the pathway in the absence of IRAK. An important downstream target for TRAFs is likely to be NIK, a common mediator in the activation of NF-κB in response to IL-1, TNF-α, and other stimuli (41). As shown in Fig. 1, TRAFs may also activate MEKK1, which in turn activates Jun kinase and IKK (30, 32, 35, 50, 64, 76). However, it is not yet clear how the TRAFs are activated. Since our study shows that the overexpression of TRAF6 can activate NF-κB constitutively even in the absence of IRAK, IRAK might activate TRAF6 simply by facilitating its aggregation.
The kinase activity of IRAK is not necessary for it to function in IL-1 signaling. Although this result is somewhat surprising, it was not totally unexpected since a similar observation has been made for receptor interacting protein, a serine-threonine kinase in TNF-α signaling (67). The ability of mutant kinases without catalytic activity still to function has also been observed in interferon signaling. Although the receptor-associated protein tyrosine kinase JAK1 is required for the gamma interferon (IFN-γ) response, a kinase-dead mutant of JAK1 can restore IFN-γ-induced gene expression but not the antiviral state to a JAK1-null mutant cell line (9). Also, although Tyk2 is required for the IFN-α response, a kinase-dead mutant of Tyk2 can restore IFN-α-induced gene expression in Tyk2-null cells (18).
It is not known whether IRAK is phosphorylated at the receptor by itself, another kinase, or both. Using IRAK-deficient cells, we show that a kinase-dead mutant of IRAK can still be phosphorylated upon IL-1 stimulation (Fig. 10B and E), revealing that another kinase must be capable of phosphorylating IRAK at least in part. Although the mechanistic role of IRAK phosphorylation is not clear, its state of phosphorylation does affect its affinity for MyD88 (71). The high affinity of MyD88 for underphosphorylated IRAK is consistent with its role in recruiting IRAK to the receptor, and the inability of MyD88 to bind to phosphorylated IRAK may explain how IRAK leaves the receptor complex after activation.
IRAK2, a homolog of IRAK lacking apparent kinase activity, may also be involved in IL-1 signaling since it interacts with IL-1R and forms a complex with MyD88 and TRAF6 (48). The level of IRAK2 expression in 293 cells seems to be much lower than that of IRAK. Since overexpression of IRAK2 restores IL-1 responsiveness to I1A cells (Fig. 11), it is possible that IRAK and IRAK2 are differentially expressed functional alternatives, explaining the small residual IL-1 response that we sometimes observe in IRAK-deficient cells since these cells have a small amount of IRAK2. However, we still cannot exclude the possibility that IRAK and IRAK2 have somewhat different functions.
Novel components in IL-1 signaling.
Analyses of three mutant cell lines in complementation groups I2 and I3 show that in response to IL-1, that the activation of both NFκB and Jun kinase is abolished, that IRAK is neither phosphorylated nor degraded, and that overexpressed MyD88 can still activate NF-κB constitutively, probably by interacting with IRAK. These results strongly suggest that these mutants are likely defective in components upstream of IRAK. However, the known upstream components IL-1R1, IL-1RAcP, and MyD88 are all expressed normally in all three mutant cell lines. ICR191 is a frameshift mutagen, and we have found that it rarely leads to mutations that allow the protein to be expressed; almost always, both the target mRNA and protein are missing (unpublished results). Therefore, it is very likely that mutants in complementation groups I2 and I3 are defective in components of the IL-1 signaling pathway that have not yet been identified. One possibility is an additional receptor component. IL-1R is a member of a family that includes IL-1Rrp2, T1/ST2, and rsc786/TIL (37, 42, 46, 53, 77). MyD88, also a member of this family, was only recently found to play a role in IL-1 signaling (71). It is also possible that the mutation in complementation group I2 or I3 affects the kinase that phosphorylates IRAK-K239A. In such a mutant, IRAK would still be recruited to the receptor complex but not phosphorylated. Complementation of these mutant cell lines with expression libraries will advance our understanding of IL-1 signaling considerably.
