Abstract
Understanding sex differences in behavioral and molecular effects of stress has important implications for understanding the vulnerability to chronic psychiatric disorders associated with stress response circuitry. The amygdala is critical for emotional learning and generating behavioral responses to stressful stimuli, and preclinical studies indicate that amygdalar endocannabinoid (eCB) signaling regulates emotional states. This study measured eCB contents in the basolateral (BLA) and central (CeA) amygdala of male and female rats exposed to predator odor stress (bobcat urine) and tested for contextual avoidance 24 h later. Stressed females had lower levels of 2-arachidonoyl glycerol (2-AG) in the BLA and higher levels of anandamide (AEA) in the CeA, while exposure to bobcat urine did not affect amygdalar eCB contents in males. We previously reported that female rats exposed to bobcat urine exhibit blunted acoustic startle reactivity (ASR) 48 h after predator odor stress. Therefore, we tested the hypothesis that intra-BLA injection of a diacylglycerol lipase (DAGL) inhibitor (which would be expected to reduce 2-AG levels in BLA) and intra-CeA injection of a fatty acid amide hydrolase (FAAH) inhibitor (which would be expected to increase AEA levels in CeA) would mimic previously observed predator odor stress-induced reductions in ASR. Contrary to our hypothesis, microinjections of either the DAGL inhibitor DO34 into the BLA or the FAAH inhibitor URB597 into the CeA significantly increased ASR in females compared to vehicle-treated rats. These findings describe sex-specific effects of predator odor stress on amygdalar eCBs, and new roles for amygdalar eCBs in regulating behavior in females.
Keywords: Sex difference, Predator odor, Startle reactivity, Endocannabinoid, Amygdala
1. Introduction
Stress is associated with the onset and severity of several psychiatric disorders, including post-traumatic stress disorder (PTSD) and depression (Bangasser and Valentino, 2014). Notably, women are two to three times more at risk to develop these disorders (Breslau, 2009; Kessler et al., 2005) and the symptomatology, development, and responsiveness to treatment differ between genders (Altemus et al., 2104; Young and Pfaff, 2014). Unfortunately, the current biological knowledge of the mechanisms behind sex disparities is scarce compared with the abundant clinical evidence, which remains mostly unexplained.
The endocannabinoid (eCB) system has attracted attention for its role in numerous behavioral and brain functions, and as a therapeutic target in neuropsychiatric disease states (Patel et al., 2017). The eCB system is composed of two cannabinoid receptors, CB1 and CB2 (Matsuda et al., 1990; Munro et al., 1993); two major endogenous lipid ligands, N-arachidonoyl ethanolamine (anandamide, AEA (Devane et al., 1992);) and 2-arachidonoyl glycerol (2-AG (Sugiura et al., 1995);); and the enzymes involved in biosynthesis, transportation, and degradation of endogenous ligands (Hu and Mackie, 2015; Micale and Drago, 2018). The biosynthesis of 2-AG is mediated by the conversion of diacylglycerol to 2-AG by the enzyme diacylglycerol lipase (DAGL), and its metabolism is primarily driven by the enzyme monoacylglycerol lipase (Blankman and Cravatt, 2013). The biosynthesis of AEA, on the other hand, is complex and seems to involve multiple redundant pathways, whereas its metabolism is almost entirely mediated by the enzyme fatty acid amide hydrolase (FAAH (Blankman and Cravatt, 2013);). In addition to binding to cannabinoid receptors, AEA is also an endogenous ligand for the nonselective cation channel, transient receptor potential vanilloid type 1 (TRPV1) (Zygmunt et al., 1999).
