Abstract
Snakebite incidence at least partly depends on the biology of the snakes involved. However, studies of snake biology have been largely neglected in favour of anthropic factors, with the exception of taxonomy, which has been recognised for some decades to affect the design of antivenoms. Despite this, within-species venom variation and the unpredictability of the correlation with antivenom cross-reactivity has continued to be problematic. Meanwhile, other aspects of snake biology, including behaviour, spatial ecology and activity patterns, distribution, and population demography, which can contribute to snakebite mitigation and prevention, remain underfunded and understudied. Here, we review the literature relevant to these aspects of snakebite and illustrate how demographic, spatial, and behavioural studies can improve our understanding of why snakebites occur and provide evidence for prevention strategies. We identify the large gaps that remain to be filled and urge that, in the future, data and relevant metadata be shared openly via public data repositories so that studies can be properly replicated and data used in future meta-analyses.
Keywords: Snakebite mitigation, Conservation, Ecology, Behaviour, Risk mapping, Snake rescue networks
Graphical abstract
Highlights
-
•
Snakebite is a function of the abundance of both humans and snakes.
-
•
The probability of bites and envenoming resulting from encounters is mediated by snake traits.
-
•
Data on behaviour, activity patterns, distribution, and population demography of most medically important snakes is lacking.
-
•
Funding for studying these aspects of the biological agents of snakebite has lagged far behind that for other aspects.
1. Introduction
Snakebite envenoming is a public health problem that affects more than 2.5 million people globally. It also has significant socio-economic repercussions on vulnerable sectors, since the prevalence of snakebite is the highest in the poorest areas of any given community where snake-human conflict occurs (Mohapatra et al., 2011; Mise et al., 2016; Gutiérrez et al., 2017). The World Health Organization has set the ambitious goal of reducing the incidence of death and disability from snakebite by 50% by 2030. Snakebite prevention is a key component of this strategy and should be given equal if not higher priority than snakebite treatment, and the required research supported accordingly. Snakebite incidence varies on a geographical and temporal scale, resulting from the interaction of anthropic (Harrison et al., 2009; Mise et al., 2016) and environmental (Chaves et al., 2015, Ferreira et al., 2020) drivers. Using a meta-analysis approach, Luiselli et al. (2020) found no difference in the proportion of venomous snake species richness or abundance between tropical and temperate snake assemblages, and, conversely, not all poor rural populations are affected. Higher snakebite incidence observed in many tropical regions is therefore not simply a function of higher snake relative abundance or diversity or high rates of rural poverty, but rather the product of a range of factors acting at the local scale. An excellent example is provided by Udyawer et al., (2021), who highlight peaceful co-existence between highly venomous snakes and people in the marine environment in New Caledonia. Clearly, snakebite is fundamentally a socio-ecological process (Goldstein et al., 2021).
Understanding the distribution of venomous snakes and their impact on health systems, human populations, and the snakebite burden on people at national, regional, and global levels is important for effective reduction and mitigation of snakebite (WHO, 2017). Obtaining distribution data for snakes has become easier over the past few years with the assistance of open-source platforms such as Global Biodiversity Information Framework, iNaturalist, more thorough IUCN Red List assessments, and collaborative efforts such as the Global Atlas of Reptile Distribution project (http://www.gardinitiative.org/; Roll et al., 2017). However, it is equally important to understand the correlation between the various ecological factors driving snake behaviour and activity in relation to snakebite (Murray et al., 2020). This matters especially in that venomous snakes, despite the medical threat posed by some species, are also objects of conservation concern, unlike many anthropophilic invertebrate animal vectors of disease (e.g., Anopheles gambiae, Aedes aegypti mosquitoes).
The WHO has classified venomous snakes into two categories. Category 1 are snakes of highest medical importance and include highly venomous snakes that are common and/or widespread, causing numerous snakebites that result in death or disability (WHO, 2018). Category 2 are secondary medically important snakes that can cause morbidity, death, or disability, but for which exact epidemiological or clinical data may be lacking, and/or are less implicated in snakebite because of their behaviour, activity cycles, remote locations, habitat preferences or small range sizes. Approximately 5.80 billion people live within the range of Category 1 species, while 5.53 billion people live within the range of Category 2 species. The main areas of concern are primarily located in the tropics, particularly in regions where populations are most economically vulnerable, including central and west Africa, South Asia and South America (Longbottom et al., 2018). Longbottom et al. (2018) present a comprehensive global perspective on snakebite risk, assessing vulnerable populations based on their overlap with venomous snake species and their access to healthcare. Nevertheless, consideration of the behavioural and ecological traits of species that contribute to the likelihood of different snakes delivering envenoming bites remains missing from this, and most other, global burden/risk assessments.
It is evident that much of the work on medically important snakes across the globe has focused on understanding their venom (Oh et al., 2017; Williams et al., 2011; Bittenbinder et al., 2018; Harris et al., 2020). While several ecological studies have been conducted on North American and European vipers and some Bothrops species (Martins et al., 2001; Monteiro et al., 2006; Bisneto and Kaefer, 2019), similar studies on venomous snakes in Africa and Asia are limited. For example, ecological studies on Bungarus species in Asia are few (Pandey et al., 2020; Goldstein et al., 2021). There are also gaps in our knowledge of how the behavioural and ecological characteristics of snakes may vary within a species across their ranges spanning several regions (e.g., southern Africa vs. central Africa).
In this paper, we review the existing literature to highlight that while a greater understanding of snake taxonomy is essential for determining the distribution and identity of medically significant species (Wüster and Thorpe, 1991; Wüster, 1996), it has largely failed to deliver the initial expectation of serving as a guideline for antivenom design. Instead, the large gaps in our understanding of venomous snake ecology (including demography, activity patterns and behaviour) need to be addressed, and crucially need more funding directed towards this area of research.
2. Can snake biology predict venom variation and antivenom neutralisation?
Due to their complexity, snake venoms are almost infinitely variable. This variation is one of the key obstacles to the design of universal, or at least broad-spectrum, treatments for snakebite envenoming (Gutiérrez et al., 2017; Casewell et al., 2020), which has led to increasing research interest in the causes and mechanisms of venom evolution. While it was once believed that a better understanding of taxonomy would serve as a roadmap to understanding venom variation (Wüster et al., 1997), it has become clear that the causes of venom variation are a good deal more complex. Broadly, they can be subdivided into selectively neutral and selection-driven categories. Selectively neutral drivers of variation primarily include evolutionary divergence (most basically, taxonomic affinities) and, intraspecifically, gene flow, whereas potential selective pressures primarily include optimisation of venom to diet, or for defence. Co-evolution between venomous snake species and their prey, either through arms races or phenotype matching (Holding et al., 2016) potentially generates a great deal of diversity at small geographical scales.
A few instances of neutral factors explaining the observed variability in venom composition have emerged (Williams et al., 1988; Lomonte et al., 2014; Margres et al., 2019). However, most studies comparing the impact of neutral and selective drivers of venom evolution have found little evidence for neutral processes. Snakes use their venoms primarily in a foraging role, to immobilise prey prior to ingestion. Numerous case studies have demonstrated correlations between venom composition and diet (Daltry et al., 1996), prey-specific venom lethality (e.g., Jorge da Silva and Aird, 2001; Barlow et al., 2009; Gibbs and Mackessy, 2009; Holding et al., 2016), or prey-specific toxins (e.g., Pawlak et al., 2009; Modahl et al., 2018). Over the last few years, case studies have been joined by meta-analyses seeking to discern broad patterns of association between ecological traits, phylogeny and aspects of venom phenotype (Davies and Arbuckle, 2019; Healy et al., 2019; Lyons et al., 2020; Holding et al., 2021). While this approach appears highly attractive, the underlying biological data on snake ecology are often weak. Detailed information on natural diet is available for few species (Glaudas et al., 2019), patterns of prey resistance to venoms are rarely known, even though this may be a common phenomenon (Arbuckle et al., 2017), and lethality data from anything other than natural prey might be highly unrepresentative (Richards et al., 2012; Smiley-Walters et al., 2018). In addition, by usually treating species as single data points, much intraspecific variation is missed. Consequently, the results of meta-analyses are best treated with caution. New technologies, such as assays that can detect prey-specific binding of toxins to the target receptors of different species, promise to further enhance our ability to investigate the interactions between venom composition and diet (Zdenek et al., 2019).
Besides foraging, snakes also use venom in defence against potential predators. However, few studies have addressed the role of defence in driving venom evolution. Mathematical modelling (Gangur et al., 2018) suggests that the presence of predators could result in the evolution of greater lethal potential in a venomous mesopredator. The detection of specific pain-causing toxins in a few snakes (Bohlen et al., 2011; Zhang et al., 2017) suggests selection for defence in some species, but a survey of herpetologists envenomed by a wide variety of caenophidians provided no evidence of the pattern of widespread, rapid-onset pain predicted by selection for defence models (Ward-Smith et al., 2020). Nonetheless, in spitting cobras, the three instances of evolution of defensive spitting were accompanied by identical shifts in venom composition and increased algesic activity (Kazandjian et al., 2021).
An improved understanding of the selective pressures driving venom evolution requires, above all else, more detailed data on snake diet, predators, the outcomes of encounters and their determinants, and details of the role of venom in those encounters (Whitford et al., 2019). Citizen scientists can potentially contribute precious data on rarely observed phenomena, such as these, that are not amenable to targeted study (Maritz and Maritz, 2020).
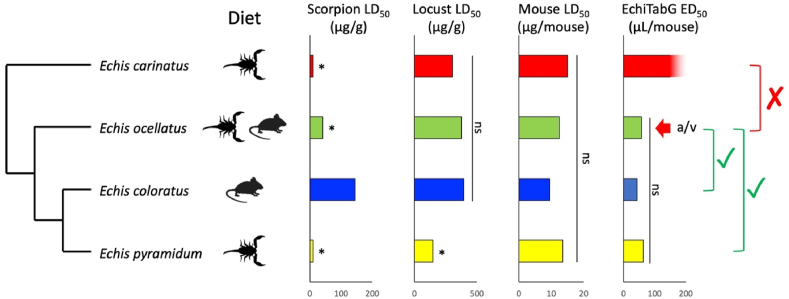
What do these results tell us about the predictability of snakebite symptoms and treatment? In some instances, phylogenetic relationships can successfully predict clinical syndromes (e.g., Lesser Antillean Bothrops; Wüster et al., [2002]). In other cases, clinically relevant geographic variation in venom composition exists within species, independent of phylogeny (e.g., Thorpe et al., 2007; Oh et al., 2021) and even in the face of continuing gene flow (e.g., Zancolli et al., 2019). As a result, the ability of antivenoms to neutralise different venoms can vary in unpredictable ways at all taxonomic levels (Williams et al., 2011). At the individual level, ontogenetic variation in the venom of Crotalus simus, resulting from selective translation from similar transcriptomes, affects the ability of antivenoms to neutralise juvenile and adult venoms of the same snakes (Saravia et al., 2002; Durban et al., 2013). Intraspecific geographic variation hinders antivenom effectiveness in a number of examples in elapids (e.g., Senji Laxme et al., 2021), Old World vipers (e.g., Rogalski et al., 2017; Pla et al., 2019) and pitvipers (e.g., Sousa et al., 2018). In contrast, some antivenoms display remarkable paraspecific activities against some venom, such as cross-neutralisation of procoagulant venom activities between vipers and some colubrines (Ainsworth et al., 2018). The complexity of the relationship between venom activities specific to different prey and the treatment of snakebite is illustrated by the genus Echis (Fig. 1): profound interspecific differences in lethality to natural scorpion prey (Barlow et al., 2009) are only weakly reflected in a convenient model laboratory arthropod (Richards et al., 2012), and the same species do not differ in their lethality to mice (Casewell et al., 2014). Despite this, the ability of an antivenom raised against Echis ocellatus to neutralise the venoms of other Echis was best predicted by phylogeny, not diet or prey-specific lethality (Casewell et al., 2014). For public health policy and the formulation of antivenom strategies, the frequency of selection-driven venom variation, and the often poor correlation between taxonomy, selection and antivenom cross-reactivity suggest that taxonomy and ecological data do not provide reliable short-cuts to antivenom formulation and distribution. Rather, antivenoms need to be tested against venoms from a variety of provenances for each medically relevant species. The unpredictability of antivenom cross-reactivity emphasises the need for novel approaches to snakebite treatment that circumvent the constraints of venom specificity that bedevil conventional antivenoms, such as novel, toxin-specific antibodies (de la Rosa et al., 2019; Ratanabanangkoon et al., 2020), repurposed small-molecule treatments (e.g., Albulescu et al., 2020) and other technological developments (e.g., Knudsen et al., 2019). In conclusion, phylogenetic affinities and taxonomy are most usefully interpreted as initial roadmaps for research, and as null hypotheses when investigating the causes and drivers of venom variation, but not as definitive guides for snakebite treatment strategies.
Fig. 1.
The complex interplay of phylogeny, diet and other factors in shaping the prey-specific lethality of Echis venoms and the cross-reactivity of antivenom. Viper diet predicts specific venom lethality to natural prey (scorpions), but this is poorly replicated in comparable assays run on a convenient model arthropod, and the species do not differ significantly in their mouse lethality. The cross-neutralisation capability of EchiTabG, raised against E. ocellatus (red arrow) is predicted by phylogeny but not diet or any prey-specific lethality. The ED50 for E. carinatus was above the maximum test threshold of 150 μL/mouse. Redrawn from Casewell et al. (2014). * = significantly (p<0.5) more toxic than next most toxic venom; ns = non-significant.
3. Snake population ecology is vital to an understanding of snakebite
The presence and abundance of snakes influences the probability of encountering a venomous snake and consequently, of envenoming. Human population density and poverty are well-known correlates of snakebite, which could be mechanistically linked with incidence by a large number of more specific mechanisms (e.g., education, PPE, occupation, housing quality and so on). However, we lack a concrete mechanistic understanding of the relative contributions of these varying factors to the overall burden of snakebite and how they interact with the physical presence and abundance of snakes. Nevertheless, more mechanistic approaches to understanding snakebite are beginning to emerge. For example, snakebite incidence can theoretically be modelled using snake population parameters, and the predictive ability of these models could highlight areas that require more attention from health authorities. A previous study suggested that snakebite incidence can be inferred using compartmental modelling (Bravo-Vega et al., 2019), as it is commonly done with infectious diseases (Siettos and Russo, 2013; Luz et al., 2010). In contrast to classical statistical analyses, epidemiological models rely on the processes underlying the interactions between the populations involved in disease dynamics. Thus, the model applied to snakebite is:
| (1) |
Where is the conditional probability that snakebite occurs after an encounter, is the contact rate between human population density () and venomous snake population density (). Subindex i denotes the geographic unit in which the model is going to be applied. If population density is not available, we can modify equation (1) into the following expression:
| (2) |
Here, denotes the encounter frequency between humans and venomous snakes, a parameter that is easier to measure in the field by assessing snake frequency per search effort.
This model was validated in Costa Rica, using the encounter rate of venomous snakes measured in the field and estimates of the human population. When compared with reported snakebite incidence (Bravo-Vega et al., 2019), the model showed good potential as an estimator by successfully capturing geographic variation in incidence. These models could be applied in regions with inadequate epidemiological surveillance of snakebite (see Fig. 1 in Kasturiratne et al., 2008). They can also estimate underreporting (Tchoffo et al., 2019) by comparing the model's estimated results with the official snakebite records. However, these applications require reliable estimates of population density or encounter rates for snakes, which requires field surveys.