IL-1-unresponsive mutants not defective in NF-κB activation.
Since the E-selectin promoter contains binding sites for both NF-κB and ATF, and since mutation of either site abolishes IL-1-induced promoter activity, one would expect to isolate IL-1-unresponsive mutant clones that are defective in activating NF-κB or ATF, or both. Mutants defective in both pathways would most likely have defects in upstream components, whereas mutants defective only in one pathway would most likely have defects in downstream components. Furthermore, it has been shown recently that the liberation of NF-κB from IκB and its translocation into the nucleus may not be sufficient for the full activation of NF-κB. The transcriptional activity of NF-κB is also regulated by IκB-associated protein kinase A, leading to phosphorylation of the NF-κB p65 subunit and to its binding to the transcriptional coactivator CBP/p300 (Fig. 1 and references 20, 55, 79, and 80). Phosphatidylcholine-specific phospholipase C and protein kinase C have also been implicated in regulating the transcriptional activity of NF-κB (7, 14, 19, 26, 38, 62, 81). Recently, Sizemore and Stark (63b) have found that inhibitors of PI3K block NFκB-dependent transcription by blocking the IL-1-stimulated phosphorylation of NF-κB but do not affect the IL-1-stimulated degradation of IκBα, the nuclear translocation of NF-κB, or the ability of NF-κB to bind to DNA. Therefore, mutant clones in which NF-κB is activated for DNA binding may still be defective in activating transcription through the phosphorylation of NF-κB itself. Only 10 to 30% of the clones selected from each of four mutagenized pools have lost the ability to activate NF-κB after IL-1 treatment; the remaining clones still induce the DNA binding activity of NF-κB (as shown by gel shift analysis), and some of these may be defective in other IL-1-mediated pathways.
Current state of obtaining recessive mammalian cell mutants.
In this report, we have described a novel genetic approach to generate mutant cell lines defective in specific components of IL-1 signaling, employing a double drug selection with the Zeo and herpesvirus TK genes as markers. Cells die in gancyclovir when TK is expressed, and cells that express the Zeo gene survive exposure to zeocin. An obvious advantage of lethal selection over using the fluorescence-activated cell sorter to separate cells on the basis of surface expression of CD2 or CD4 is the saving of time and money. A more important advantage is that we have not encountered any metastable mutants with the lethal selection, a serious problem with IL-1-unresponsive mutants obtained by cell sorting. Another major advantage of the TK-gancyclovir selection is that, in contrast to the gpt–6-thioguanine selection, the concentration of selective drug can be manipulated to allow cells with a lower level of constitutive marker gene expression to survive but still to kill cells with an induced level of expression. Also, since gancyclovir is a poor substrate for mammalian TK, the selection does not require the use of a TK-null cell line. Therefore, the TK selection has the potential to become a general method for isolating mammalian cell mutants in different signaling pathways.
As illustrated here by the IRAK-null cells, mutant cell lines become extremely valuable when they can be complemented by a specific cDNA since one then can pursue a detailed structure-function analysis of a single protein in a null background. Complementation of mutant cell lines defective in unknown components will enable us to identify new participants of the pathway and is a major goal of the genetic approach. Successful complementation requires introducing libraries into mutant cells with high efficiency, an appropriate level of expression of the transfected gene, and stringent selection of the complemented cells. Retroviral cDNA expression libraries used very successfully by others (74) are our first choice in attempting to complement the IL-1-unresponsive mutant cell lines. The genetic system described here also has limitations. For example, it would be very difficult to isolate mutants in redundant branches of a pathway unless the redundant molecules are expressed differentially, as are IRAK and IRAK2. Finally, extensive development will be required to set up a system that would enable one to isolate mutant mammalian cell lines defective in essential genes.
ACKNOWLEDGMENTS
We thank Stewart Leung for helpful discussion, Mary B. Stark and Michael Haag for technical assistance, members of the Stark lab for scientific input, and Jan Vilcek for the IL-8 cDNA.