The amygdala plays a critical role in promoting homeostasis by regulating physiological and behavioral responses to stress (Zhang et al., 2021), and a growing body of work demonstrates that amygdalar eCB signaling is involved in the regulation of emotional states (Di et al., 2016; Gray et al., 2015; Gunduz-Cinar et al., 2013; Lim et al., 2016; Marsicano et al., 2002; Morena et al., 2019). In humans, FAAH inhibition attenuated the activation of the amygdala, which is consistent with effects observed with anxiolytic agents (Paulus et al., 2021). In a preclinical model of traumatic stress, rats that exhibited prolonged anxiety-like behavior after exposure to the fox pheromone 2,5-dihydro-2,4,5-trimethylthiazoline (TMT) also had an increase in 2-AG levels within the amygdala (Lim et al., 2016). In contrast, systemic 2-AG augmentation was associated with a stress-resilient phenotype in mice, and BLA 2-AG signaling was required for adaptation to repeated traumatic stress (Bluett et al., 2017). Despite increasing evidence that eCBs are implicated in the modulation of behavioral and emotional responses, results are controversial and much remains unknown about the specific roles of individual eCB ligands within the amygdala, especially in females.
Our laboratory uses predator odor stress (bobcat urine) to divide adult Wistar rats into groups that exhibit high (Avoiders) or low (Non-Avoiders) avoidance of a predator odor-paired context, mirroring avoidance symptoms in some but not all humans exposed to stress (Albrechet-Souza and Gilpin, 2019). Prior work from our group has shown clear differences in some behaviors (e.g., alcohol drinking (Edwards et al., 2013; Weera et al., 2020), nociception (Itoga et al., 2016)) but not others (e.g., anxiety-like behavior (Whitaker and Gilpin, 2015)) after exposure to predator odor stress in Avoider versus Non-Avoider animals. In a study published recently, we reported that female rats, regardless of their status as Avoiders or Non-Avoider, exhibit blunted acoustic startle reactivity (ASR) 2 days after exposure to bobcat urine (Albrechet-Souza et al., 2020). The current study was designed to extend upon those findings by testing the hypotheses that 1) predator odor (i.e., bobcat urine) stress would alter eCB levels in amygdala sub-nuclei (i.e., BLA and CeA) of adult male and female Wistar rats and 2) drug manipulations of BLA and CeA eCB enzymes would mimic stress effects on ASR in female rats.
2. Material and methods
2.1. Animals
Eight-week-old male and female Wistar rats (N = 80; Charles River; Raleigh-NC, USA) were housed in same-sex pairs in a humidity- and temperature-controlled (22 °C) vivarium on a 12-h reversed light-dark cycle (lights off at 7:00 a.m.). Animals had ad libitum access to food and water throughout the experiments and were handled daily for 3 min for 7–10 days before the initiation of experimental protocols. Neither gonadal hormones (estrogen or testosterone) nor estrus cycles were tracked. All procedures were approved by the Institutional Animal Care and Use Committee of the Louisiana State University Health Sciences Center and were in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). All efforts were made to minimize animal suffering and to reduce the number of animals used.
2.2. Study design
Rats were exposed to bobcat urine and tested for avoidance of the odor-paired context 24 h later (n = 17 males and 16 females); unstressed Controls were never exposed to predator odor (n = 7 males and 8 females). At day 2 post-stress, rats were euthanized by decapitation under isoflurane anesthesia and brains were rapidly collected for analysis of eCB contents in the amygdala. Separate cohorts of experimentally naïve females (n = 32) received intra-BLA microinjections of the DAGL inhibitor (DO34) or intra-CeA microinjections of the FAAH inhibitor (URB597) and were tested for ASR. At the end of the experiment, rats were euthanized by decapitation under isoflurane anesthesia and brains were sliced in cryostat for verification of cannula placement.