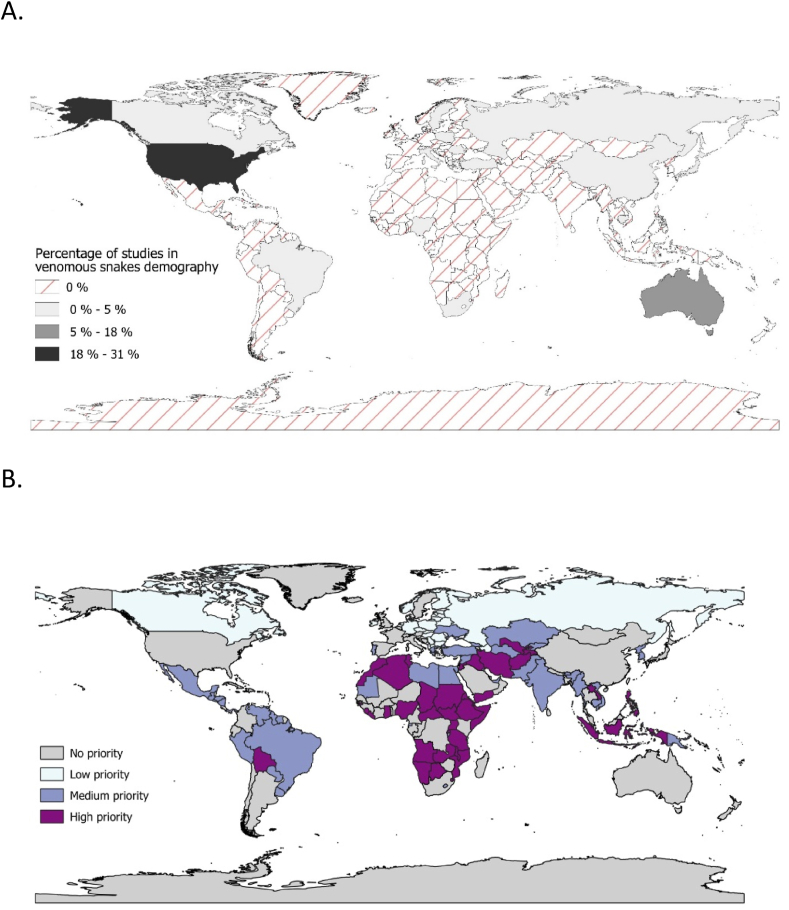
To determine the availability of information on snake demographics and identify knowledge gaps, we reviewed references in PubMed and Science Direct, using population density OR population size AND snake as search terms. We also retrieved data available in the TetraDensity dataset (Santini et al., 2018). Our search resulted in 236 entries from 106 studies reporting one of the following parameters: population size, population density (per area, linear kilometre, or effort unit). We found that capture-recapture methods dominate the investigations although other approaches are also employed (Supplementary Table 1). Regions with most information about snake demography do not match those with the highest snakebite risk (Fig. 2A).
Fig. 2.
A. Geographical distribution of the number of reports of the demography of venomous snakes. The scale represents the percentage of demography reports found for all venomous snakes. Countries that report the most data are the United States of America, Australia and Costa Rica. B. Priority regions for studies of venomous snake demography and snakebite incidence modelling. The scale represents the priority level based on the per-country diversity of venomous species, the percentage of these with demographic coverage, and the unavailability of snakebite data.
Out of 107 snake species representing eleven families, only 9 elapids and 20 vipers of medical importance were represented, less than a third of species with available estimates of population density, likely reflecting a bias towards abundant species in selection of focal species. Of these, eleven species (Agkistrodon contortrix, A. piscivorus, Crotalus adamanteus, C. horridus, C. oreganus, C. viridis, Bitis gabonica, B. nasicornis, Bothrops asper, Notechis scutatus and N. ater) are included in WHO's list of venomous snakes of greatest medical importance (WHO, 2018). Estimates of population size were included in 25% of studies, whereas density estimates appear in 73%. The highest values for population size were reported for aquatic snakes, or for species inhabiting islands, such as the vipers Gloydius shedaoensis on She Dao, China, and Macrovipera schweizeri on Milos, Greece. Close to 18% of the reported species showed densities that we have arbitrarily classed as very high (D> 500 ind/ha) or high (100 <D <500 ind/ha). This group comprises primarily aquatic species, most of them piscivorous, but also some semi-fossorial species. Only one venomous species, Gloydius shedaoensis, is included in this group. This species' abundance probably relates to the availability of food during the spring and autumn bird migrations, and the lack of predators on islands (Li, 1995; Lillywhite and Martins, 2019).
Another 12 species, again mostly aquatic or insular, in the reviewed studies can be considered abundant (defined here as 20 < D < 100 ind/ha). Several venomous species on islands stand out in this category, such as Bothrops insularis (from the Brazilian Quemada Grande), Agkistrodon piscivorus (from the Cedar Keys in Florida), and Notechis scutulatus (Carnac Island, Australia). As is the case with G. shedaoensis, these species inhabit islands with no human populations, thus their contribution to the regional burden of snakebite is negligible. On the other hand, more than half of the studies examined correspond to species with moderate (1 < 20 ind/ha) and low densities (<1 ind/ha). This last category includes terrestrial and arboreal snakes with larger body mass, among them large constrictors (Pythonidae and Boidae), racers and kingsnakes (Colubridae), and the larger vipers on the list of medically important species (Bitis gabonica, Crotalus adamanteus, C. horridus, and Bothrops asper).
Only 16% of studies examined report encounters per unit of effort, a relatively low percentage considering that effort-corrected estimates are more useful than uncorrected ones (Dorcas and Willson, 2009). Using this indicator, the most abundant snakes sampled (>1 ind/hour) are again aquatic, including some marine species. The venomous rattlesnake Crotalus viridis is also included in this group; however, the rest of the venomous species with available information showed very low encounter rates (Supplementary Table 1). It should be noted that sighting rates do not necessarily correlate well with estimates of actual population density in snakes (Rodda, 2012; Boback et al., 2020), and accurate estimates of population parameters such as density often require a large investment of time and resources and an understanding of the biases of the methods used (Dorcas and Willson, 2009; Rodda, 2012).
From the surveys reviewed here, it appears that continental populations of medically important venomous snakes occur at lower densities than those exhibited by venomous species in islands or by several common species of non-venomous snakes. This in turn reinforces the notion that snakebite results from the interaction of density with other factors. Efforts must be made in order to establish these demographic parameters for most venomous species, allowing not only greater insight into venomous snake ecology, but also potentially permitting some inference of envenoming incidence in areas where other information is unavailable. We developed an index (see Supplementary Methods) to identify countries where this approach (Bravo-Vega et al., 2019) would be most valuable, which takes into account the number of snake species classified as “greatest threat to public health” (Categories 1 and 2), the demographic information available for them, and the quality of available data that reports snakebite incidence (Kasturiratne et al., 2008). Using this index, we can highlight countries with available information about snake demography but with unreliable estimates of snakebite incidence. We were able to identify Bolivia, the Indonesian archipelago, and several countries in central Asia and central and east African regions as priorities for this kind of intervention (Fig. 2B).
4. Radio-telemetry studies can improve our understanding of why snakebites occur and provide evidence for prevention strategies
In-situ study of snakes is generally difficult because of their highly cryptic nature, tendency to move infrequently, use of inaccessible microhabitats, and, as mentioned in Section 3, often relatively low population densities (Dorcas and Willson, 2009; Steen, 2010). Radio-telemetry provides a method for relocating individual snakes in the field (Reinert and Cundall, 1982; Újvári and Korsós, 2000; Boback et al., 2020). As well as a few other methods, such as use of harmonic tags and satellite tracking that generally have more limited applications (e.g. Gourret et al., 2011; Sperry et al., 2013; Robinson et al., 2021; Whitney et al., 2021), this improves our understanding of spatial ecology and resource requirements (Macartney et al., 1988; Ettling et al., 2016; Lomas et al., 2019), movement patterns (Shipley et al., 2013; Marshall et al., 2020), temporal activity patterns (Whitaker and Shine, 2002; DeGregorio et al., 2018; Siers et al., 2018), habitat selection (Moore and Gillingham, 2006; Sutton et al., 2017; Shelton et al., 2020), behaviour (Clark, 2005; Putman et al., 2016), physiology (Holding et al., 2014; Capehart et al., 2016), and natural history (Sasa et al., 2009; Devan-Song et al., 2017). All these factors can help provide important insight into how human-snake conflicts arise and thus how to prevent them. Additionally, radio-telemetry can be used to evaluate snake-human interactions (Whitaker and Shine, 1999; Glaudas, 2021), including conflict mitigation and prevention techniques (Devan-Song et al., 2016; Maida et al., 2020).
We carried out a systematic online structured review of the published literature on venomous snake radio-telemetry studies, supplemented with relevant publications from the dataset used by Crane et al. (2021). We examined a total of 101 published studies from the past 20 years (see Supplementary Information for sampling methods and the final dataset). We found that the majority (70.3%) of the studies are carried out in developed countries where snakebite incidence and mortality is relatively low (Table 1), most notably the United States (n=53), Australia (n=11), and Canada (n=4), although there were exceptions among a few developing countries (e.g., Thailand n=9, South Africa n=7). Only 17 of the 101 studies (16.8%) occurred within geographic regions where snakebite incidence and mortality is high (Swaroop and Grab, 1954; Chippaux, 1998; Kasturiratne et al., 2008). Furthermore, only 36 of the publications appeared to have had study sites which at least partially included human-modified areas (such as agriculture or settlements), whereas the remaining 65 studies appeared to have taken place completely within natural landscapes (often large protected areas) with apparently little or no human activity. As a result, only 23 of the 101 examined studies (22.8%) made any mention of snake-human interactions, and only 9 attempted to apply their findings to snakebite or conflict prevention.
Table 1.
Demographic data and characteristics of the 101 examined published studies on terrestrial venomous snakes which used radio-telemetry. High and low snakebite incidence regions were determined using estimates by Kasturiratne et al. (2008).
| Characteristics | Number (%) |
|---|---|
| Taxa | |
| Viperidae | 84 (83.17%) |
| Elapidae | 17 (16.83%) |
| Low snakebite incidence regions | |
| Australia | 11(10.89%) |
| North America | 57 (56.44%) |
| Europe | 3 (2.97%) |
| East Asia | 4 (3.96%) |
| West Asia | 2 (1.98%) |
| Southern Africa | 7 (6.93%, all South Africa) |
| LIR Total | 84 (83.17%) |
| High snakebite incidence regions | |
| Central America | 4 (3.96%) |
| Tropical South America | 2 (1.98%) |
| Southeast Asia | 9 (8.90%, all Thailand) |
| South Asia | 1 (0.99%) |
| Sub-Saharan Africa | 1 (0.99%) |
| HIR Total | 17 (16.83%) |
| Study site landscape | |
| Completely natural landscape | 65 (64.36%) |
| Some human-modified areas | 36 (35.64%) |
| Mention of shared space with humans | 23 (22.77%) |
| Suggestions made | |
| For snakebite/conflict prevention (explicit) | 9 (8.91%) |
| Increased community education | 7 (6.93%) |
| Further research to promote coexistence | 12 (11.88%) |
Most of the studies that examine snake-human interactions evaluate snake translocation, a commonly used technique for conflict mitigation, where the “nuisance” snake is relocated to a new location, away from people. These studies are extremely valuable, and have demonstrated that translocation of snakes over great distances (where the snake is removed from its home range) results in many issues, including decreased fitness and higher mortality (Nowak et al., 2002; Butler et al., 2005; Sullivan et al., 2015; Devan-Song et al., 2016; Wolfe et al., 2018). In contrast, short-distance translocations (where the individual is moved to a new location within its home range) appear to result in fewer issues, and have been suggested as a viable conflict mitigation technique (Hardy et al., 2001; Brown et al., 2009; Heiken et al., 2016), which, under certain circumstances, might be able to reduce chances of snakebites in the short term (Brown et al., 2009). This highlights the importance of general spatial ecology studies which improve our understanding of the spatial requirements of a species, thus helping inform management regarding suitable translocation distances. However, short-distance translocations only provide short-term solutions, as the relocated individuals are known to sometimes return to their original capture locations (see Box A) or other nearby residential areas (Hardy et al., 2001; Butler et al., 2005; Sullivan et al., 2015; Hodges et al., 2021), emphasising the need for increased education and other conflict prevention methods.
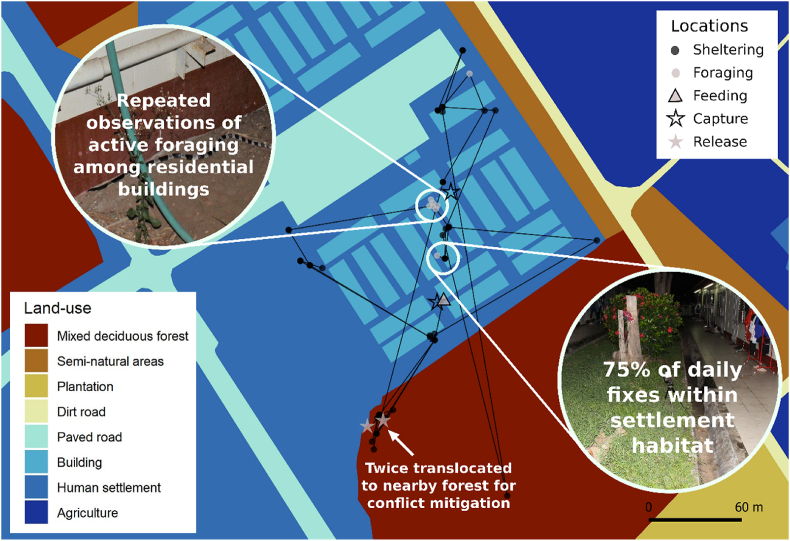
BoxA. Spatial telemetry studies can guide the most suitable mitigation measures: a case study of a Malayan krait in Thailand.
A recent study (Hodges et al., 2021) examining the movements and behaviour of a telemetered focal Malayan krait (Bungarus candidus) living among a university dormitory complex alongside 466 students for just over 100 days revealed that the focal snake was located within settlement habitat on 75 of the 100 daily location checks despite being translocated to a nearby forest fragment after students encountered the snake among their rooms on two occasions. The snake was also observed to commonly forage among the residential buildings and sidewalks shortly after dusk. These findings provide a better understanding of why bites by kraits (Bungarus spp.) commonly occur inside households (Kularatne, 2002; Chappuis et al., 2007; Warrell, 2010; Tongpoo et al., 2018) and highlight the need to build awareness among communities in order to inform people about the potential dangers and the need to practice appropriate prevention measures (Chappuis et al., 2007; Hodges et al., 2021).
Alt-text: BoxA
Some radio-telemetry studies provide important insight into snake-human interactions, demonstrating the true nature, and often mild dispositions, of venomous species (Whitaker and Shine, 2000; Andrews and Gibbons, 2005; Sasa et al., 2009; Wasko and Sasa, 2009). For example, Whitaker and Shine (1999) demonstrated that Australian brown snakes (Pseudonaja textilis) generally avoided encounters with people by fleeing from approaching humans, often evading the detection of the researchers entirely. Their findings also suggested that the shade of clothing worn by observers impacted encounter rates with brown snakes. This was translated into specific suggestions for reducing potentially dangerous encounters with brown snakes as wearing dark-coloured clothing, which contrasts with the sky and surroundings, may alert the snakes to approaching humans, and provides ample opportunity for the snakes to move away before a conflict arises. Alternatively, some species have been found to rely on other avoidance strategies, such as crypsis (Andrews and Gibbons, 2005; Sasa et al., 2009; Wasko and Sasa, 2009), requiring awareness of surroundings to minimize the chances of people stepping on snakes.
Despite the clear potential for radio-telemetry studies to evaluate conflict prevention strategies, few studies have attempted to do so thus far. Some spatial ecology studies ascertain temporal activity patterns of potentially dangerous snakes, which can identify specific periods of time when people may be most at risk of encountering venomous snakes (Whitaker and Shine, 2002; Waldron et al., 2013). Other studies improve our knowledge of species habitat use and selection, which under certain circumstances may help reduce frequency of encounters by either keeping humans away from areas “preferred” by snakes (Fraga et al., 2013), or by altering habitats to deter snakes from areas where humans are active (Carter et al., 2014). Some attempts have been made to assess the effectiveness of linear barriers (fence-lines) in deterring telemetered rattlesnakes from entering developed areas (Colley et al., 2017; Maida et al., 2020; Laidig and Golden, 2004). Such barriers can be costly and difficult to implement and maintain effectively (Laidig and Golden, 2004), with examples of snakes finding openings in fencing and subsequently becoming stuck within these “undesirable” areas (Baxter-Gilbert et al., 2015). There is thus a great need to evaluate conflict prevention strategies and to examine the impacts such measures may have on other local wildlife species.
Though important, spatial ecology and other systematic field studies using radio-telemetry can be particularly complex and costly. Space use, temporal activity patterns, habitat use, and behaviour of a species vary geographically, seasonally, ontogenetically, sexually, and between individuals, requiring a large sample of individuals to be examined across multiple seasons in order to reach clear conclusions. In addition, results from even thorough studies are generally limited to the population and demographics examined, therefore it is imperative that multiple studies contribute to a broader understanding of any species (Nichols et al., 2019). While studies which implement statistics with robust samples are ideal, smaller focal animal studies are more feasible for gaining higher resolution data and can still provide valuable insight and preliminary information (e.g. Barve et al., 2013; Knierim et al., 2019; Hodges et al., 2021), especially when data are shared via public data repositories (Marshall and Strine, 2021).