This work was supported by a Human Frontiers of Science Program grant and by NIH/NCI grant P01-CA62220.
REFERENCES
- 1.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1 and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 2.Aizawa S, Nakano H, Ishida T, Horie R, Nagai M, Ito K, Yagita H, Okumura K, Inoue J, Watanabe T. Tumor necrosis factor receptor-associated factor (TRAF) 5 and TRAF2 are involved in CD30-mediated NFκB activation. J Biol Chem. 1997;272:2042–2045. doi: 10.1074/jbc.272.4.2042. [DOI] [PubMed] [Google Scholar]
- 3.Askew G R, Doetschman T, Lingrel J B. Site-directed point mutations in embryonic stem cells: a gene-targeting tag-and-exchange strategy. Mol Cell Biol. 1993;13:4115–4124. doi: 10.1128/mcb.13.7.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeuerle P A, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 5.Baeuerle P A, Henkel T. Function and activation of NFκB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 6.Barnes P J, Karin M. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 7.Bergmann M, Hart L, Lindsay M, Barnes P J, Newton R. IκBα degradation and nuclear factor-B DNA binding are insufficient for interleukin-1β and tumor necrosis factor-α-induced B-dependent transcription. J Biol Chem. 1998;273:6607–6610. doi: 10.1074/jbc.273.12.6607. [DOI] [PubMed] [Google Scholar]
- 8.Brenner D A, O’Hara M, Angel P, Chojkier M, Karin M. Prolonged activation of jun and collagenase genes by tumour necrosis factor-alpha. Nature. 1989;337:661–663. doi: 10.1038/337661a0. [DOI] [PubMed] [Google Scholar]
- 9.Briscoe J, Rogers N C, Witthuhn B A, Watling D, Harpur A G, Wilks A F, Stark G R, Ihle J N, Kerr I M. Kinase-negative mutants of JAK1 can sustain interferon-gamma-inducible gene expression but not an antiviral state. EMBO J. 1996;15:799–809. [PMC free article] [PubMed] [Google Scholar]
- 9a.Cao, Z. Unpublished data.
- 10.Cao Z, Henzel W J, Gao X. IRAK: a kinase associated with the interleukin-1 receptor. Science. 1996;271:1126–1131. doi: 10.1126/science.271.5252.1128. [DOI] [PubMed] [Google Scholar]
- 11.Cao Z, Ziong J, Takeuchi M, Kurama T, Goeddel D V. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 12.Cohen L, Henzel W J, Baeuerle P A. IKAP is a scaffold protein of the IκB complex. Nature. 1998;395:292–296. doi: 10.1038/26254. [DOI] [PubMed] [Google Scholar]
- 13.Darnell J E, Jr, Kerr I M, Stark G R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 14.Diaz-Meco M T, Dominguez I, Sanz L, Dent P, Lozano J, Municio M M, Berra E, Hay R T, Sturgill T W, Moscat J. Zeta PKC induces phosphorylation and inactivation of IκB-alpha in vitro. EMBO J. 1994;13:2842–2848. doi: 10.1002/j.1460-2075.1994.tb06578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 16.Dinarello C A. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 17.Duckett C S, Gedrich R W, Gilfillan M C, Thompson C B. Induction of nuclear factor κB by the CD30 receptor is mediated by TRAF1 and TRAF2. Mol Cell Biol. 1997;17:1535–1542. doi: 10.1128/mcb.17.3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gauzzi M C, Velazquez L, McKendry R, Mogensen K E, Fellous M, Pellegrini S. Interferon-α-dependent activation of Tyk2 requires phosphorylation of positive regulatory tyrosines by another kinase. J Biol Chem. 1996;271:20494–20500. doi: 10.1074/jbc.271.34.20494. [DOI] [PubMed] [Google Scholar]