2.3. Predator odor stress
Rats were tested in a 5-day place conditioning procedure (Albrechet-Souza and Gilpin, 2019; Albrechet-Souza et al., 2020) that began after the acclimatization period. Rats were allowed 5 min of free exploration of the apparatus (3-chamber pre-test session), which consisted of three large chambers (36 cm length × 30 cm width × 34 cm height) with different types of floor texture (circles, grid or rod floor) and patterned walls (circles, white or stripes), separated by a small triangular connecting chamber. The apparatus was thoroughly cleaned between animals with Quatricide® PV in water at a concentration of 1:64 (Pharmacal Research Labs; Waterbury-CT, USA). For each rat, the chamber that exhibited the most deviant time score of the three (i.e., highly preferred or highly avoided) was excluded from all future sessions for that rat. On the next day (day −2 in Fig. 1A), each rat was allowed 5 min to explore the two chambers that would eventually be used for the conditioning procedure for that rat. Rats were assigned to Stress or Control groups that were counterbalanced for magnitude of baseline preference for one chamber versus the other (i.e., groups were assigned such that mean pre-existing preference for each of the two chambers was approximately zero for Stress and Control groups). For rats in the Stress group, an unbiased and counterbalanced design was used to determine which chamber (i.e., more preferred or less preferred) would be paired with bobcat urine for each rat. On the next day (day −1 in Fig. 1A), each rat was placed in one of the two chambers with the guillotine door shut without urine for 15 min (neutral exposure). On day 0, rats were placed in the other chamber with the guillotine door shut and a sponge soaked with 3 ml of bobcat urine (Lynx rufus; Maine Outdoor Solutions; Hermon-ME, USA) was placed under the floor (rats could not contact the sponge) for 15 min (odor exposure). Controls were treated identically to odor-exposed rats, but the sponges did not contain bobcat urine. On day 1, rats were allowed to explore the two chambers for 5 min (post-exposure session). All testing was conducted using indirect dim illumination (one 60W white light facing the wall providing approximately 10 lux in the apparatus) and all sessions were recorded. Avoidance was quantified as a difference score calculated as time spent in odor-paired chamber on day 1 minus time spent in the same chamber on day −2. Rats that exhibited >10-s decrease in time spent in the odor-paired context were classified as Avoiders; all other bobcat urine-exposed rats were classified as Non-Avoiders. All animals were tested during the dark phase of the light-dark cycle, between 9:00 a.m.–12:00 p.m.
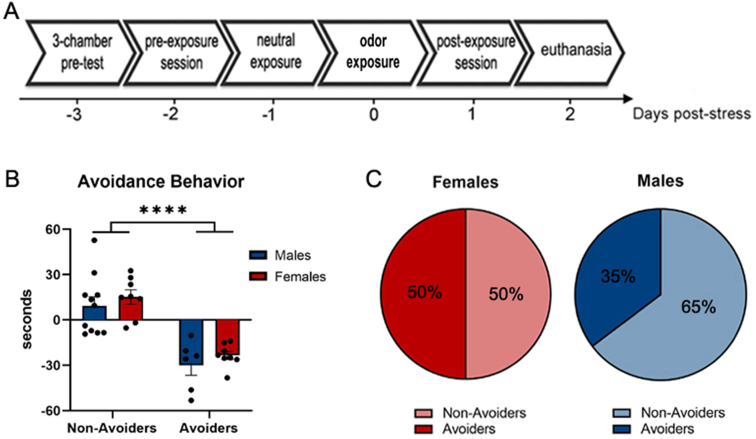
Fig. 1.
Avoidance behavior in male and female rats exposed to predator odor stress. A. Experimental design. Rats underwent the conditioned place aversion paradigm using bobcat urine. Controls were never exposed to predator odor stress. Avoidance behavior was measured 24 h post-stress (day 1). At day 2 post-stress, rats were euthanized and brains were collected for analysis of endocannabinoid content in the amygdala. B. Change in time (mean ± SEM) spent in bobcat urine-paired chamber in rats indexed as Avoiders or Non-Avoiders. C. Avoidance distribution of stressed rats (Avoiders × Non-Avoiders). **** denotes P < .0001.
2.4. Endocannabinoid extraction and analysis
Rats were euthanized by decapitation under isoflurane anesthesia 2 days after exposure to bobcat urine during the dark phase of the light-dark cycle, between 9:00 a.m.–11:00 a.m. Brains were rapidly dissected, snap-frozen in −30 °C isopentane, and stored at −80 °C until microdissection. BLA and CeA tissue samples were taken from frozen coronal brain sections (500 μm thick) using a 17-gauge punch tool on a cryostat, in accordance with Paxinos and Watson (2007). Tissue punches were stored at −80 °C until homogenization. The lipid extraction process was performed as described by Morena et al. (2015). Briefly, brain tissue was weighed and placed into borosilicate glass culture tubes containing 2 ml of acetonitrile with 5 pmol of [2H8] AEA and 5 nmol of [2H8] 2-AG for extraction, and homogenized with a glass rod. Tissue was sonicated for 30 min on ice water and incubated overnight at −20 °C to precipitate proteins, then centrifuged at 1500×g to remove particulates. The supernatants were transferred to a new glass tube and evaporated to dryness under N2 gas. The samples were reconstituted in 200 μl of acetonitrile and dried again under N2 gas. Lipid extracts were suspended in 200 μl of acetonitrile and stored at −80 °C until analysis. Measurements of 2-AG and AEA were performed by liquid chromatography mass spectrometry as previously detailed (Qi et al., 2015).