5. The overlap between snake and human activities is context-dependent
Snake behaviour is a crucial part of why snakes bite. For example, Boomslangs (Dispholidus typus) have powerfully haemotoxic venom and are more frequently encountered within their range than potently neurotoxic black mambas (Dendroaspis polylepis) (e.g., 491 versus 127 iNaturalist records as of 28 June 2021). Generally shy and unobtrusive (Marais, 2011) despite their broad distribution in sub-Saharan Africa only eight deaths due to Boomslang envenomation have been recorded since 1957 in South Africa (Krüger and Lemke, 2019). Black mambas, with a similarly broad distribution, account for far more deaths from bites (Ochola et al., 2019; Laustsen et al., 2015). However, the numbers of cases of bites from black mambas also vary across Africa (Erulu et al., 2018) and the driving factors behind this are unclear. As well as variation in human and snake population densities, rates of reporting, and habitat and activity patterns, variation in snake defensiveness may also occur. Controlled experiments studying reactions of vipers to being stepped on show that they are unlikely to bite (Gibbons and Dorcas, 2002; Glaudas et al., 2005; Adams et al., 2020); however, similar studies are lacking for elapids and other groups. Future studies could focus on how existing knowledge on populations vulnerable to snakebite (Longbottom et al., 2018) relate to the behaviour of venomous snakes present in the area.
Goldstein et al. (2021) used agent-based models to integrate human behaviour and snake ecology in high-risk landscapes, using Sri Lanka as a case study and demonstrating the importance of focusing on locally-specific factors. Other important factors to consider include habitat transformation due to climate change (Zacarias and Loyola, 2019), agricultural expansion (Akani et al., 2008, 2018) and urbanisation (Lowry et al., 2013; Devan-Song et al., 2017), which may have either positive or negative impacts on the distribution, movement and behaviour of venomous snakes, and which will ultimately impact snakebite risk (Yañez-Arenas et al., 2016). Thus, by contextualising contemporary knowledge about snake distributions and ecology, we can put better mitigation measures in place to avoid bites, cases in the first place, Moreover, it is important to also understand the beneficial roles that snakes might play in controlling the spread of other diseases (Hafidzi and Saayon, 2001), and this may best be accomplished within a One Health approach (Babo Martins et al., 2019).
A crucial aspect of understanding snakebite is the overlap between snake activities and human activities (Longkumer et al., 2016; Ediriweera et al., 2018; Glaudas, 2021; Goldstein et al., 2021). However, very few studies have looked at both snake ecology and distribution to assess the risk factors (Akani et al., 2013; Nori et al., 2014; Yañez-Arenas et al., 2014; Yañez-Arenas et al., 2016; Angarita-Gerlein et al., 2017). Along with the absence of distributional data, there is a paucity of data regarding where the conflict occurs within the confines of rural landscapes (but see Box B). Most studies that have developed methodology to estimate high risk areas of human-snake conflict do not use distributional information of the snakes (Leynaud and Reati, 2009; Akani et al., 2013), or do not analyse this at an appropriate scale (Hansson et al., 2013). More recent studies that have incorporated snake distributional data have not used data collected in the field (Akani et al., 2013; Nori et al., 2014; Goldstein et al., 2021).
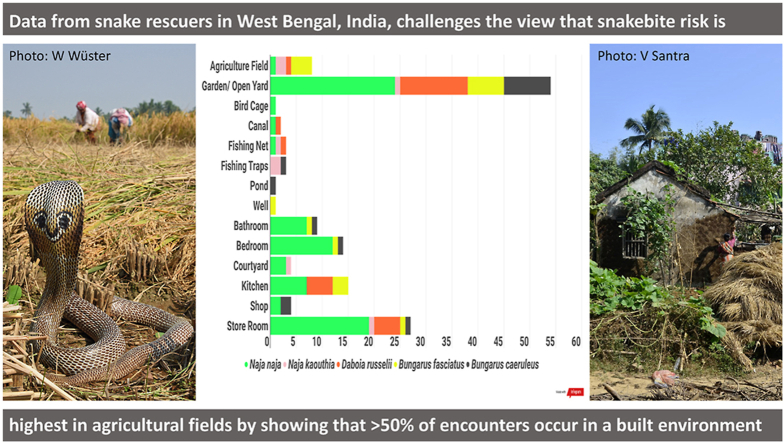
BoxB. Utilizing Snake Rescue Networks: a case study from Hooghly District, West Bengal, India.
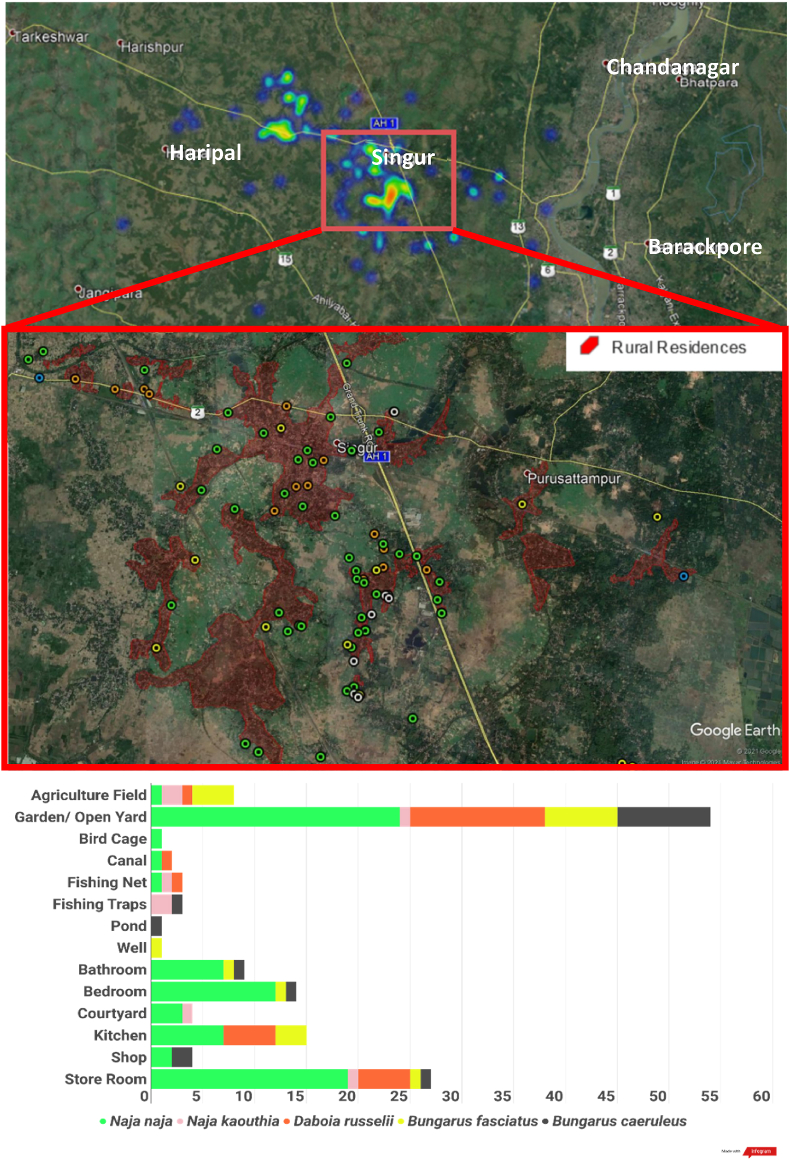
This study was conducted in the district of Hooghly, West Bengal, by a licensed local conservation not-for-profit organisation. Hooghly district lies along the south-eastern margin of the Hooghly River and is part of the lower Gangetic Delta (Patra et al., 2018), with a mainly rural population engaged primarily in agriculture as the main source of livelihood. The village residences form ‘islands’ surrounded by medium to large tracts of agricultural fields that grow a variety of seasonal crops including rice, corn, jute, bamboo, and various vegetables (Kelly, 1981). Venomous snakes present include Naja naja, Naja kaouthia, Daboia russelii and Bungarus caeruleus. The banded krait, Bungarus fasciatus, is also commonly found in human settlements but there are no records or anecdotal reports of bites from this species. Between July 2020 and February 2021, 210 rescues were completed (see Supplementary Methods for details), of which 61 were non-venomous snakes and 147 were venomous snakes. The most rescues were conducted in August (n=49) and the fewest in January (n=3), although N. naja, N. kaouthia and D. russelii were rescued the most during October and B. caeruleus in September. Most rescues happened between 9 p.m. and midnight and snakes were most frequently rescued from residential gardens/open yards (37%), storerooms (18.5%), kitchens (10.3%), bedrooms (9.6%), bathrooms (6.2%) and agricultural fields (5.5%), with all other habitats representing under 5% of all rescues. 50% of the rescues were made inside buildings. A drastic increase in snake rescues, including non-venomous species, occurs during the Indian summer monsoon (June to September), with a decrease in winter (December to February). Crops such as rice, maize, groundnut, sesame, and various gourds are harvested in October and November, which matches with peak rescue calls for D. russelii. While these patterns of human-snake interaction agree with previous studies and reviews of snakebite in the region (Alirol et al., 2010; Warrell, 2010; Vaiyapuri et al., 2013; Ghosh et al., 2016), they deviate from these in that 87% of the recorded interactions occurs in residential areas (indicated in red on the inset map below), including open spaces such as gardens and yards within these areas, which further illustrates that human-snake conflict arises out of a combination of snake distribution and human behaviour (Goldstein et al., 2021). The current study, while limited by the finances and manpower of the rescue network, has yielded a significant amount of data in a short time that visualizes aspects of the conflict such as the species diversity of the region, habitat choices of various snake species, seasonal activity pattern changes, and potential for human-snake conflict.
Alt-text: BoxB
Taking India as an example, considering the limitations of the polyvalent antivenom made for treating snakebite (Kochar et al., 2007; Kumar and Sabitha, 2011; Togridou et al., 2019), and the lack of access to treatment facilities in several regions of the country (Suchithra et al., 2008; Mohapatra et al., 2011; Gutiérrez et al., 2017), there is a need to improve knowledge of the distribution of venomous snakes in anthropic habitats. Snake rescue networks can potentially provide a large amount of data due to the number of individual snake-human encounters involved (Fearn et al., 2001; Pyke and Szabo, 2018). Each rescued animal provides data on species, sex, age (within broad categories), size at a particular location, date, and time when a potential human-snake conflict occurred (Koenig et al., 2002; Shine and Koenig, 2001). In India, snake rescue networks are active across various states, including Assam (Soud, 2010), Gujarat (Vyas, 2013), Kerala (Roshnath, 2017), Madhya Pradesh (Husain, 2006) and Tamil Nadu (Janani et al., 2016). Research that uses data collected from rescues (see Box B) is rarely used to create conflict mitigation strategies (Lunney et al., 2007; Thomas et al., 2013); however, as demonstrated here, such data can help in visualizing crucial aspects of the potential for snakebite conflict, such as the anthropic habitat choices of various snake species, as well as seasonal use of these habitats and potentially seasonal encounter rate changes. Such programs could also assist in obtaining relevant samples for phylogenetic, population genetic, and venomic studies. Rescue networks are also vital to the mitigation of snakebite since they are in direct contact with the stakeholders who are most affected by snakebite conflict after a rescue, and can communicate information about the important ecological roles of snakes, how to avoid future conflict and what to do in case of a snakebite accident. While most such networks are voluntary and may involve a variable degree of training for the role, in some cases these are being formalised (Box C) with many additional benefits, including ensuring the legality of the activities.
BoxC. Establishing a centralized and technology-supported Human-Snake Conflict Mitigation Network: A case study from Kerala, India.
The Government of Kerala recognizes snakebite as one of the major causes of human-wildlife conflict, and provides the kin of snakebite victims with monetary compensation. The health infrastructure in Kerala State is rated as the best across the country (Healthy States Progressive India, 2019) and free treatment for snakebite is available at sub-district level government hospitals. The state supports widespread populations of the “Big 4” venomous snakes (Naja naja, Daboia russelii, Bungarus caeruleus, Echis carinatus) as well as other potentially significant venomous snakes due to suitable climatic conditions, and as 69.5% of the land is utilized for farming purposes (with rice, coconut, rubber, cocoa, cardamom, tea, coffee and other spices being the major crops), human-snake interaction is very common. In 2019, a team of experts was constituted, under the leadership of the State Chief Wildlife Warden, to examine human-snake conflict and find solutions to reduce risk of snakebite across the State. They proposed a statewide Human- Snake Conflict Mitigation Team comprising Forest Department personnel as well as trained and certified volunteers assigned to deal with human-snake conflict situations and to educate communities about snakes and snakebite. This was subsequently adopted by the Kerala State Forest Department and initiated in July 2020. The establishment of the Kerala State-level Snake Rescue Network, involving Government Officials and volunteers from General Public, is the first of its kind in India. The Centralized Information Management and Monitoring mechanism makes it efficient to monitor, and the data collected from public users and rescuers will provide accurate information about human-snake conflict across the state. This system is conceptualized as a replicable model suitable for implementation in other regions, with appropriate customization, that will significantly aid the reduction of the occurrence of snakebite. Additional advantages of the active recruitment and management of trained rescuers include the prevention of illegal activities involving snakes, including sale of the animals and their venom.
Alt-text: BoxC
6. Promoting co-existence with venomous snakes: evaluating mitigation methods for human-snake conflict
Human-Snake Conflict (HSC) includes not just mortality and morbidity of humans but also mortality of livestock and domestic animals due to snakebites, as well as mortality of snakes (many of them non-venomous) that are killed in retaliation and/or out of fear. While many studies have looked at the problem of this Neglected Tropical Disease, there are very few published studies which have looked at solutions, specifically in terms of outreach and raising awareness (Roshnath and Divakar, 2019).
Again, using India as a globally significant example (accounting for over 50% of global snakebite deaths; Suraweera et al., 2020), the National Snakebite Management guidelines in India (National Snakebite Management Protocol, 2008), as well as other papers, have repeatedly cited various mitigation techniques (Ralph et al., 2019). These include using protective gear (gumboots, long trousers) when working in the fields, maintaining hygienic surroundings, using mosquito nets, and not sleeping on the ground (Jacobsen, 2014; Rodrigo et al., 2017; Samuel et al., 2020). Other techniques also identified as contributing to high mortality include application of incorrect first-aid methods such as cutting or burning the bite site, attempting to suck the venom out, or going to faith-healers instead of trained medical professionals (Bhargava et al., 2020), incorrect identification of snakes and inability to distinguish between venomous and non-venomous snakes (Longkumer et al., 2016; Pandey et al., 2016; Bolon et al., 2020; Durso et al., 2021). However, given the large number of snakebites, which will be much greater than the number of deaths resulting, there is surprisingly little published information on which mitigation techniques have been most useful in practice.
We conducted a literature review to assess mitigation tactics for HSC in India and the rest of the world, published in journals and articles between 2010 and 2020 (for methods and a list of reviewed papers, see Supplementary Information). The 68 selected papers were broken down into five categories corresponding with the region, species and the themes/recommendations identified within each (Table 2). To facilitate greater understanding of past, present and possible future human-snake conflict problems, these were further placed into three broad categories of current HSC issues including human mortalities due to snakebites, livestock/domestical animal mortality due to snakebites and modelling and predictions of human-snake conflict. The majority of papers (over 73%) reviewed focussed on human mortality (Williams et al., 2019), with almost all papers focussed on problem identification. Training of doctors to treat snakebites was included in 24 papers (over 35%), with detailed infographics on identifying snakebites based on the symptoms given (Jesudasan and Abhilash, 2019; Michael et al., 2019; Musah et al., 2019). A majority (over 60%) of those 24 papers also included recommendations for more research and ramping up infrastructure for production and distribution of antivenoms.
Table 2.
Results of a literature review of mitigation tactics for Human-Snake Conflict (HSC) published in journals and articles between 2010 and 2020. Figures indicate the number of times a theme has been covered in 69 selected articles (note that a single article may include more than one theme).
| A |
B |
C |
D |
E |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Models of outreach &awareness/mitigation |
Rescue, translocation, relocation |
Need for more community outreach/intervention |
Need for training doctors/first responders/investing in medical infrastructure |
Sustainable co-existence |
||||||
| India | Global | India | Global | India | Global | India | Global | India | Global | |
| 1.Snakes | 4 | 6 | 3 | 4 | 14 | 25 | 8 | 16 | 4 | 1 |
| 2. Wildlife (incl. snakes) | 4 | 3 | ||||||||
While many studies focussed on snakebite management (Michael et al., 2019), few included the concept of promoting sustainable co-existence (Narayanan and Bindumadhav, 2019) or snake conservation (Parkin et al., 2020). Although 10 papers in total touched upon working and theoretical models of mitigation, it is significant that not a single study addressed the long-term impact or efficacy of those methods.