- 19.Genot E M, Parker P J, Cantrell D A. Analysis of the role of protein kinase C-α, -ɛ, and -ζ, in T cell activation. J Biol Chem. 1995;270:9833–9839. doi: 10.1074/jbc.270.17.9833. [DOI] [PubMed] [Google Scholar]
- 20.Gerritsen M E, Williams A J, Neish A S, Moore S, Shi Y, Collins T. CREB-binding protein/p300 are transcriptional coactivators of p65. Proc Natl Acad Sci USA. 1997;94:2927–2932. doi: 10.1073/pnas.94.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenfeder S A, Nunes P, Kwee L, Labow M, Chizzonite R A, Ju G. Molecular cloning and characterization of a second subunit of the interleukin 1 receptor complex. J Biol Chem. 1995;270:13757–13765. doi: 10.1074/jbc.270.23.13757. [DOI] [PubMed] [Google Scholar]
- 22.Hsu H, Shu H B, Pan M G, Goeddel D V. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 23.Hsu H, Solovyev I, Colombero A, Elliott R, Kelley M, Boyle W J. ATAR, a novel tumor necrosis factor receptor family member, signals through TRAF2 and TRAF5. J Biol Chem. 1997;272:13471–13474. doi: 10.1074/jbc.272.21.13471. [DOI] [PubMed] [Google Scholar]
- 24.Huang J, Gao X, Li S, Cao Z. Recruitment of IRAK to the interleukin-1 receptor complex requires interleukin-1 receptor accessory protein. Proc Natl Acad Sci USA. 1997;94:12829–12832. doi: 10.1073/pnas.94.24.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishida T K, Tojo T, Aoki T, Kobayashi N, Ohishi T, Watanabe T, Yamamoto T, Inoue J. TRAF5, a novel tumor necrosis factor receptor-associated factor family protein, mediates CD40 signaling. Proc Natl Acad Sci USA. 1996;93:9437–9442. doi: 10.1073/pnas.93.18.9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janosch P, Schellerer M, Seitz T, Reim P, Eulitz M, Brielmeier M, Kolch W, Sedivy J M, Mischak H. Characterization of IκB kinases. IκB-α is not phosphorylated by Raf-1 or protein kinase C isozymes, but is a casein kinase II substrate. J Biol Chem. 1996;271:13868–13874. doi: 10.1074/jbc.271.23.13868. [DOI] [PubMed] [Google Scholar]
- 27.Kessler D S, Veals S A, Fu X Y, Levy D E. Interferon-α regulated nuclear translocation and DNA-binding affinity of ISGF3, a multimeric transcriptional activator. Genes Dev. 1990;4:1753–1765. doi: 10.1101/gad.4.10.1753. [DOI] [PubMed] [Google Scholar]
- 28.Kojima H, Takeuchi M, Ohta T, Nishida Y, Arai N, Ikeda M, Ikegami H, Kurimoto M. Interleukin-18 activates the IRAK-TRAF6 pathway in mouse EL-4 cells. Biochem Biophys Res Commun. 1998;244:183–186. doi: 10.1006/bbrc.1998.8236. [DOI] [PubMed] [Google Scholar]
- 29.Korherr C, Hofmeister R, Wesche H, Falk W. A critical role for interleukin-1 receptor accessory protein in interleukin-1 signaling. Eur J Immunol. 1997;27:262–267. doi: 10.1002/eji.1830270139. [DOI] [PubMed] [Google Scholar]
- 30.Lange-Carter C A, Pleiman C M, Gardner A M, Blumer K J, Johnson G L. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science. 1993;260:315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- 31.Lee S Y, Lee S, Kandala G, Liou M L, Liou H C, Choi Y. CD30/TNF receptor-associated factor interaction: NF-κB activation and binding specificity. Proc Natl Acad Sci USA. 1996;93:9699–9703. doi: 10.1073/pnas.93.18.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee S Y, Reichlin A, Santana A, Sokol K A, Nussenzweig M C, Choi Y. TRAF2 is essential for JNK for not NF-κB activation and regulates lymphocyte proliferation and survival. Immunity. 1997;7:703–713. doi: 10.1016/s1074-7613(00)80390-8. [DOI] [PubMed] [Google Scholar]