2.5. Stereotaxic surgery
Separate cohorts of experimentally naïve female rats were anesthetized with isoflurane and surgically implanted with 26-gauge cannulas (Plastics One; Roanoke-VA, USA) aimed at the BLA or CeA. The stereotaxic coordinates, according with Paxinos and Watson (2007), were as follows: BLA = 2.4 mm posterior to bregma, ± 4.9 mm lateral to the midline, and 7.7 mm ventral to the skull; CeA = 2.3 mm posterior to bregma, ± 4.0 mm lateral to the midline, and 7.4 mm ventral to the skull. At the end of surgeries, rats were monitored to ensure recovery from anesthesia and were given 5–7 days to recover before the start of behavioral procedures. Rats were treated with the analgesic flunixin (2.5 mg/kg, s.c.) and the antibiotic cefazolin (20 mg/kg, i.m.) before the start of surgeries and once the following day.
2.6. Drug treatment
The DAGL inhibitor DO34 (1 μg in 0.3 μl; Sigma-Aldrich, catalog #SML2732) was dissolved in 70% dimethyl sulfoxide (McReynolds et al., 2018). The FAAH inhibitor URB597 (10 ng in 0.2 μl; Sigma-Aldrich, catalog #U4133) was dissolved in 5% polyethylene glycol, 5% Tween-80, and 90% saline (Gray et al., 2015; Morena et al., 2014, 2016). On the day before the test day, rats received 1 sham injection, consisting of insertion of the 30-gauge injection needles into the cannulas for 2 min. On the test day, rats received bilateral intra-BLA infusions of DO34 or an equivalent volume of vehicle or intra-CeA infusions of URB597 or an equivalent volume of vehicle by using the injection needles connected by polyethylene tubing (PE-20) to 10-μl Hamilton microsyringes driven by an infusion syringe pump (KD Scientific Inc.; Holliston-MA, USA). The injection needles protruded 1 mm beyond the tips of the cannulas, and the injection volume was infused over a period of 30 s. The injection needles were retained within the cannulas for an additional minute after drug infusion to maximize diffusion and to prevent drug backflow into the cannulas.
2.7. Acoustic startle response test
Female rats were tested for ASR 45–60 min after vehicle or drug infusions. Rats were placed in a Plexiglas tube attached to an accelerometer inside a dark, soundproof chamber (San Diego Instruments; San Diego-CA, USA) and allowed to acclimate for 5 min (75-dB background noise) before the test session (Albrechet-Souza et al., 2020). This background white noise was present throughout the session. The chamber and Plexiglas tube were cleaned with Quatricide between each animal. Before testing, an S-R calibrator tube was used to calibrate the chambers. The test session consisted of 30 trials with startle stimuli of three different decibel levels: 750-ms bursts of 90 dB, 105 dB or 115 dB white noise were randomly presented 10 times each, with each pair of presentations separated by a 30-s fixed inter-trial interval. The maximum startle response (Vmax, arbitrary units) during the first 100 ms of each trial was recorded.
2.8. Histology
Female rats were euthanized by decapitation under isoflurane anesthesia. The brains were rapidly dissected, snap-frozen in −30 °C isopentane, and stored at −20 °C until sectioning. Coronal sections of 50 μm were cut on a cryostat, mounted on gelatin-coated slides, and stained with cresyl violet. The sections were examined under a light microscope, and determination of the location of infusion needle tips within the BLA or CeA was made according with Paxinos and Watson (2007). Only rats with bilaterally accurate cannula placements were included in the data analysis. Three animals were excluded because of cannula misplacement.