In India, where there are a combination of different issues contributing to mortality in different regions, and a number of not-for-profit organizations running snakebite mitigation projects in different regions over a significant number of years (Whitaker, 2018; Togridou et al., 2019), there is still no published data to assess the efficacy of any of these methods (Ralph et al., 2019). Many papers discuss the need for community awareness and outreach, without going as far as recommending working solutions for these, or repeated the global guidelines, without adapting them to the regional circumstances. The most common problems in designing mitigation tactics and assessing impact were identified as the lack of funding and/or the paucity of time to either expand the project region or follow up on the retention and implementation of outreach programmes (Samuel et al., 2020). Rescue, relocation and translocation are some of the most common methods of short-term conflict resolution (Harvey et al., 2014; Keener-Eck, Morzillo and Christoffel, 2020a); however, there are increasing numbers of studies pointing to the futility of such exercises (also see Box A), especially when undertaken without a thorough understanding of snake ecology, population density, and behaviour (Ramesh and Nehru, 2019; Blackwell et al., 2016). Similarly, papers reviewed could not demonstrate the efficacy of rescues with the given data (Low, 2018; Harvey et al., 2014). Only 7 papers discussed partnering with the Forest Department or other relevant authorities to extricate snakes out of situations which could prove harmful to the snakes as well as humans, as short-term solutions (Bhattarai et al., 2017; Togridou et al., 2019; Low, 2018). No study provided data on the number of snakes killed in retaliation in the project area (which can be collected as demonstrated in Whitaker and Shine [2000]), showed the impact of rescues on reducing snakebites or retaliatory killings, mentioned a centralised database of snake species encountered during rescues (see Boxes B and C) or gave an idea of the number of rescuers involved. These data are relevant to understanding the scale of rescue methods being applied and to monitor the impact on both humans and snakes, since snakes are a protected group in India (Wild Life (Protection), 2016). The survival and return rates of snakes that have undergone long-distance translocation, a common practice among snake rescuers in India, has only been monitored for a few select species from other countries and is not necessarily applicable to the Indian scenario (Ramesh and Nehru, 2019).
Only three papers analysed mass livestock mortality and morbidity due to snakebites, with one article (Herrera et al., 2017) suggesting the annual figures to be as high as 10,000 for cows in Costa Rica, while discussing the use of active immunisation of cattle to increase survival of envenomation. The recent, and first, global scoping review of snakebite in domestic animals (Bolon et al., 2019) highlighted lack of data, especially from developing countries. These data are needed to fully understand the extent of livestock mortalities, particularly in agrarian economies, and therefore analyse the true societal cost. No data is available for India, nor has any compensation been discussed even though various compensation schemes for domestic animals lost to predation by large carnivores is a high priority (Karanth et al., 2018). Compensation is available for human deaths due to snakebite in several States in India, and this was discussed in a few papers; however, there has been no impact study to show awareness of, and the feasibility of claiming, such compensation in the cases of snakebite deaths (Roshnath et al., 2018).
There is a lack of studies on perceptions of extrinsic and intrinsic value of snakes by the public, and lack of data from agricultural systems regarding the effectiveness of snakes as rodent control agents (Ramesh and Nehru, 2019). Snakes were classified as problem animals by respondents in locally based studies in other regions (Western et al., 2019; Keener-Eck, Morzillo and Christoffel, 2020b), which showed a correlation between occupation (farming) and negative perception. A few studies also looked at fear and disgust of snakes as evolutionary or taught responses, hypothesising that those who feared snakes the most also had trouble correctly identifying them (Henke et al., 2019) and were less likely to absorb corrective information (Castillo-Huitrón et al., 2020). One study concluded that using audio-visual aids can be effective in changing the perception of people about snakes (Quesada-Acuña and Pérez-Gómez, 2020), though the sample size was limited. These studies are highly pertinent since snakes occupy a unique position among dangerous wildlife of being present in both urban and rural human-dominated landscapes and have one of the highest mortality rates of humans caused by any dangerous wildlife species (Tumram et al., 2017; Conover, 2019). A one-size-fits-all approach cannot be taken, and projects that evaluate responses pre, during and post-intervention over a longer period of time need to be designed after taking stock of the issues in the area (Johansson et al., 2016). Few studies have addressed the socio-economic, gender and literacy-related complexities (Vaiyapuri et al., 2013; Kasturiratne et al., 2017; Dandona et al., 2018) that need to be understood for every region before implementing mitigation methods. This applies even if they seem simple, like the recommendation to wear boots (Ralph et al., 2019), which risks ignoring differences between the feet of habitual barefoot walkers and habitual shoe-wearers even within the same population (D’Août et al., 2009), suitability of available boots to resist bites by the most dangerous local species (Pe et al., 1998), and likelihood of long-term behavioural change (Box D). A multidisciplinary approach is required, especially in countries like India, which have varied cultures, traditions and beliefs in different geographical regions, as well as diverse socio-economic conditions wherein it may seem more economically effective to eliminate problem animals (Soto-Shoender and Giuliano, 2011), particularly within economically challenged communities (St John et al., 2012). Until we have a clear understanding of where, how, why and to what extent the conflict is occurring, successful mitigation models cannot be fully designed, executed or replicated in other areas (Box E).
BoxD. Uptake of snakebite mitigation measures: case study from Tamil Nadu, India.
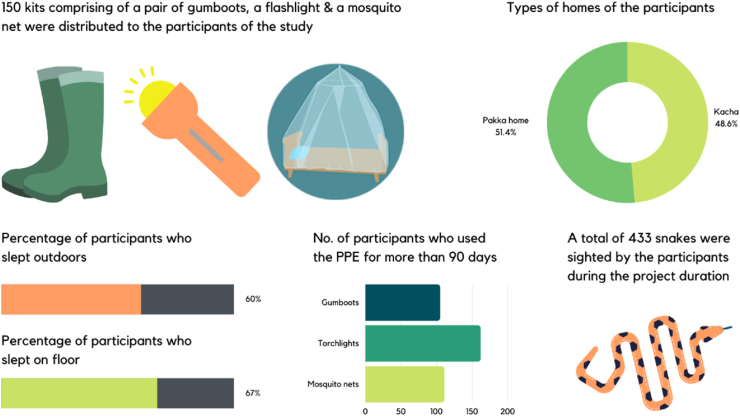
This project was initiated by the Centre for Herpetology at the Madras Crocodile Bank, which has been working in the field of snakebite mitigation and outreach models across India since the 1980s. Three taluks (an administrative subdivision, usually comprising several villages) with agriculture as the primary source of income were chosen in the region around Thirukkarankudi, a town in the district of Tirunelveli, Tamil Nadu. Through hospital visits and surveys, the medical facilities in the Thirukkurungudi region were estimated to receive between 120 & 150 snakebites annually with 8–10 deaths (even though validated data was not available from two government hospitals). As part of the overall objective of educating people on snakebite avoidance and the proper first aid and medical treatment for snakebite, their willingness to accept the usage of snakebite prevention tools such gum (Wellington) boots, flashlights (torches) and mosquito nets was assessed. The project was funded by the Srinivasan Services Trust and as conducted between December 2019 and May 2020.
A total of 150 kits of Personal Protective Equipment (PPE), costing between INR 1400 and 2600 (c. USD 20–35), were distributed free of cost among agrarian communities in the selected taluks. Consequently, surveys were conducted to evaluate the level of adoption of this equipment among users during the implementation period of 169 days. The results showed that there is a willingness among people to adopt safety measures against snakebite, despite some limitations such as one particular brand of gumboot being found to be uncomfortably heavy, resulting in some participants not using the boots while actually undertaking farming activities. The majority incorporated the use of the PPE in their daily practices, and six individuals reported encounters with snakes where they believed the use of the kit had prevented a snakebite from occurring. This also motivated more farmers from the surrounding villages to inquire about adopting this model. However, willingness to buy these kits was not tested in the present study. This could be tested further and even if villagers are disinclined to buy such kits, models could be used where such kits are provided free or at subsidized rates, similar to those used in the distribution of mosquito nets to prevent malaria.
Alt-text: BoxD
BoxE. RECOMMENDATIONS.
-
•
Regularly inspect houses for routes for snakes to enter. Seal gaps under housing walls and doors, and cover plumbing and drain pipelines leading to the exterior.
-
•
Reduce the abundance of snake prey items in the vicinity of houses by removing the attractants of those prey (uneaten food, stores of grain or other crops, trash, debris, wood piles, sources of water).
-
•
Promote the use of PPE including flashlights, good shoes which cover feet and ankles, and mosquito nets at night and, if appropriate, subsidise or distribute these free of cost.
-
•
The effectiveness of these interventions should be evaluated in the same way as other public health interventions.
-
•
Incorporate education and awareness programs for people living in afflicted areas into public health programs, including school health curricula, taking into account regional variation in cultural and agricultural practices, and snake species distribution.
-
•
Integrate herpetologists into public health networks.
-
•
Make additional funding available to extend the evidence base on the distribution, demography, ecology and behaviour of venomous snakes.
Alt-text: BoxE
The need for understanding HSC is especially pressing in the context of climate change, since several recent studies suggest possible increases in HSC as humans and snakes respond via shifting distributions and behaviours (Yousefi et al., 2020). Where range extensions occur in areas where existing levels of conflict is low, the necessary medical infrastructure may be lacking [Zacarias and Loyola, 2019]) and will require increased training for doctors, scaling up of medical infrastructure and instigation of outreach programmes, concurrent with collecting more precise data for monitoring and mapping predicted and current areas of human-snake conflict (Heathcote et al., 2019; Longbottom et al., 2018; Rifaie et al., 2017).
7. Funding imbalances in snakebite research
In 2019, Policy Cures Research (PCR) was tasked by the Wellcome Trust (WT) to assess snakebite-related funding awarded between the years of 2007 and 2018. This analysis was carried out using data obtained through the G-Finder survey for neglected disease biomedical research and development (R&D; Policy Cures Research, 2019a). The G-Finder data include biomedical research and development (basic research and product, e.g., drug or diagnosis development) and research for implementation (operational, implementation, health systems and policy research) but not specifically ecology, biodiversity or similar fields. In total, 47 of 62 organizations responded to the PCR survey and reported data. Of these responses, 37 funders and 1298 grants were identified with a total value of USD 55m awarded over this period ( Policy Cures Research, 2019b).
PCR concluded that “More basic research is needed to accurately estimate the burden of snakebite envenoming, and to understand the natural history and pathogenesis of disease, and the structure and properties of toxins and their variability between regions and species” (Policy Cures Research, no date). While the PCR survey included characterisation of vector behaviour and ecology under ‘vector biology’, ‘biochemistry’ and ‘genetics’, all terms included under the ‘basic research’ category, many of these studies focused on characterising venom profiles.
Here, we focused solely upon research funding for studies of snake ecology, including behaviour and biodiversity (EBB). Upon completion of the search (see Supplementary Information for Methods), only ten of the 1298 awarded grants fulfilled the criteria. A total of USD 947,492 funding for EBB was identified between the years of 1998–2020 after accounting for annual inflation. Of these, six were awarded between 2007 and 2018 to the value of USD 682,854, accounting for 1.23% of all snakebite research funding during this period (Supplementary Figure 1). However, the results are likely to provide an accurate picture of the funding imbalance for EBB when compared with the other snakebite research fields. Ecologically focused studies have been subject to the least amount of funding over the previous two decades and likely prior to that as well, with EBB research receiving just 1.23% of all snakebite funding between 2007 and 2018. Further studies are required to determine which factors are most responsible for funding allocation and which research fields require adjustments in available funding. To determine if future funding for snakebite research is allocated to fields that facilitate a reduction in snakebite incidence or increase in treatment quality, the following questions need to be addressed: What is the total value of available funding for snakebite focused research and to which research fields is this available? Is there a chronic lack of EBB snakebite research interest which subsequently reduces funding availability? If so, is this due to the unavailability of funding? What are the relative costs of EBB research compared to other snakebite-related fields (e.g., fieldwork to determine snake-human conflict hotspots vs the cost of antivenom development and clinical testing)? What are the outputs of each research field and how does each impact snakebite? Can their impact be quantified, and if so, would this reveal a need for reallocation of funding within snakebite research?
8. Conclusion
While the Wellcome Trust website states that “Snakebites are inevitable, but the resulting deaths and morbidity are not” (Wellcome (no date) Our Work: Snakebites,), it can be argued that snakebite is only currently inevitable due to the current lack of ecological understanding of venomous snakes and the social infrastructure which places the poorest of people in the most dangerous of situations. While research into venom and effective treatments of bites is, without a doubt, important in saving lives and comes with its own logistical, social, and financial difficulties (Chippaux, 2017), these should not be considered in isolation and understanding the ecology and behaviour of the vector for this neglected tropical disease (NTD) and how various factors such as climate, geography and human behaviour in snake-human interactions are likely to affect it, has tremendous potential to both prevent bites and protect venomous snakes (Nori et al., 2014; Yañez-Arenas et al., 2014; Angarita-Gerlein et al., 2017; Ediriweera et al., 2018; Goldstein et al., 2021). Even with widely available and highly effective treatments, snakebite will always be a highly traumatic and painful experience entailing expensive medical treatment. As stated by Murray et al. (2020) “This imbalance is akin to trying to combat malaria while overlooking mosquitoes”. Together with improved medical treatment, prevention must be a priority if the problem of snakebite is to be tackled (Togridou et al., 2019), and if the WHO target to reduce the toll of death and disability by 50% by 2030 is to be met. Not only will an increase in such studies provide useful insight for management regarding snake-human conflict prevention and mitigation, but it will undoubtedly provide vital information for conservation efforts. The literature searches undertaken in this review have highlighted the many limitations and biases inherent in studies on snakes, many of which are the result of lack of transparency and reporting in publications, and lack of availability of the data. We urge that, in the future, data is made available via public data repositories with full reporting (e.g. of criteria used to select the study species and site) to facilitate meta-analyses. Furthermore, there is an urgent need for studies to evaluate snake-human conflict prevention measures and to disseminate the results of these to the public through education programs, in order to help promote coexistence between snakes and humans, particularly in regions that are currently most vulnerable.
Author contributions
Anita Malhotra: Conceptualization, Writing - Original Draft, Writing - Review and Editing; Visualizaton. Wolfgang Wüster: Writing – Original Draft; Writing - Review and Editing; Writing - Review and Editing; Cameron Wesley Hodges: Data curation; Investigation; Writing – Original Draft; Writing - Review and Editing; Visualizaton; John Benjamin Owens: Writing – Original Draft; Writing - Review and Editing; Data curation; Allwin Jesudasan: Writing – Original Draft; Investigation; Writing - Review and Editing. Gnaneswar Ch: Writing – Original Draft; Investigation; Visualizaton; Writing - Review and Editing, Ajay Kartik: Writing – Original Draft; Investigation; Peter Christopher: Writing – Original Draft; Investigation; Writing - Review and Editing, Jose Louies: Writing – Original Draft; Hiral Naik: Writing – Original Draft; Writing - Review and Editing. Vishal Santra: Investigation; Writing - Review and Editing. Sourish Rajagopalan Kuttalam: Writing – Original Draft; Data curation; Investigation; Writing - Review and Editing; Visualizaton. Shaleen Attre: Writing – Original Draft; Writing - Review and Editing; Data curation; Mahmood Sasa: Writing – Original Draft; Writing - Review and Editing; Data curation; Investigation; Carlos Bravo-Vega: Writing – Original Draft; Writing - Review and Editing; Data curation; Investigation; Visualizaton.
Ethical statement
Hereby, the authors assure that for the submitted manuscript the following is fulfilled:
-
1)
This material is the authors' own original work, which has not been previously published elsewhere.
-
2)
The paper is not currently being considered for publication elsewhere.
-
3)
The paper reflects the authors' own research and analysis in a truthful and complete manner.
-
4)
The paper properly credits the meaningful contributions of co-authors and co-researchers.
-
5)
The results are appropriately placed in the context of prior and existing research.
-
6)
All sources used are properly disclosed (correct citation).