- 33.Levy D E, Kessler D S, Pine R, Darnell J E., Jr Cytoplasmic activation of ISGF3, the positive regulator of interferon-α-stimulated transcription, reconstituted in vitro. Genes Dev. 1989;3:1362–1371. doi: 10.1101/gad.3.9.1362. [DOI] [PubMed] [Google Scholar]
- 34.Ling L, Cao Z, Goeddel D V. NF-κB-inducing kinase activates IKK-α by phosphorylation of Ser-176. Proc Natl Acad Sci USA. 1998;95:3792–3797. doi: 10.1073/pnas.95.7.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Z-G, Hsu H, Goeddel D V, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 36.Lord K A, Hoffman-Liebermann B, Liebermann D A. Nucleotide sequence and expression of a cDNA encoding MyD88, a novel myeloid differentiation primary response gene induced by IL6. Oncogene. 1990;5:1095–1097. [PubMed] [Google Scholar]
- 37.Lovenberg T W, Crowe P D, Liu C, Chalmers D T, Liu X J, Liaw C, Clevenger W, Oltersdorf T, De Souza E B, Maki R A. Cloning of a cDNA encoding a novel interleukin-1 receptor related protein (IL 1R-rp2) J Neuroimmunol. 1996;70:113–122. doi: 10.1016/s0165-5728(96)00047-1. [DOI] [PubMed] [Google Scholar]
- 38.Lozano J, Berra E, Municio M M, Diaz-Meco M T, Dominguez I, Sanz L, Moscat J. Protein kinase C ζ isoform is critical for κB-dependent promoter activation by sphingomyelinase. J Biol Chem. 1994;269:19200–19202. [PubMed] [Google Scholar]
- 39.Lupton S D, Brunton L L, Kalberg V A, Overell R W. Dominant positive and negative selection using a hygromycin phosphotransferase-thymidine kinase fusion gene. Mol Cell Biol. 1991;11:3374–3378. doi: 10.1128/mcb.11.6.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Majumder S, Zhou L Z-H, Chaturvedi R, Babcock G, Aras S, Ransohoff R M. Regulation of human IP-10 gene expression in astrocytoma cells by inflammatory cytokines. J Neurosci Res. 1998;54:169–180. doi: 10.1002/(SICI)1097-4547(19981015)54:2<169::AID-JNR5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 41.Malinin N L, Boldin M P, Kovalenko A V, Wallach D. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 42.Medzhitov R, Preston-Hurlburt P, Janeway C A., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 43.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway C A., Jr MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 44.Mercurio F, Zhu H, Murray B W, Shevchenko A, Bennett B L, Li J, Young D B, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 45.Minden A, Lin A, Claret F-X, Lange-Carter C, Derijard B, Davis R J, Johnson G L, Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 46.Mitcham J L, Parnet P, Bonnert T P, Garka K E, Gerhart M J, Slack J L, Gayle M A, Dower S K, Sims J E. T1/ST2 signaling establishes it as a member of an expanding interleukin-1 receptor family. J Biol Chem. 1996;271:5777–5783. doi: 10.1074/jbc.271.10.5777. [DOI] [PubMed] [Google Scholar]
- 47.Muzio M, Natoli G, Saccani S, Levrero M, Mantovani A. The human toll signaling pathway: divergence of nuclear factor κB and JNK/SAPK activation upstream of tumor necrosis factor receptor-associated factory 6 (TRAF6) J Exp Med. 1998;187:2097–2101. doi: 10.1084/jem.187.12.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muzio M, Ni J, Feng P, Dixit V M. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science. 1997;278:1612–1615. doi: 10.1126/science.278.5343.1612. [DOI] [PubMed] [Google Scholar]