2.9. Statistical analysis
Data are presented as mean and standard error of the mean (SEM), except where otherwise indicated. Statistical analysis and graph construction were performed using Graphpad Prism 9 (GraphPad; La Jolla-CA, USA). Effect size partial eta squared (ηp2) was calculated for significant main effects and interactions using IBM SPSS Statistics 27 (IBM; New York-NY, USA). Two-way analysis of variance (ANOVA) was performed to analyze change in time spent in odor-paired chamber and eCB contents in amygdala subnuclei – the variables in all cases were sex and stress condition. Startle reactivity was analyzed with two-way repeated measures (RM) ANOVAs – the variables were drug dose (between-subject) and decibel level (within-subject). Fisher's exact test was used to analyze differences in the proportion of Avoiders and Non-Avoiders in each sex. Student's unpaired t-test was used to compare the magnitude of avoidance in male and female Avoiders. A priori Student's unpaired t-tests were used to compare eCB contents between Avoiders and Non-Avoiders in each sex. In cases of significant ANOVA effects, appropriate post hoc comparisons were performed. Statistical significance threshold was set at P < .05.
3. Results
3.1. Avoidance behavior in male and female rats
Timeline of the experimental procedure is shown in Fig. 1A. Independent of sex, Avoiders exhibited significantly greater avoidance of the odor-paired chamber at 24 h post-exposure (F (1, 29) = 50.30, P < .0001; ηp2 = 0.634) relative to Non-Avoiders (Fig. 1B). There was no significant difference in the magnitude of avoidance between male and female Avoiders (t = 1.00, P = .33) or in the proportion of animals that met Avoider criteria in both sexes (Fig. 1C; P = .49).
3.2. Stress effects on endocannabinoid content in the amygdala of male and female rats
Because we did not find significant differences in amygdalar eCB contents between Avoider and Non-Avoider males (BLA 2-AG: t = 0.59, P = .56; BLA AEA: t = 0.93, P = .36; CeA 2-AG: t = 1.47, P = .16; CeA AEA: t = 0.47, P = .64) nor between Avoider and Non-Avoider females (BLA 2-AG: t = 1.51, P = .16; BLA AEA: t = 1.23, P = .25; CeA 2-AG: t = 0.96, P = .35; CeA AEA: t = 0.34, P = .73), rats exposed to bobcat urine were pooled into a group designated Stress and compared to unstressed Controls, segregated by sex.
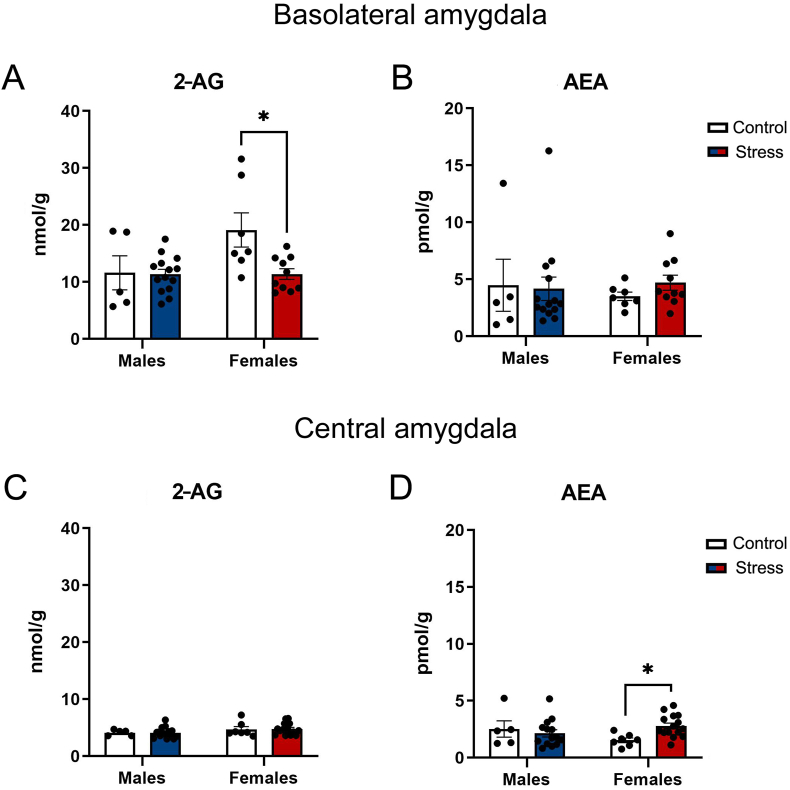
A two-way ANOVA yielded significant main effects of sex (F (1, 32) = 4.58, P = .04; ηp2 = 0.125) and stress (F (1, 32) = 5.10, P = .03; ηp2 = 0.138), as well as a sex × stress interaction effect (F (1, 32) = 4.54, P = .04; ηp2 = 0.124) on 2-AG levels in the BLA following exposure to bobcat urine. Tukey's post-hoc comparisons revealed that stressed females exhibited lower levels of 2-AG in the BLA relative to Control females (P = .01) (Fig. 2A). There was also a significant sex × stress interaction effect (F (1, 37) = 4.67, P = .03; ηp2 = 0.112) on AEA levels in the CeA. Tukey's post-hoc comparisons revealed that stressed females exhibited higher levels of AEA in the CeA relative to Control females (P = .01) (Fig. 2D).