-
7)
All authors have been personally and actively involved in substantial work leading to the paper, and will take public responsibility for its content.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Vishal Santra and Sourish Kuttalam would like to acknowledge the efforts of the 11 members of the wildlife rescue team from CONCERN who volunteer their time to preserve lives of both wildlife and human on a daily basis in Hooghly district. The team from Madras Crocodile Bank Trust (Centre for Herpetology) gratefully acknowledge funding for the mitigation project from TVS Motor. KAM would like to acknowledge Joint Centre funding from the UK Medical Research Council (MRC) and Department for International Development (MR/R015600/1) and an MRC Global Challenges Research Fund award (MP/P024513/1). SA would like to acknowledge the University of Kent for the Global Challenges Doctoral Centre Award, funded by the UKRI Global Challenges Research Fund. Apart from these, this research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Handling Editor: Glenn King
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.toxcx.2021.100081.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Adams A., Garrison J., Mcdaniel S., Bueche E., Howell H. Don't tread on me: an examination of the anti-predatory behavior of Eastern Copperheads (Agkistrodon contortrix) Acta Herpetol. 2020;15:31–37. [Google Scholar]
- Ainsworth S., Slagboom J., Alomran N., Pla D., Alhamdi Y., King S.I., Bolton F.M.S., Gutiérrez J.M., Vonk F.J., Toh C.-H., Calvete J.J., Kool J., Harrison R.A., Casewell N.R. The paraspecific neutralisation of snake venom induced coagulopathy by antivenoms. Communications Biology. 2018;1:1–14. doi: 10.1038/s42003-018-0039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akani G.C., Amadi N., Dendi D., Luiselli L. Do community metrics vary in reptile communities from Niger Delta forests subjected to slash‐and‐burn agricultural practices? Afr. J. Ecol. 2018;56:1044–1048. [Google Scholar]
- Akani G.C., Ebere N., Luiselli L., Eniang E.A. Community structure and ecology of snakes in fields of oil palm trees (Elaeis guineensis) in the Niger Delta, southern Nigeria. Afr. J. Ecol. 2008;46:500–506. [Google Scholar]
- Akani G.C., Ebere N., Franco D., Eniang E.A., Petrozzi F., Politano E., Luiselli L. Correlation between annual activity patterns of venomous snakes and rural people in the Niger Delta, southern Nigeria. J. Venom. Anim. Toxins Incl. Trop. Dis. 2013;19(1):2. doi: 10.1186/1678-9199-19-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albulescu L.-O., Xie C., Ainsworth S., Alsolaiss J., Crittenden E., Dawson C.A., Softley R., Bartlett K.E., Harrison R.A., Kool J., Casewell N.R. A therapeutic combination of two small molecule toxin inhibitors provides broad preclinical efficacy against viper snakebite. Nat. Commun. 2020;11:6094. doi: 10.1038/s41467-020-19981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alirol E., Sharma S.K., Bawaskar H.S., Kuch U., Chappuis F. Snake bite in South Asia: a review. PLoS Neglected Trop. Dis. 2010;4(1):e603. doi: 10.1371/journal.pntd.0000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews K.M., Gibbons J.W. How do highways influence snake movement? Behavioral responses to roads and vehicles. Copeia. 2005;2005(4):772–782. doi: 10.1643/0045-8511(2005)005[0772:HDHISM]2.0.CO;2. [DOI] [Google Scholar]
- Angarita-Gerlein D., Bravo-Vega C.A., Cruz C., Forero-Muñoz N.R., Navas-Zuloaga M.G., Umaña-Caro J.D. IBIO4299 International Research Experience for Students 2017. 2017. Snakebite dynamics in Colombia: effects of precipitation seasonality on incidence; pp. 1–4.https://bit.ly/3i2pCcp Available from: [Google Scholar]
- Arbuckle K., Rodríguez de la Vega R.C., Casewell N.R. Coevolution takes the sting out of it: evolutionary biology and mechanisms of toxin resistance in animals. Toxicon. 2017;140:118–131. doi: 10.1016/j.toxicon.2017.10.026. [DOI] [PubMed] [Google Scholar]
- Babo Martins S., Bolon I., Chappuis F., Ray N., Alcoba G., Ochoa C., Kumar Sharma S., Nkwescheu A.S., Wanda F., Durso A.M., Ruiz de Castañeda R. Snakebite and its impact in rural communities: the need for a one health approach. PLoS Neglected Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow A., Pook C.E., Harrison R.A., Wüster W. Coevolution of diet and prey-specific venom activity supports the role of selection in snake venom evolution. Proc. R. Soc. B Biol. Sci. 2009;276:2443–2449. doi: 10.1098/rspb.2009.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barve S, Bhaisare D, Giri A, Shankar P.G., Whitaker R, Goode M. A preliminary study on translocation of “rescued” King Cobras (Ophiophagus hannah) Hamadryad. 2013;36(2):80–86. [Google Scholar]
- Baxter-Gilbert J.H., Riley J.L., Lesbarrères D., Litzgus J.D. Mitigating reptile road mortality: fence failures compromise ecopassage effectiveness. PloS One. 2015;10(3) doi: 10.1371/journal.pone.0120537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava S., Kumari K., Sarin R.K., Singh R. First-hand knowledge about snakes and snake-bite management: an urgent need. Nagoya J. Med. Sci. 2020;82(4):763–774. doi: 10.18999/nagjms.82.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai S., Pokheral C.P., Lamichhane B.R. Death feigning behavior in the Burmese Python Python bivittatus kuhl, 1820 in chitwan national park, Nepal. Russ. J. Herpetol. 2017;24:323–326. doi: 10.30906/1026-2296-2017-24-4-323-326. [DOI] [Google Scholar]
- Bisneto P.F., Kaefer I.L. Reproductive and feeding biology of the common lancehead Bothrops atrox (Serpentes, Viperidae) from central and southwestern Brazilian Amazonia. Acta Amazonica. 2019;49(2):105–113. doi: 10.1590/1809-4392201802371. [DOI] [Google Scholar]
- Bittenbinder M.A., Zdenek C.N., Op den Brouw B., Youngman N.J., Dobson J.S., Naude A., Vonk F.J., Fry B.G. Coagulotoxic cobras: clinical implications of strong anticoagulant actions of African spitting Naja venoms that are not neutralised by antivenom but are by LY315920 (Varespladib) Toxins. 2018;10(12):516. doi: 10.3390/toxins10120516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell B.F., DeVault T.L., Fernández-Juricic E., Gese E.M., Gilbert-Norton L., Breck S.W. No single solution: application of behavioural principles in mitigating human-wildlife conflict. Anim. Behav. 2016;120:245–254. doi: 10.1016/j.anbehav.2016.07.013. [DOI] [Google Scholar]
- Boback S.M., Nafus M.G., Yackel Adams A.A., Reed R.N. Use of visual surveys and radiotelemetry reveals sources of detection bias for a cryptic snake at low densities. Ecosphere. 2020;11(1) doi: 10.1002/ecs2.3000. [DOI] [Google Scholar]
- Bohlen C.J., Chesler A.T., Sharif-Naeini R., Medzihradszky K.F., Zhou S., King D., Sánchez E.E., Burlingame A.L., Basbaum A.I., Julius D. A heteromeric Texas coral snake toxin targets acid-sensing ion channels to produce pain. Nature. 2011;479:410–414. doi: 10.1038/nature10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolon I., Finat M., Herrera M., Nickerson A., Grace D., Schütte S., Babo Martins S., Ruiz de Castañeda R. Snakebite in domestic animals: first global scoping review. Prev. Vet. Med. 2019;170:104729. doi: 10.1016/j.prevetmed.2019.104729. [DOI] [PubMed] [Google Scholar]
- Bolon I., Durso A.M., Botero Mesa S., Ray N., Alcoba G., Chappuis F., Ruiz de Castañeda R. Identifying the snake: first scoping review on practices of communities and healthcare providers confronted with snakebite across the world. PloS One. 2020;15 doi: 10.1371/journal.pone.0229989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo-Vega C.A., Cordovez J.M., Renjifo-Ibáñez C., Santos-Vega M., Sasa M. Estimating snakebite incidence from mathematical models: a test in Costa Rica. PLoS Neglected Trop. Dis. 2019;13(12) doi: 10.1371/journal.pntd.0007914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.R., Bishop C.A., Brooks R.J. Effectiveness of short‐distance translocation and its effects on western rattlesnakes. J. Wildl. Manag. 2009;73(3):419–425. doi: 10.2193/2007-558. [DOI] [Google Scholar]
- Butler H., Malone B., Clemann N. The effects of translocation on the spatial ecology of tiger snakes (Notechis scutatus) in a suburban landscape. Wildl. Res. 2005;32(2):165–171. doi: 10.1071/WR04020. [DOI] [Google Scholar]
- Capehart G.D., Escallón C., Vernasco B.J., Moore I.T., Taylor E.N. No drought about it: effects of supplemental hydration on the ecology, behavior, and physiology of free-ranging rattlesnakes. J. Arid Environ. 2016;134:79–86. doi: 10.1016/j.jaridenv.2016.06.018. [DOI] [Google Scholar]
- Carter E.T., Attum O., Eads B.C., Hoffman A.S., Kingsbury B.A. Reducing the potential for human–snake encounters in a recreational park. Human–Wildlife Interactions. 2014;8(2):2. doi: 10.26077/ggt7-vr34. [DOI] [Google Scholar]
- Casewell N.R., Jackson T.N.W., Laustsen A.H., Sunagar K. Causes and consequences of snake venom variation. Trends Pharmacol. Sci. 2020;41:570–581. doi: 10.1016/j.tips.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casewell N.R., Wagstaff S.C., Wüster W., Cook D.A.N., Bolton F.M.S., King S.I., Pla D., Sanz L., Calvete J.J., Harrison R.A. Medically important differences in snake venom composition are dictated by distinct postgenomic mechanisms. Proc. Natl. Acad. Sci. Unit. States Am. 2014;111:9205–9210. doi: 10.1073/pnas.1405484111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Huitrón N.M., Naranjo E.J., Santos-Fita D., Estrada-Lugo E. The importance of human emotions for wildlife conservation. Front. Psychol. 2020;11 doi: 10.3389/fpsyg.2020.01277. 1277-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappuis F., Sharma S.K., Jha N., Loutan L., Bovier P.A. Protection against snake bites by sleeping under a bed net in southeastern Nepal. Am. J. Trop. Med. Hyg. 2007;77(1):197–199. doi: 10.4269/ajtmh.2007.77.197. [DOI] [PubMed] [Google Scholar]
- Chaves L.F., Chuang T.W., Sasa M., Gutiérrez J.M. Snakebites are associated with poverty, weather fluctuations, and El Niño. Science Advances. 2015;1 doi: 10.1126/sciadv.1500249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippaux J.P. Snake-bites: appraisal of the global situation. Bull. World Health Organ. 1998;76(5):515. [PMC free article] [PubMed] [Google Scholar]
- Chippaux J.P. Snakebite envenomation turns again into a neglected tropical disease! J. Venom. Anim. Toxins Incl. Trop. Dis. 2017;23:38. doi: 10.1186/s40409-017-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R.W. Pursuit-deterrent communication between prey animals and timber rattlesnakes (Crotalus horridus): the response of snakes to harassment displays. Behav. Ecol. Sociobiol. 2005;59(2):258–261. doi: 10.1007/s00265-005-0032-9. [DOI] [Google Scholar]
- Colley M., Lougheed S.C., Otterbein K., Litzgus J.D. Mitigation reduces road mortality of a threatened rattlesnake. Wildl. Res. 2017;44(1):48–59. doi: 10.1071/WR16130. [DOI] [Google Scholar]
- Conover M.R. Numbers of human fatalities, injuries, and illnesses in the United States due to wildlife. Human–Wildlife Interactions. 2019;13(2):264–276. [Google Scholar]
- Crane M., Silva I., Marshall B.M., Strine C.T. Lots of movement, little progress: A review of reptile home range literature. Peer J. 2021;9 doi: 10.7717/peerj.11742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Août K., Pataky T.C., De Clercq D., Aerts P. The effects of habitual footwear use: foot shape and function in native barefoot walkers. Footwear Sci. 2009;1(2):81–94. doi: 10.1080/19424280903386411. [DOI] [Google Scholar]
- Daltry J.C., Wüster W., Thorpe R.S. Diet and snake venom evolution. Nature. 1996;379:537–540. doi: 10.1038/379537a0. [DOI] [PubMed] [Google Scholar]
- Dandona R., Kumar G.A., Kharyal A., George S., Akbar M., Dandona L. Mortality due to snakebite and other venomous animals in the Indian state of Bihar: findings from a representative mortality study. PloS One. 2018;13 doi: 10.1371/journal.pone.0198900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E.-L., Arbuckle K. Coevolution of snake venom toxic activities and diet: evidence that ecological generalism favours toxicological diversity. Toxins. 2019;11:711. doi: 10.3390/toxins11120711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Rosa G., Olvera F., Archundia I.G., Lomonte B., Alagón A., Corzo G. Horse immunization with short-chain consensus α-neurotoxin generates antibodies against broad spectrum of elapid venomous species. Nat. Commun. 2019;10:3642. doi: 10.1038/s41467-019-11639-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGregorio B.A., Ravesi M., Sperry J.H., Tetzlaff S.J., Josimovich J., Matthews M., Kingsbury B.A. Daily and seasonal activity patterns of the Massasauga (Sistrurus catenatus): an automated radio-telemetry study. Herpetol. Conserv. Biol. 2018;13(1):10–16. [Google Scholar]
- Devan-Song A., Martelli P., Dudgeon D., Crow P., Ades G., Karraker N.E. Is long-distance translocation an effective mitigation tool for white-lipped pit vipers (Trimeresurus albolabris) in South China? Biol. Conserv. 2016;204:212–220. doi: 10.1016/j.biocon.2016.10.013. [DOI] [Google Scholar]
- Devan-Song A., Martelli P., Karraker N.E. Reproductive biology and natural history of the white-lipped pit viper (Trimeresurus albolabris gray, 1842) in Hong Kong. Herpetol. Conserv. Biol. 2017;12:41–55. [Google Scholar]
- Dorcas M.E., Willson J.D. In: Snakes: Ecology and Conservation. Mullin S.J., Seigel R.A., editors. Cornell University; New York: 2009. Innovative methods for studies of snake ecology and conservation; pp. 5–37. [Google Scholar]
- Durban J., Pérez A., Sanz L., Gómez A., Bonilla F., Rodríguez S., Chacón D., Sasa M., Angulo Y., Gutiérrez J.M., Calvete J.J. Integrated “omics” profiling indicates that miRNAs are modulators of the ontogenetic venom composition shift in the Central American rattlesnake, Crotalus simus simus. BMC Genom. 2013;14:234. doi: 10.1186/1471-2164-14-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durso A.M., Bolon I., Kleinhesselink A.R., Mondardini M.R., Fernandez-Marquez J.L., Gutsche-Jones F., Gwilliams C., Tanner M., Smith C.E., Wüster W., Grey F. Crowdsourcing snake identification with online communities of professional herpetologists and avocational snake enthusiasts. Royal Society Open Science. 2021;8(1):201273. doi: 10.1098/rsos.201273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ediriweera D.S., Diggle P.J., Kasturiratne A., Pathmeswaran A., Gunawardena N.K., Jayamanne S.F., Isbister G.K., Dawson A., Lalloo D.G., de Silva H.J. Evaluating temporal patterns of snakebite in Sri Lanka: the potential for higher snakebite burdens with climate change. Int. J. Epidemiol. 2018;47(6):2049–2058. doi: 10.1093/ije/dyy188/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erulu V.E., Okumu M.O., Ochola F.O., Gikunju J.K. Revered but poorly understood: a case report of Dendroaspis polylepis (black mamba) envenomation in watamu, malindi Kenya, and a review of the literature. Tropical medicine and infectious disease. 2018;3(3):104. doi: 10.3390/tropicalmed3030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettling J.A., Aghasyan L.A., Aghasyan A.L., Parker P.G. Spatial ecology of Armenian vipers, Montivipera raddei, in two different landscapes: human-modified vs. recovered-natural. Russ. J. Herpetol. 2016;23(2):93–102. doi: 10.30906/1026-2296-2016-23-2-93-102. [DOI] [Google Scholar]