- 49.Nakano H, Oshima H, Chung W, Williams-Abbott L, Ware C F, Yagita H, Okumura K. TRAF5, an activator of NF-κB and putative signal transducer for the lymphotoxin-β receptor. J Biol Chem. 1996;271:14661–14664. doi: 10.1074/jbc.271.25.14661. [DOI] [PubMed] [Google Scholar]
- 50.Nakano H, Shindo M, Sakon S, Nishinaka S, Mihara M, Yagita H, Okumura K. Differential regulation of κB kinase α and β by two upstream kinases, NF-κB inducing kinase and mitogen-activated protein kinase/ERK kinase kinase-1. Proc Natl Acad Sci USA. 1998;95:3537–3542. doi: 10.1073/pnas.95.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Neill L A J. Towards an understanding of the signal transduction pathways for interleukin 1. Biochim Biophys Acta. 1995;1266:31–44. doi: 10.1016/0167-4889(94)00217-3. [DOI] [PubMed] [Google Scholar]
- 52.O’Neill L A J. Molecular mechanisms underlying the actions of the pro-inflammatory cytokine interleukin 1. Biochem Soc Trans. 1997;25:295–302. doi: 10.1042/bst0250295. [DOI] [PubMed] [Google Scholar]
- 53.Parnet P, Garka K E, Bonnert T P, Dower S K, Sims J E. IL-1Rrp is a novel receptor-like molecule similar to the type I interleukin-1 receptor and its homologues T1/ST2 and IL-1R AcP. J Biol Chem. 1996;271:3967–3970. doi: 10.1074/jbc.271.8.3967. [DOI] [PubMed] [Google Scholar]
- 54.Pellegrini S, John J, Shearer M, Kerr I M, Stark G R. Use of a selectable marker regulated by alpha interferon to obtain mutations in the signaling pathway. Mol Cell Biol. 1989;9:4605–4612. doi: 10.1128/mcb.9.11.4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perkins N D, Felzien L K, Betts J C, Leung K, Beach D H, Nabel G J. Regulation of NF-κB by cyclin-dependent kinases associated with the p300 coactivator. Science. 1997;275:523–527. doi: 10.1126/science.275.5299.523. [DOI] [PubMed] [Google Scholar]
- 56.Reddy S A, Huang J H, Liao W S. Phosphatidylinositol 3-kinase in interleukin 1 signaling. J Biol Chem. 1997;272:29167–29173. doi: 10.1074/jbc.272.46.29167. [DOI] [PubMed] [Google Scholar]
- 57.Regnier C H, Song H Y, Gao X, Goeddel D V, Cao Z, Rothe M. Identification and characterization of an IκB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 58.Rothe M, Sarma V, Dixit V M, Goeddel D V. TRAF2-mediated activation of NF-κB by TNF receptor 2 and CD40. Science. 1995;269:1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- 59.Rothwarf D M, Zandi E, Natoli G, Karin M. IKK-γ is an essential regulatory subunit of the IκB kinase complex. Nature. 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 60.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor University Press; 1989. [Google Scholar]
- 61.Schindler U, Baichwal V R. Three NF-κB binding sites in the human E-selectin gene required for maximal tumor necrosis factor alpha-induced expression. Mol Cell Biol. 1994;14:5820–5831. doi: 10.1128/mcb.14.9.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schutze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Kronke M. TNF activated NFκB by phosphatidylcholine-specific phospholipase C-induced “acidic” sphingomyelin breakdown. Cell. 1992;71:765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- 63.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-κB. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 63a.Sizemore, N., S. Leung, and G. R. Stark. Unpublished data.
- 63b.Sizemore, N., and G. R. Stark. Unpublished data.