- Fearn S., Robinson B., Sambono J., Shine R. Pythons in the pergola: the ecology of 'nuisance' carpet pythons (Morelia spilota) from suburban habitats in south-eastern Queensland. Wildl. Res. 2001;28:573–579. [Google Scholar]
- Ferreira A.A., Reis V.P.D., Boeno C.N., Evangelista J.R., Santana H.M., Serrath S.N., Lopes J.A., Rego C.M.A., Tavares M.N.M., Paloschi M.V., Nery N.M., Dantas A.S., Rodrigues M.M., Zuliani J.P. Increase in the risk of snakebites incidence due to changes in humidity levels: a time series study in four municipalities of the state of Rondônia. Rev. Soc. Bras. Med. Trop. 2020;53 doi: 10.1590/0037-8682-0377-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga R.D., Magnusson W.E., Abrahão C.R., Sanaiotti T., Lima A.P. Habitat selection by Bothrops atrox (serpentes: viperidae) in central amazonia, Brazil. Copeia. 2013;4:684–690. doi: 10.1643/CE-11-098. [DOI] [Google Scholar]
- Gangur A.N., Seymour J.E., Liddell M.J., Wilson D., Smout M.J., Northfield T.D. When is overkill optimal? Tritrophic interactions reveal new insights into venom evolution. Theor. Ecol. 2018;11:141–149. doi: 10.1007/s12080-017-0354-z. [DOI] [Google Scholar]
- Ghosh S., Mukhopadhyay P., Chatterjee T. Management of snake bite in India. J. Assoc. Phys. India. 2016;64:209–218. [PubMed] [Google Scholar]
- Gibbs H.L., Mackessy S.P. Functional basis of a molecular adaptation: prey-specific toxic effects of venom from Sistrurus rattlesnakes. Toxicon. 2009;53:672–679. doi: 10.1016/j.toxicon.2009.01.034. [DOI] [PubMed] [Google Scholar]
- Gibbons J.W., Dorcas M.E. Defensive behavior of cottonmouths (Agkistrodon piscivorus) toward humans. Copeia. 2002:195–198. 2002. [Google Scholar]
- Glaudas X. Proximity between humans and a highly medically significant snake, Russell's viper, in a tropical rural community. Ecol. Appl. 2021;31(4) doi: 10.1002/eap.2330. [DOI] [PubMed] [Google Scholar]
- Glaudas X., Farrell T.M., May P.G. 2005. Defensive Behavior of Free-Ranging Pygmy Rattlesnakes (Sistrurus Miliarius) pp. 196–200. Copeia, 2005. [Google Scholar]
- Glaudas X., Glennon K.L., Martins M., Luiselli L., Fearn S., Trembath D.F., Jelić D., Alexander G.J. Foraging mode, relative prey size and diet breadth: a phylogenetically explicit analysis of snake feeding ecology. J. Anim. Ecol. 2019;88:757–767. doi: 10.1111/1365-2656.12972. [DOI] [PubMed] [Google Scholar]
- Goldstein E., Erinjery J.J., Martin G., Kasturiratne A., Ediriweera D.S., de Silva H.J., Diggle P., Lalloo D.G., Murray K.A., Iwamura T. Integrating human behavior and snake ecology with agent-based models to predict snakebite in high risk landscapes. PLoS Neglected Trop. Dis. 2021;15(1) doi: 10.1371/journal.pntd.0009047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourret A, Alford R, Schwarzkopf L. Very small, light dipole harmonic tags for tracking small animals. Herpetol. Rev. 2011;42:522–525. [Google Scholar]
- Gutiérrez J.M., Calvete J.J., Habib A.G., Harrison R.A., Williams D.J., Warrell D.A. Snakebite envenoming. Nat. Rev. Dis. Primer. 2017;3:17063. doi: 10.1038/nrdp.2017.63. [DOI] [PubMed] [Google Scholar]
- Hafidzi M., Saayon M. Status of rat infestation and recent control strategies in oil palm plantations in Peninsular Malaysia. Pertanika J. Trop. Agric. Sci. 2001;24:109–114. [Google Scholar]
- Hansson E., Sasa M., Mattisson K., Robles A., Gutiérrez J.M. Using geographical information systems to identify populations in need of improved accessibility to antivenom treatment for snakebite envenoming in Costa Rica. PLoS Neglected Trop. Dis. 2013;7(1) doi: 10.1371/journal.pntd.0002009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy D.L., Greene H.W., Tomberlin B., Webster M. Relocation of nuisance rattlesnakes: problems using short-distance translocation in a small rural community. Sonoran Herpetol. 2001;14(6):1–3. [Google Scholar]
- Harris R.J., Zdenek C.N., Harrich D., Frank N., Fry B.G. An appetite for destruction: detecting prey-selective binding of α-neurotoxins in the venom of Afro-Asian elapids. Toxins. 2020;12(3):205. doi: 10.3390/toxins12030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R.A., Hargreaves A., Wagstaff S.C., Faragher B., Lalloo D.G. Snake envenoming: a disease of poverty. PLoS Neglected Trop. Dis. 2009;3(12):e569. doi: 10.1371/journal.pntd.0000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey D.S., Lentini A.M., Cedar K., Weatherhead P.J. Moving massasaugas: insight into rattlesnake relocation using Sistrurus c. catenatus. Herpetol. Conserv. Biol. 2014;9:67–75. [Google Scholar]
- Healthy States Progressive India . World Bank Group; Washington, D.C: 2019. Reports on the Ranks of States and Union Territories (English)http://documents.worldbank.org/curated/en/437741588839001002/Healthy-States-Progressive-India-Reports-on-the-Ranks-of-States-and-Union-Territories Available from: [Google Scholar]
- Healy K., Carbone C., Jackson A.L. Snake venom potency and yield are associated with prey-evolution, predator metabolism and habitat structure. Ecol. Lett. 2019;22:527–537. doi: 10.1111/ele.13216. [DOI] [PubMed] [Google Scholar]
- Heathcote G., Hobday A.J., Spaulding M., Gard M., Irons G. Citizen reporting of wildlife interactions can improve impact-reduction programs and support wildlife carers. Wildl. Res. 2019;46:415–428. doi: 10.1071/WR18127. [DOI] [Google Scholar]
- Heiken K.H., Brusch IV G.A., Gartland S., Escallón C., Moore I.T., Taylor E.N. Effects of long distance translocation on corticosterone and testosterone levels in male rattlesnakes. Gen. Comp. Endocrinol. 2016;237:27–33. doi: 10.1016/j.ygcen.2016.07.023. [DOI] [PubMed] [Google Scholar]
- Henke S.E., Kahl S.S., Wester D.B., Perry G., Britton D. Efficacy of an online native snake identification search engine for public use. Human–Wildlife Interactions. 2019;13:14. doi: 10.26077/pg70-1r55. [DOI] [Google Scholar]
- Herrera M., González K., Rodríguez C., Gómez A., Segura Á., Vargas M., Villalta M., Estrada R., León G. Active immunization of cattle with a bothropic toxoid does not abrogate envenomation by Bothrops asper venom, but increases the likelihood of survival. Biologicals. 2017;46:1–5. doi: 10.1016/j.biologicals.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Hodges C.W., Barnes C.H., Patungtaro P., Strine C.T. Deadly dormmate: a case study on Bungarus candidus living among a student dormitory with implications for human safety. Ecol. Solut. Evidence. 2021;2(e12047):1–10. doi: 10.1002/2688-8319.12047. [DOI] [Google Scholar]
- Holding M.L., Biardi J.E., Gibbs H.L. Coevolution of venom function and venom resistance in a rattlesnake predator and its squirrel prey. Proc. R. Soc. B Biol. Sci. 2016;283:20152841. doi: 10.1098/rspb.2015.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holding M.L., Frazier J.A., Dorr S.W., Henningsen S.N., Moore I.T., Taylor E.N. Physiological and behavioral effects of repeated handling and short-distance translocations on free-ranging Northern Pacific rattlesnakes (Crotalus oreganus oreganus) J. Herpetol. 2014;48(2):233–239. doi: 10.1670/11-314. [DOI] [Google Scholar]
- Holding M.L., Strickland J.L., Rautsaw R.M., Hofmann E.P., Mason A.J., Hogan M.P., Nystrom G.S., Ellsworth S.A., Colston T.J., Borja M. Phylogenetically diverse diets favor more complex venoms in North American pitvipers. Proc. Natl. Acad. Sci. Unit. States Am. 2021;118(17) doi: 10.1073/pnas.2015579118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain H. IFAW-WTI Emergency Relief Network Digest; 2006. Snake Research Organization, Ujjain, Madhya Pradesh, 114-117. 2007. [Google Scholar]
- Jacobsen K.S. vol. 45. 2014. Snake conflict-mitigation in India; pp. 108–111. (The Knowledge of the Irula Tribe). Asian Affairs. [DOI] [Google Scholar]
- Janani S., Maheshwaran E.G., Leenu J., Samuel T., Raveen R. Diversity of snakes rescued at Chennai, Tamil Nadu, India. International Journal of Fauna and Biological Studies. 2016;3(5):81–86. [Google Scholar]
- Jesudasan J.E., Abhilash K.P.P. Venomous snakebites: management and anti-snake venom. Current Medical Issues. 2019;17:66. doi: 10.4103/cmi.cmi_33_19. [DOI] [Google Scholar]
- Johansson M., Ferreira I.A., Støen O., Frank J., Flykt A. Targeting human fear of large carnivores — many ideas but few known effects. Biol. Conserv. 2016;201:261–269. doi: 10.1016/j.biocon.2016.07.010. [DOI] [Google Scholar]
- Jorge da Silva N., Aird S.D. Prey specificity, comparative lethality and compositional differences of coral snake venoms. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001;128:425–456. doi: 10.1016/S1532-0456(00)00215-5. [DOI] [PubMed] [Google Scholar]
- Karanth K.K., Gupta S., Vanamamalai A. Compensation payments, procedures and policies towards human-wildlife conflict management: insights from India. Biol. Conserv. 2018;227:383–389. doi: 10.1016/j.biocon.2018.07.006. [DOI] [Google Scholar]
- Kasturiratne A., Pathmeswaran A., Wickremasinghe A.R., Jayamanne S.F., Dawson A., Isbister G.K., de Silva H.J., Lalloo D.G. The socio-economic burden of snakebite in Sri Lanka. PLoS Neglected Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasturiratne A., Wickremasinghe A.R., de Silva N., Gunawardena N.K., Pathmeswaran A., Premaratna R., Savioli L., Lallo D.G., de Silva H.J. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5(11):e218. doi: 10.1371/journal.pmed.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazandjian T.D., Petras D., Robinson S.D., van Thiel J., Greene H.W., Arbuckle K., Barlow A., Carter D.A., Wouters R.M., Whiteley G., Wagstaff S.C., Arias A.S., Albulescu L.-O., Plettenberg Laing A., Hall C., Heap A., Penrhyn-Lowe S., McCabe C.V., Ainsworth S., da Silva R.R., Dorrestein P.C., Richardson M.K., Gutiérrez J.M., Calvete J.J., Harrison R.A., Vetter I., Undheim E.A.B., Wüster W., Casewell N.R. Convergent evolution of pain-inducing defensive venom components in spitting cobras. Science. 2021;371:386–390. doi: 10.1126/science.abb9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keener-Eck L., Morzillo A.T., Christoffel R.A. Resident attitudes toward timber rattlesnakes (Crotalus horridus) Soc. Nat. Resour. 2020;33(9):1073–1091. doi: 10.1080/08941920.2019.1695989. [DOI] [Google Scholar]
- Keener-Eck L., Morzillo A.T., Christoffel R.A. A comparison of wildlife value orientations and attitudes toward timber rattlesnakes (Crotalus horridus) Hum. Dimens. Wildl. 2020;25(1):47–61. doi: 10.1080/10871209.2019.1694108. [DOI] [Google Scholar]
- Kelly K. Agricultural change in hooghly, 1850–1910. Ann. Assoc. Am. Geogr. 1981;71(2):237–252. doi: 10.1111/j.1467-8306.1981.tb01350.x. [DOI] [Google Scholar]
- Knierim T.K., Strine C.T., Suwanwaree P., Hill J.G. Spatial ecology study reveals nest attendance and habitat preference of banded kraits (Bungarus fasciatus) Herpetol. Bull. 2019;150:6–13. [Google Scholar]
- Knudsen C., Ledsgaard L., Dehli R.I., Ahmadi S., Sørensen C.V., Laustsen A.H. Engineering and design considerations for next-generation snakebite antivenoms. Toxicon. 2019;167:67–75. doi: 10.1016/j.toxicon.2019.06.005. [DOI] [PubMed] [Google Scholar]
- Kochar D.K., Tanwar P.D., Norris R.L., Sabir M., Nayak K.C., Agrawal T.D., Purohit V.P., Kochar A., Simpson I.D. Rediscovery of severe saw-scaled viper (Echis sochureki) envenoming in the Thar desert region of Rajasthan, India. Wilderness Environ. Med. 2007;18(2):75–85. doi: 10.1580/06-WEME-OR-078R.1. [DOI] [PubMed] [Google Scholar]
- Koenig J., Shine R., Shea G. The dangers of life in the city: patterns of activity, injury and mortality in suburban lizards (Tiliqua scincoides) J. Herpetol. 2002:62–68. doi: 10.1670/0022-1511(2002)036[0062:TDOLIT]2.0.CO;2. [DOI] [Google Scholar]
- Krüger H.J., Lemke F.G. Fatal Boomslang bite in the northern cape. African journal of emergency medicine : Revue africaine de la medecine d'urgence. 2019;9(1):53–55. doi: 10.1016/j.afjem.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kularatne S.A.M. Common krait (Bungarus caeruleus) bite in Anuradhapura, Sri Lanka: a prospective clinical study, 1996–98. Postgrad. Med. 2002;78(919):276–280. doi: 10.1136/pmj.78.919.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Sabitha P. Inadequacy of present polyspecific anti snakevenom—a study from central Kerala. Indian J. Pediatr. 2011;78(10):1225–1228. doi: 10.1007/s12098-011-0396-y. [DOI] [PubMed] [Google Scholar]
- Laidig K.J., Golden D.M. Pinelands Commission; New Jersey: 2004. Assessing Timber Rattlesnake Movements Near a Residential Development and Locating New Hibernacula in the New Jersey Pinelands. [DOI] [Google Scholar]
- Laustsen A.H., Lomonte B., Lohse B., Fernández J., Gutiérrez J.M. Unveiling the nature of black mamba (Dendroaspis polylepis) venom through venomics and antivenom immunoprofiling: identification of key toxin targets for antivenom development. Journal of Proteomics. 2015;119:126–142. doi: 10.1016/j.jprot.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Leynaud G.C., Reati G.J. Identificación de las zonas de riesgo ofídico en Córdoba, Argentina, mediante el programa SIGEpi. Rev. Panam. Salud Públic. 2009;26:64–69. doi: 10.1590/s1020-49892009000700010. http://www.scielosp.org/scielo.php?script=sci_arttext&pid=S1020-49892009000700010 Available from. : [DOI] [PubMed] [Google Scholar]
- Li J.-L. Liaoning Science and Technology Press; Dalian, People’s Republic of China: 1995. China Snake Island. [Google Scholar]
- Lillywhite H.B., Martins M., editors. Islands and Snakes: Isolation and Adaptive Evolution. Oxford University Press; New York: 2019. [Google Scholar]
- Lomas E., Maida J.R., Bishop C.A., Larsen K.W. Movement ecology of northern Pacific rattlesnakes (Crotalus o. oreganus) in response to disturbance. Herpetologica. 2019;75(2):153–161. doi: 10.1655/D-17-00060. [DOI] [Google Scholar]
- Lomonte B., Tsai W.-C., Ureña-Diaz J.M., Sanz L., Mora-Obando D., Sánchez E.E., Fry B.G., Gutiérrez J.M., Gibbs H.L., Sovic M.G., Calvete J.J. Venomics of New World pit vipers: genus-wide comparisons of venom proteomes across Agkistrodon. J. Proteomics. 2014;96:103–116. doi: 10.1016/j.jprot.2013.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longbottom J., Shearer F.M., Devine M., Alcoba G., Chappuis F., Weiss D.J., Ray S.E., Ray N., Warrell D.A., Ruiz de Castañeda R., Williams D.J., Hay S.I., Pigott D.M. Vulnerability to snakebite envenoming: a global mapping of hotspots. Lancet. 2018;392:673–684. doi: 10.1016/S0140-6736(18)31224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longkumer T., Armstrong L.J., Santra V., Finny P. Human, snake, and environmental factors in human-snake conflict in North Bihar-A one-year descriptive study. Christian Journal for Global Health. 2016;3:36–45. [Google Scholar]
- Low M. In: Global Reintroduction Perspectives: 2018. Case Studies from Around the Globe. Soorae P.S., editor. IUCN SSC Reintroduction Specialist Group, and Abu Dhabi, AE : Environment Agency-Abu Dhabi; Gland, Switzerland: 2018. Rescue, rehabilitation and release of reticulated pythons in Singapore; pp. 78–81. [DOI] [Google Scholar]
- Lowry H., Lill A., Wong B.B.M. Behavioural responses of wildlife to urban environments. Biol. Rev. 2013;88:537–549. doi: 10.1111/brv.12012. [DOI] [PubMed] [Google Scholar]
- Luiselli L., Sale L., Akani G.C., Amori G. Venomous snake abundance within snake species' assemblages worldwide. Diversity. 2020;12(2):69. doi: 10.3390/d12020069. [DOI] [Google Scholar]
- Lunney D., Gresser S., O'neill L.E., Matthews A., Rhodes J. The impact of fire and dogs on koalas at Port Stephens, New South Wales, using population viability analysis. Pac. Conserv. Biol. 2007;13(3):189–201. doi: 10.1071/PC070189. [DOI] [Google Scholar]
- Luz P.M., Struchiner C.J., Galvani A.P. Modeling transmission dynamics and control of vector-borne neglected tropical diseases. PLoS Neglected Trop. Dis. 2010;4(10) doi: 10.1371/journal.pntd.0000761. http://dx.plos.org/10.1371/journal.pntd.0000761_PMID:21049062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons K., Dugon M.M., Healy K. Diet breadth mediates the prey specificity of venom potency in snakes. Toxins. 2020;12:74. doi: 10.3390/toxins12020074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macartney J.M., Gregory P.T., Larsen K.W. A tabular survey of data on movements and home ranges of snakes. J. Herpetol. 1988;22:61–73. [Google Scholar]
- Maida J.R., Bishop C.A., Larsen K.W. Migration and disturbance: impact of fencing and development on Western Rattlesnake (Crotalus oreganus) spring movements in British Columbia. Can. J. Zool. 2020;98(1):1–12. doi: 10.1139/cjz-2019-0110. [DOI] [Google Scholar]
- Marais J. A complete guide to the snakes of southern Africa. Penguin Random House; South Africa: 2011. [Google Scholar]
- Margres M.J., Patton A., Wray K.P., Hassinger A.T.B., Ward M.J., Lemmon E.M., Lemmon A.R., Rokyta D.R. Tipping the scales: the migration–selection balance leans toward selection in snake venoms. Mol. Biol. Evol. 2019;36:271–282. doi: 10.1093/molbev/msy207. [DOI] [PubMed] [Google Scholar]
- Maritz R.A., Maritz B. Sharing for science: high-resolution trophic interactions revealed rapidly by social media. PeerJ. 2020;8:e9485. doi: 10.7717/peerj.9485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B.M., Crane M., Silva I., Strine C.T., Jones M.D., Hodges C.W., Suwanwaree P., Artchawakom T., Waengsothorn S., Goode M. No room to roam: King Cobras reduce movement in agriculture. Mov. Ecol. 2020;8(33):1–14. doi: 10.1186/s40462-020-00219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B.M., Strine C.T. Make like a glass frog: In support of increased transparency in herpetology. Herpetol. J. 2021;31(1):35–45. doi: 10.33256/31.1.3545. [DOI] [Google Scholar]
- Martins M., Araujo M.S., Sawaya R.J., Nunes R. Diversity and evolution of macrohabitat use, body size and morphology in a monophyletic group of Neotropical pitvipers (Bothrops) J. Zool. 2001;254(4):529–538. doi: 10.1017/S0952836901001030. [DOI] [Google Scholar]
- Michael G.C., Aliyu I., Grema B.A. Primary prevention of snakebite envenoming in resource-limited settings: a narrative review. Environmental Disease. 2019;4:37. doi: 10.4103/ed.ed_11_19. [DOI] [Google Scholar]
- Mise Y.F., Lira-da-Silva R.M., Carvalho F.M. Agriculture and snakebite in Bahia, Brazil-an ecological study. Ann. Agric. Environ. Med. 2016;23(3):467–470. doi: 10.5604/12321966.1219179. [DOI] [PubMed] [Google Scholar]
- Modahl C.M., Mrinalini, Frietze S., Mackessy S.P. Adaptive evolution of distinct prey-specific toxin genes in rear-fanged snake venom. Proc. R. Soc. B Biol. Sci. 2018;285:20181003. doi: 10.1098/rspb.2018.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra B., Warrell D.A., Suraweera W., Bhatia P., Dhingra N., Jotkar R.M., Rodriguez P.S., Mishra K., Whitaker R., Jha P., Million Death Study Collaborators Snakebite mortality in India: a nationally representative mortality survey. PLoS Neglected Trop. Dis. 2011;5(4):e1018. doi: 10.1371/journal.pntd.0001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro C., Montgomery C.E., Spina F., Sawaya R.J., Martins M. Feeding, reproduction, and morphology of Bothrops mattogrossensis (serpentes, viperidae, crotalinae) in the Brazilian pantanal. J. Herpetol. 2006;40(3):408–413. doi: 10.1670/0022-1511. [DOI] [Google Scholar]
- Moore J.A., Gillingham J.C. Spatial ecology and multi-scale habitat selection by a threatened rattlesnake: the eastern massasauga (Sistrurus catenatus catenatus) Copeia. 2006;2006(4):742–751. doi: 10.1643/0045-8511(2006)6[742:SEAMHS]2.0.CO;2. [DOI] [Google Scholar]
- Murray K.A., Martin G., Iwamura T. Focus on snake ecology to fight snakebite. Lancet. 2020;395(10220):e14. doi: 10.1016/S0140-6736(19)32510-3. [DOI] [PubMed] [Google Scholar]
- Musah Y., Ameade E.P.K., Attuquayefio D.K., Holbech L.H. Epidemiology, ecology and human perceptions of snakebites in a savanna community of northern Ghana. PLoS Neglected Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan Y., Bindumadhav S. ‘Posthuman cosmopolitanism’ for the anthropocene in India: urbanism and human-snake relations in the kali yuga. Geoforum. 2019;106:402–410. doi: 10.1016/j.geoforum.2018.04.020. [DOI] [Google Scholar]
- National snakebite management protocol, India . Ministry of Health and Family Welfare; India: 2008. Directorate General of Health and Family Welfare.www://mohfw.nic Available from. : [Google Scholar]
- Nichols J.D., Kendall W.L., Boomer G.S. Accumulating evidence in ecology: once is not enough. Ecol. Evol. 2019;9(24):13991–14004. doi: 10.1002/ece3.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nori J., Carrasco P.A., Leynaud G.C. Venomous snakes and climate change: ophidism as a dynamic problem. Climatic Change. 2014;122(1):67–80. doi: 10.1007/s10584-013-1019-6. [DOI] [Google Scholar]
- Nowak E.M., Hare T., McNally J. In: Biology of the Vipers. Schuett G.W., Höggren M., Douglas M.E., Greene H.W., editors. Eagle Mountain Publishing; LC: 2002. Management of “nuisance” vipers: effects of translocation on western diamond-backed rattlesnakes (Crotalus atrox) pp. 533–560. [Google Scholar]
- Ochola F.O., Okumu M.O., Gikunju J.K., Mbaria J.M., Muchemi G.M., Nderitu J.G. Neutralization of the lethality of the venom of Dendroaspis polylepis (black mamba) in mice by two polyvalent antivenoms used in Kenyan hospitals: results of a 2009–2011 study. Scientific African. 2019;5 doi: 10.1016/j.sciaf.2019.e00118. [DOI] [Google Scholar]
- Oh A.M.F., Tan C.H., Ariaranee G.C., Quraishi N., Tan N.H. Venomics of Bungarus caeruleus (Indian krait): comparable venom profiles, variable immunoreactivities among specimens from Sri Lanka, India and Pakistan. Journal of proteomics. 2017;164:1–18. doi: 10.1016/j.jprot.2017.04.018. [DOI] [PubMed] [Google Scholar]
- Oh A.M.F., Tan K.Y., Tan N.H., Tan C.H. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 2021. Proteomics and neutralization of Bungarus multicinctus (Many-banded krait) venom: intra-specific comparisons between specimens from China and taiwan. [DOI] [PubMed] [Google Scholar]
- Pandey D.P., Subedi Pandey G., Devkota K., Goode M. Public perceptions of snakes and snakebite management: implications for conservation and human health in southern Nepal. J. Ethnobiol. Ethnomed. 2016;12:2210. doi: 10.1186/s13002-016-0092-0. https://doi.org/1186/s13002-016-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey D.P., Bhattarai P., Piya R.C. Food spectrum of common kraits (Bungarus caeruleus): an implication for snakebite prevention and snake conservation. J. Herpetol. 2020;54(1):87–96. doi: 10.1670/18-054. [DOI] [Google Scholar]
- Parkin T., Jolly C.J., de Laive A., von Takach B. Snakes on an urban plain: temporal patterns of snake activity and human–snake conflict in Darwin, Australia. Austral Ecol. 2020 doi: 10.1111/aec.12990. [DOI] [Google Scholar]
- Patra S., Mishra P., Mahapatra S.C. Delineation of groundwater potential zone for sustainable development: a case study from Ganga Alluvial Plain covering Hooghly district of India using remote sensing, geographic information system and analytic hierarchy process. J. Clean. Prod. 2018;172:2485–2502. doi: 10.1016/j.jclepro.2017.11.161. [DOI] [Google Scholar]
- Pawlak J., Mackessy S.P., Sixberry N.M., Stura E.A., Le Du M.H., Ménez R., Foo C.S., Ménez A., Nirthanan S., Kini R.M. Irditoxin, a novel covalently linked heterodimeric three‐finger toxin with high taxon‐specific neurotoxicity. Faseb. J. 2009;23:534–545. doi: 10.1096/fj.08-113555. [DOI] [PubMed] [Google Scholar]
- Pe T., Myint A.-A., Kyu K.-A., Toe M.-M. Acceptability study of protective boots among farmers of Taungdwingyi Township. Myanmar Health Sciences Research Journal. 1998;10(2):57–60. [Google Scholar]
- Pla D., Sanz L., Quesada-Bernat S., Villalta M., Baal J., Chowdhury M.A.W., León G., Gutiérrez J.M., Kuch U., Calvete J.J. Phylovenomics of Daboia russelii across the Indian subcontinent. Bioactivities and comparative in vivo neutralization and in vitro third-generation antivenomics of antivenoms against venoms from India, Bangladesh and Sri Lanka. Journal of Proteomics. 2019;207:103443. doi: 10.1016/j.jprot.2019.103443. [DOI] [PubMed] [Google Scholar]
- Policy Cures Research . 2019. Analysis.https://www.policycuresresearch.org/analysis Available at: [Google Scholar]
- Policy Cures Research . 2019. Global Funding for Snakebite Envenoming Research 2007-2018.https://s3-ap-southeast-2.amazonaws.com/policy-cures-website-assets/app/uploads/2020/01/09162927/2019-Snakebite-envenoming-report.pdf Available at: [Google Scholar]
- Policy Cures Research (no date) R & D Needs for Global Health. Available from: https://www.policycuresresearch.org/r-and-d-needs-for-global-health (accessed on 01 February 2021).
- Putman B.J., Barbour M.A., Clark R.W. The foraging behavior of free-ranging rattlesnakes (Crotalus oreganus) in California ground squirrel (Otospermophilus beecheyi) colonies. Herpetologica. 2016;72(1):55–63. doi: 10.1655/HERPETOLOGICA-D-15-00045. [DOI] [Google Scholar]
- Pyke G.H., Szabo J.K. Conservation and the 4 Rs, which are rescue, rehabilitation, release, and research. Conserv. Biol. 2018;32(1):50–59. doi: 10.1111/cobi.12937. [DOI] [PubMed] [Google Scholar]
- Quesada-Acuña S.G., Pérez-Gómez G. The video as a tool to change perceptions and knowledge about snakes in adults with a high academic level in Costa Rica. UNP Res. J. 2020;12(2) doi: 10.22458/urj.v12i2.3033. [DOI] [Google Scholar]
- Ralph R., Sharma S.K., Faiz M.A., Ribeiro I., Rijal S., Chappuis F., Kuch U. The timing is right to end snakebite deaths in South Asia. BMJ. 2019;364:k5317. doi: 10.1136/bmj.k5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh C., Nehru P. Living with snakes in India: the intensifying health crisis over snakebites-challenges and way ahead. Asian Journal of Conservation Biology. 2019;8:220–223. [Google Scholar]
- Ratanabanangkoon K., Tan K.Y., Pruksaphon K., Klinpayom C., Gutiérrez J.M., Quraishi N.H., Tan C.H. A pan-specific antiserum produced by a novel immunization strategy shows a high spectrum of neutralization against neurotoxic snake venoms. Sci. Rep. 2020;10:11261. doi: 10.1038/s41598-020-66657-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert H.K., Cundall D. An improved surgical implantation method for radio-tracking snakes. Copeia. 1982;1982(3):702–705. doi: 10.2307/1444674. [DOI] [Google Scholar]
- Richards D.P., Barlow A., Wüster W. Venom lethality and diet: differential responses of natural prey and model organisms to the venom of the saw-scaled vipers (Echis) Toxicon. 2012;59:110–116. doi: 10.1016/j.toxicon.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Rifaie F., Maharani T., Hamidy A. Where did venomous snakes strike? A spatial statistical analysis of snakebite cases in bondowoso regency, Indonesia. HAYATI Journal of Biosciences. 2017;24:142–148. doi: 10.1016/j.hjb.2017.09.001. [DOI] [Google Scholar]
- Robinson D.P., Hyland K., Beukes G., Vettan A., Mabadikate A., Jabado R.W., Rohner C.A., Pierce S.J., Baverstock W. Satellite tracking of rehabilitated sea turtles suggests a high rate of short-term survival following release. PLoS One. 2021;16 doi: 10.1371/journal.pone.0246241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodda G.H. McDiarmid et al. editors. Reptile Biodiversity: Standard Methods for Inventory and Monitoring. University of California Press; Berkeley, CA: 2012. Population size and demographics; pp. 283–322. [Google Scholar]
- Rodrigo C., Kirushanthan S., Gnanathasan A. Prevention of krait bites by sleeping above ground: preliminary results from an observational pilot study. J. Occup. Med. Toxicol. 2017;12 doi: 10.1186/s12995-017-0156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski A., Soerensen C., op den Brouw B., Lister C., Dashevsky D., Arbuckle K., Gloria A., Zdenek C.N., Casewell N.R., Gutiérrez J.M., Wüster W., Ali S.A., Masci P., Rowley P., Frank N., Fry B.G. Differential procoagulant effects of saw-scaled viper (Serpentes: viperidae: Echis) snake venoms on human plasma and the narrow taxonomic ranges of antivenom efficacies. Toxicol. Lett. 2017;280:159–170. doi: 10.1016/j.toxlet.2017.08.020. [DOI] [PubMed] [Google Scholar]
- Roll U., Feldman A., Novosolov M., Allison A., Bauer A.M., Bernard R., Colli G.R. The global distribution of tetrapods reveals a need for targeted reptile conservation. Nature Ecology & Evolution. 2017;1:1677–1682. doi: 10.1038/s41559-017-0332-2. [DOI] [PubMed] [Google Scholar]
- Roshnath R. Snake rescues: a conservation effort in kannur district. Kongunadu Research Journal. 2017;4(1):161–165. doi: 10.26524/krj193. [DOI] [Google Scholar]
- Roshnath R., Divakar N. Solving species quandary: why awareness programs are pivotal in snake conservation. Herpetol. J. 2019;29:214–218. doi: 10.33256/hj29.4.214218. [DOI] [Google Scholar]
- Roshnath R., Kunhiraman E., Rajan C.V. Incidence of snake bite and corresponding compensation payments in the Kannur district of Kerala, India. Herpetol. Bull. 2018;143:27. [Google Scholar]
- Samuel S.P., Chinnaraju S., Williams H.F., Pichamuthu E., Subharao M., Vaiyapuri M., Arumugam S., Vaiyapuri R., Baksh M.F., Patel K., Trim S.A., Duncombe T.E., Vaiyapuri S. Venomous snakebites: rapid action saves lives—a multifaceted community education programme increases awareness about snakes and snakebites among the rural population of Tamil Nadu, India. PLoS Neglected Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0008911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini L., Isaac N.J., Maiorano L., Ficetola G.F., Huijbregts M.A., Carbone C., Thuiller W. Global drivers of population density in terrestrial vertebrates. Global Ecol. Biogeogr. 2018;27(8):968–979. doi: 10.1111/geb.12758. [DOI] [Google Scholar]