- 64.Song H Y, Regnier C H, Kirschning C J, Goeddel D V, Rothe M. Tumor necrosis factor (TNF)-mediated kinase cascades: bifurcation of nuclear factor-κB and c-jun N-terminal kinase (JNK-SAPK) pathways at TNF receptor-associated factor 2. Proc Natl Acad Sci USA. 1997;94:9792–9796. doi: 10.1073/pnas.94.18.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stark G R, Kerr I M, Williams B R G, Silverman R H, Schreiber R D. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 66.Thanos D, Maniatis T. NF-κB: a lesson in family values. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 67.Ting A T, Pimentel-Muinos F X, Seed B. RIP mediates tumor necrosis factor receptor 1 activation of NF-κB but not Fas/APO-1-initiated apoptosis. EMBO J. 1996;15:6189–6196. [PMC free article] [PubMed] [Google Scholar]
- 68.Velazquez L, Fellous M, Stark G R, Pellegrini S. A protein tyrosine kinase in the interferon α/β signaling pathway. Cell. 1992;70:313–322. doi: 10.1016/0092-8674(92)90105-l. [DOI] [PubMed] [Google Scholar]
- 69.Verma I M, Stevenson J K, Schwarz E M, Van Antwerp D, Miyamoto S. Rel/NF κB/IκB family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 70.Wesche H, Henzel W J, Shillinglaw W, Li S, Cao Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7:837–847. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- 71.Wesche H, Korherr C, Kracht M, Falk W, Resch K, Martin M U. The interleukin-1 receptor accessory protein (IL-1RAcP) is essential for IL-1-induced activation of interleukin-1 receptor-associated kinase (IRAK) and stress-activated protein kinase (SAP kinases) J Biol Chem. 1997;272:7727–7731. doi: 10.1074/jbc.272.12.7727. [DOI] [PubMed] [Google Scholar]
- 72.Whitley M Z, Thanos D, Read M A, Maniatis T, Collins T. A striking similarity in the organization of the E-selectin and beta interferon gene promoters. Mol Cell Biol. 1994;14:6464–6475. doi: 10.1128/mcb.14.10.6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Woronicz J D, Gao X, Cao Z, Rothe M, Goeddel D V. IκB kinase-β: NF-κB activation and complex formation with IκB kinase-α and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 74.Yamaoka S, Courtois G, Bessia C, Whiteside S T, Weil R, Agou F, Kirk H E, Kay R J, Israel A. Complementation cloning of NEMO, a component of the IκB kinase complex essential for NF-κB activation. Cell. 1998;93:1231–1240. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- 75.Yamin T T, Miller D K. The interleukin-1 receptor-associated kinase is degraded by proteasomes following its phosphorylation. J Biol Chem. 1997;272:21540–21547. doi: 10.1074/jbc.272.34.21540. [DOI] [PubMed] [Google Scholar]
- 76.Yan M, Dai T, Deak J C, Kyriakis J M, Zon L I, Woodgett J R, Templeton D J. Activation of stress-activated protein kinase by MEKK1 phosphorylation of its activator SEK1. Nature. 1994;372:798–800. doi: 10.1038/372798a0. [DOI] [PubMed] [Google Scholar]
- 77.Yanagisawa K, Takagi T, Tsukamoto T, Tetsuka T, Tominaga S. Presence of a novel primary response gene ST2L, encoding a product highly similar to the interleukin 1 receptor type 1. FEBS Lett. 1993;318:83–87. doi: 10.1016/0014-5793(93)81333-u. [DOI] [PubMed] [Google Scholar]
- 78.Zandi E, Rothwarf D M, Delhase M, Hayakawa M, Karin M. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 79.Zhong H, Suyang H, Erdjument-Bromage H, Tempst P, Ghosh S. The transcriptional activity of NFκB is regulated by the IκB-associated PKA subunit through a cyclic AMP-independent mechanism. Cell. 1997;89:413–424. doi: 10.1016/s0092-8674(00)80222-6. [DOI] [PubMed] [Google Scholar]
- 80.Zhong H, Voll R E, Ghosh S. Phosphorylation of NFκB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell. 1998;1:661–671. doi: 10.1016/s1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]
- 81.Zumbansen M, Stoffel W. Tumor necrosis factor alpha activates NF κB in acid sphingomyelinase-deficient mouse embryonic fibroblasts. J Biol Chem. 1997;272:10904–10909. doi: 10.1074/jbc.272.16.10904. [DOI] [PubMed] [Google Scholar]