- Saravia P., Rojas E., Arce V., Guevara C., López J.C., Chaves E., Velásquez R., Rojas G., Gutiérrez J.M. Geographic and ontogenic variability in the venom of the neotropical rattlesnake Crotalus durissus: pathophysiological and therapeutic implications. Rev. Biol. Trop. 2002;50:337–346. [PubMed] [Google Scholar]
- Sasa M., Wasko D.K., Lamar W.W. Natural history of the terciopelo Bothrops asper (serpentes: viperidae) in Costa Rica. Toxicon. 2009;54(7):904–922. doi: 10.1016/j.toxicon.2009.06.024. [DOI] [PubMed] [Google Scholar]
- Senji Laxme R.R., Attarde S., Khochare S., Suranse V., Martin G., Casewell N.R., Whitaker R., Sunagar K. Biogeographical venom variation in the Indian spectacled cobra (Naja naja) underscores the pressing need for pan-India efficacious snakebite therapy. PLoS Neglected Trop. Dis. 2021;15 doi: 10.1371/journal.pntd.0009150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton M.B., Phillips S.S., Goldingay R.L. Habitat requirements of an arboreal Australian snake (Hoplocephalus bitorquatus) are influenced by hollow abundance in living trees. For. Ecol. Manag. 2020;455:117675. doi: 10.1016/j.foreco.2019.117675. [DOI] [Google Scholar]
- Shine R., Koenig J. Snakes in the garden: an analysis of reptiles “rescued” by community-based wildlife carers. Biol. Conserv. 2001;102(3):271–283. doi: 10.1016/S0006-3207(01)00102-1. [DOI] [Google Scholar]
- Shipley B.K., Chiszar D., Fitzgerald K.T., Saviola A.J. Spatial ecology of prairie rattlesnakes (Crotalus viridis) associated with black-tailed prairie dog (Cynomys ludovicianus) colonies in Colorado. Herpetol. Conserv. Biol. 2013;8(1):240–250. [Google Scholar]
- Siers S.R., Yackel Adams A.A., Reed R.N. Behavioral differences following ingestion of large meals and consequences for management of a harmful invasive snake: a field experiment. Ecol. Evol. 2018;8:10075–10093. doi: 10.1002/ece3.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siettos C.I., Russo L. Mathematical modeling of infectious disease dynamics. Virulence. 2013;4(4):295–306. doi: 10.4161/viru.24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley-Walters S.A., Farrell T.M., Gibbs H.L. The importance of species: pygmy rattlesnake venom toxicity differs between native prey and related non-native species. Toxicon. 2018;144:42–47. doi: 10.1016/j.toxicon.2018.01.022. [DOI] [PubMed] [Google Scholar]
- Soto-Shoender J.R., Giuliano W.M. Predation on livestock by large carnivores in the tropical lowlands of Guatemala. Oryx. 2011;45:561–568. doi: 10.1017/S0030605310001845. [DOI] [Google Scholar]
- Soud R. Notes on a rescue of a Burmese Python Python molurus bivittatus Kuhl, 1820 (Family: Pythonidae) from an urban area of Bongaigaon District, Assam. Reptile Rap. 2010;9(11) https://zooreach.org/SARN/ReptileRap/No9_jan10.pdf Available from: [Google Scholar]
- Sousa L.F., Zdenek C.N., Dobson J.S., Op den Brouw B., Coimbra F.C.P., Gillett A., Del-Rei T.H.M., Chalkidis H.D.M., Sant'Anna S., Teixeira-da-Rocha M.M., Grego K., Travaglia Cardoso S.R., Moura da Silva A.M., Fry B.G. Coagulotoxicity of Bothrops (lancehead pit-vipers) venoms from Brazil: differential biochemistry and antivenom efficacy resulting from prey-driven venom variation. Toxins. 2018;10:411. doi: 10.3390/toxins10100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry J.H., Ward M.P., Weatherhead P.J. Effects of temperature, moon phase, and prey on nocturnal activity in ratsnakes: An automated telemetry study. J. Herpetol. 2013;47:105–111. [Google Scholar]
- St John F.A.V., Keane A.M., Edwards-Jones G., Jones L., Yarnell R.W., Jones J.P. Identifying indicators of illegal behaviour: carnivore killing in human-managed landscapes. Proc. Biol. Sci. 2012;279:804–812. doi: 10.1098/rspb.2011.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen D.A. Snakes in the grass: secretive natural histories defy both conventional and progressive statistics. Herpetol. Conserv. Biol. 2010;5(2):183–188. [Google Scholar]
- Suchithra N., Pappachan J.M., Sujathan P. Snakebite envenoming in Kerala, South India: clinical profile and factors involved in adverse outcomes. Emerg. Med. J. 2008;25(4):200–204. doi: 10.1136/emj.2007.051136. [DOI] [PubMed] [Google Scholar]
- Sullivan B.K., Nowak E.M., Kwiatkowski M.A. Problems with mitigation translocation of herpetofauna. Conserv. Biol. 2015;29(1):12–18. doi: 10.1111/cobi.12336. [DOI] [PubMed] [Google Scholar]
- Suraweera W., Warrell D., Whitaker R., Menon G., Rodrigues R., Fu S.H., Begum R., Sati P., Piyasena K., Bhatia M., Brown P., Jha P. Trends in snakebite deaths in India from 2000 to 2019 in a nationally representative mortality study. eLife. 2020;9 doi: 10.7554/eLife.54076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton W.B., Wang Y., Schweitzer C.J., McClure C.J. Spatial ecology and multi-scale habitat selection of the Copperhead (Agkistrodon contortrix) in a managed forest landscape. For. Ecol. Manag. 2017;391:469–481. doi: 10.1016/j.foreco.2017.02.041. [DOI] [Google Scholar]
- Swaroop S., Grab B. Snakebite mortality in the world. Bull. World Health Organ. 1954;10(1):35–76. [PMC free article] [PubMed] [Google Scholar]
- Tchoffo D., Kamgno J., Kekeunou S., Yadufashije C., Djeunga H.C.N., Nkwescheu A.S. High snakebite underreporting rate in the Centre Region of Cameroon: an observational study. BMC Publ. Health. 2019;19(1):1–7. doi: 10.1186/s12889-019-7363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M.J., Peterson M.L., Friedenberg N., Van Eenennaam J.P., Johnson J.R., Hoover J.J., Klimley A.P. Stranding of spawning run green sturgeon in the Sacramento River: post-rescue movements and potential population-level effects. N. Am. J. Fish. Manag. 2013;33(2):287–297. doi: 10.1080/02755947.2012.758201. [DOI] [Google Scholar]
- Thorpe R.S., Pook C.E., Malhotra A. Phylogeography of the Russell's viper (Daboia russelii) complex in relation to variation in the colour pattern and symptoms of envenoming. Herpetol. J. 2007;17:209–218. [Google Scholar]
- Togridou A., Graham S.A., Owens J.B., Santra V., Bharti O., Malhotra A. Prevention is better than cure: snakebite education in India. Επιστήμες Αγωγής. 2019;2019(4):75–96. [Google Scholar]
- Tongpoo A., Sriapha C., Pradoo A., Udomsubpayakul U., Srisuma S., Wananukul W., Trakulsrichai S. Krait envenomation in Thailand. Therapeut. Clin. Risk Manag. 2018;14:1711. doi: 10.2147/TCRM.S169581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumram N.K., Ambade V.N., Dixit P.G. Human fatalities caused by animal attacks: a six-year autopsy study. Med. Leg. J. 2017;85(4):194–199. doi: 10.1177/0025817217707166. [DOI] [PubMed] [Google Scholar]
- Udyawer V., Goiran C., Shine R. Peaceful coexistence between people and deadly wildlife: why are recreational users of the ocean so rarely bitten by sea snakes? People Nat. 2021;3:335–346. doi: 10.1002/pan3.10190. [DOI] [Google Scholar]
- Újvári B., Korsós Z. Use of radiotelemetry on snakes: a review. Acta Zool. Acad. Sci. Hungar. 2000;46(2):115–146. [Google Scholar]
- Vaiyapuri S., Vaiyapuri R., Ashokan R., Ramasamy K., Nattamaisundar K., Jeyaraj A., Chandran V., Gajjeraman P., Baksh M.F., Gibbins J.M., Hutchinson E.G. Snakebite and its socio-economic impact on the rural population of Tamil Nadu, India. PloS One. 2013;8(11) doi: 10.1371/journal.pone.0080090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas R. Snake diversity and voluntary rescue practice in the cities of Gujarat State, India: an evaluation. Reptile Rap. 2013;15:27–39. https://www.zoosprint.zooreach.org/ZoosPrintNewsLetter/Reptile%20Rap_15_Jan_13.pdf Available from: [Google Scholar]
- Waldron J.L., Welch S.M., Holloway J., Mousseau T.A. Using occupancy models to examine human–wildlife interactions. Hum. Dimens. Wildl. 2013;18(2):138–151. doi: 10.1080/10871209.2012.719173. [DOI] [Google Scholar]
- Ward-Smith H., Arbuckle K., Naude A., Wüster W. Fangs for the memories? A survey of pain in snakebite patients does not support a strong role for defence in the evolution of snake venom composition. Toxins. 2020;12:201. doi: 10.3390/toxins12030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrell D.A. Snake bite. Lancet. 2010;375(9708):77–88. doi: 10.1016/S0140-6736(09)61754-2. [DOI] [PubMed] [Google Scholar]
- Wasko D.K., Sasa M. Activity patterns of a neotropical ambush predator: spatial ecology of the Fer‐de‐lance (Bothrops asper, Serpentes: viperidae) in Costa Rica. Biotropica. 2009;41(2):241–249. doi: 10.1111/j.1744-7429.2008.00464.x. [DOI] [Google Scholar]
- Wellcome (no date) Our Work: Snakebites. Available at: https://wellcome.org/what-we-do/our-work/snakebites#what-we've-funded-b197 (accessed on 30 January 2021).
- Western D., Nightingale D.L.M., Mose V.N., Sipitiek J.O., Kimiti K.S. Variability and change in Maasai views of wildlife and the implications for conservation. Hum. Ecol. 2019;47:205–216. doi: 10.1007/s10745-019-0065-8. [DOI] [Google Scholar]
- Whitaker P.B., Shine R. When, where and why do people encounter Australian brownsnakes (Pseudonaja textilis: elapidae)? Wildl. Res. 1999;26(5):675–688. doi: 10.1071/WR98043. [DOI] [Google Scholar]
- Whitaker P.B., Shine R. Sources of mortality of large elapid snakes in an agricultural landscape. J. Herpetol. 2000;121–128 doi: 10.2307/1565247. [DOI] [Google Scholar]
- Whitaker P.B., Shine R. Thermal biology and activity patterns of the eastern brownsnake (Pseudonaja textilis): a radiotelemetric study. Herpetologica. 2002;58(4):436–452. doi: 10.1655/0018-0831(2002)058[0436:TBAAPO]2.0.CO;2. [DOI] [Google Scholar]
- Whitaker R. Snakebite mitigation project of the Madras Crocodile Bank/Centre for Herpetology, India: background and a brief summary of activities. Trans. R. Soc. Trop. Med. Hyg. 2018;113:818–819. doi: 10.1093/trstmh/try130. [DOI] [PubMed] [Google Scholar]
- Whitford M.D., Freymiller G.A., Higham T.E., Clark R.W. Determinants of predation success: how to survive an attack from a rattlesnake. Funct. Ecol. 2019;1365–2435:13318. doi: 10.1111/1365-2435.13318. [DOI] [Google Scholar]
- Whitney N.M., White C.F., Smith B.J., Cherkiss M.S., Mazzotti F.J., Hart K.M. Accelerometry to study fine-scale activity of invasive Burmese pythons (Python bivittatus) in the wild. Animal Biotelemetry. 2021;9(1):1–13. doi: 10.1186/s40317-020-00227-7. [DOI] [Google Scholar]
- WHO . World Health Organization; Geneva: 2017. Report of the Tenth Meeting of the WHO Strategic and Technical Advisory Group for Neglected Tropical Diseases. [Google Scholar]
- WHO . 2018. Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins.https://www.who.int/bloodproducts/AntivenomGLrevWHO_TRS_1004_web_Annex_5.pdf?ua=1 Available from. : [DOI] [PubMed] [Google Scholar]
- Wild Life (Protection) Act, 1972, along with Allied Rules. Universal Law Publishing Co.; New Delhi, India: 2016. [Google Scholar]
- Williams D.J., Faiz M.A., Abela-Ridder B., Ainsworth S., Bulfone T.C., Nickerson A.D., Habib A.G., Junghanss T., Fan H.W., Turner M., Harrison R.A., Warrell D.A. Strategy for a globally coordinated response to a priority neglected tropical disease: snakebite envenoming. PLoS Neglected Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D.J., Gutiérrez J.M., Calvete J.J., Wüster W., Ratanabanangkoon K., Paiva O., Brown N.I., Casewell N.R., Harrison R.A., Rowley P.D., O'Shea M., Jensen S.D., Winkel K.D., Warrell D.A. Ending the drought: new strategies for improving the flow of affordable, effective antivenoms in Asia and Africa. Journal of proteomics. 2011;74(9):1735–1767. doi: 10.1016/j.jprot.2011.05.027. [DOI] [PubMed] [Google Scholar]
- Williams V., White J., Schwaner T.D., Sparrow A. Variation in venom proteins from isolated populations of tiger snakes (Notechis ater Niger, N. scutatus) in South Australia. Toxicon. 1988;26:1067–1075. doi: 10.1016/0041-0101(88)90205-X. [DOI] [PubMed] [Google Scholar]
- Wolfe A.K., Fleming P.A., Bateman P.W. Impacts of translocation on a large urban-adapted venomous snake. Wildl. Res. 2018;45(4):316–324. doi: 10.1071/WR17166. [DOI] [Google Scholar]
- Wüster W. Taxonomic changes and toxinology: systematic revisions of the Asiatic cobras (Naja naja species complex) Toxicon. 1996;34:399–406. doi: 10.1016/0041-0101(95)00139-5. [DOI] [PubMed] [Google Scholar]
- Wüster W., Golay P., Warrell D.A. Synopsis of recent developments in venomous snake systematics. Toxicon. 1997;35:319–340. doi: 10.1016/S0041-0101(96)00152-3. [DOI] [PubMed] [Google Scholar]
- Wüster W., Thorpe R.S. Asiatic cobras: systematics and snakebite. Experientia. 1991;47:205–209. doi: 10.1007/BF01945429. [DOI] [PubMed] [Google Scholar]
- Wüster W., Thorpe R.S., Salomão M.D.G., Thomas L., Puorto G., Theakston R.D.G., Warrell D.A. Origin and phylogenetic position of the Lesser Antillean species of Bothrops (Serpentes, Viperidae): biogeographical and medical implications. Bull. Nat. Hist. Mus. Zool. 2002;68:101–106. doi: 10.1017/S0968047002000110. [DOI] [Google Scholar]
- Yañez-Arenas C., Peterson A.T., Mokondoko P., Rojas-Soto O., Martínez-Meyer E. The use of ecological niche modeling to infer potential risk areas of snakebite in the Mexican state of Veracruz. PloS One. 2014;9(6) doi: 10.1371/journal.pone.0100957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yañez-Arenas C., Peterson A.T., Rodríguez-Medina K., Barve N. Mapping current and future potential snakebite risk in the new world. Climatic Change. 2016;134(4):697–711. doi: 10.1007/s10584-015-1544-6. [DOI] [Google Scholar]
- Yousefi M., Kafash A., Khani A., Nabati N. Applying species distribution models in public health research by predicting snakebite risk using venomous snakes' habitat suitability as an indicating factor. Sci. Rep. 2020;10:18073. doi: 10.1038/s41598-020-74682-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacarias D., Loyola R. Climate change impacts on the distribution of venomous snakes and snakebite risk in Mozambique. Climatic Change. 2019;152:195–207. doi: 10.1007/s10584-018-2338-4. [DOI] [Google Scholar]
- Zancolli G., Calvete J.J., Cardwell M.D., Greene H.W., Hayes W.K., Hegarty M.J., Herrmann H.-W., Holycross A.T., Lannutti D.I., Mulley J.F., Sanz L., Travis Z.D., Whorley J.R., Wüster C.E., Wüster W. When one phenotype is not enough: divergent evolutionary trajectories govern venom variation in a widespread rattlesnake species. Proc. R. Soc. B Biol. Sci. 2019;286:20182735. doi: 10.1098/rspb.2018.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdenek C.N., Harris R.J., Kuruppu S., Youngman N.J., Dobson J.S., Debono J., Khan M., Smith I., Yarski M., Harrich D., Sweeney C., Dunstan N., Allen L., Fry B.G. A taxon-specific and high-throughput method for measuring ligand binding to nicotinic acetylcholine receptors. Toxins. 2019;11:600. doi: 10.3390/toxins11100600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Medzihradszky K.F., Sánchez E.E., Basbaum A.I., Julius D. Lys49 myotoxin from the Brazilian lancehead pit viper elicits pain through regulated ATP release. Proc. Natl. Acad. Sci. Unit. States Am. 2017;114:E2524–E2532. doi: 10.1073/pnas.1615484114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.