Fig. 2.
Endocannabinoid content in the amygdala of male and female rats exposed to predator odor stress. At day 2 post-stress, rats were euthanized, and brains were rapidly dissected and snap-frozen. Basolateral (BLA) and central (CeA) amygdala tissue samples were taken from frozen coronal sections; analyses of 2-arachidonoyl glycerol (2-AG) and anandamide (AEA) were performed by liquid chromatography mass spectrometry. A. 2-AG levels in the BLA of Controls and stressed rats. B. AEA levels in the BLA of Controls and stressed rats. C. 2-AG levels in the CeA of Controls and stressed rats. D. AEA levels in the CeA of Controls and stressed rats. Data presented as mean ± SEM. * denotes P < .05.
There were no effects of sex (F (1, 32) = 0.03, P = .85) or stress (F (1, 32) = 0.14, P = .70) on AEA levels in the BLA (Fig. 2B), nor were there effects of sex (F (1, 37) = 3.18, P = .08) or stress (F (1, 37) = 0.01, P = .91) on 2-AG levels in the CeA (Fig. 2C).
3.3. Startle reactivity in female rats injected with intra-BLA DO34
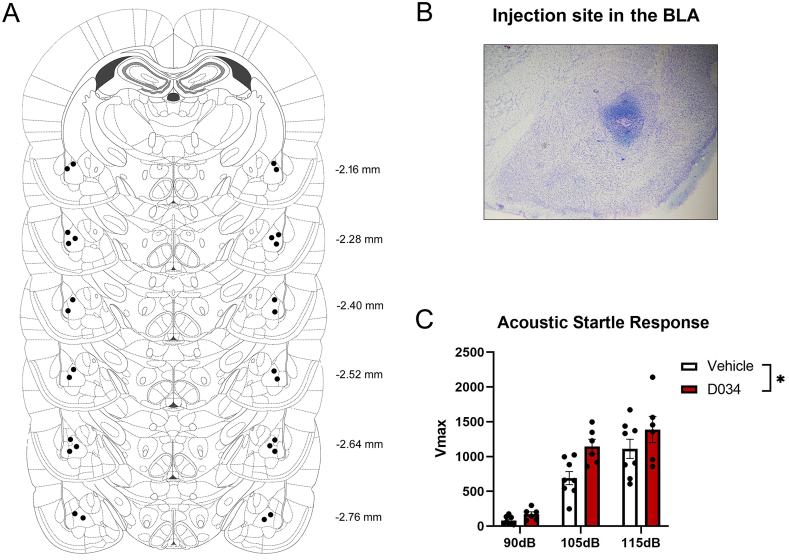
Fig. 3A shows correct placements of intra-BLA cannulas. Fig. 3B is a representative photomicrograph of the drug injection site in the BLA. A two-way RM ANOVA showed that females that received intra-BLA microinjections of DO34 exhibited significantly higher ASR (F (1, 12) = 5.34, P = .03; ηp2 = 0.308) relative to vehicle-injected females. As expected, we also found a significant decibel effect (F (2, 24) = 86.61, P < .0001; ηp2 = 0.878) (Fig. 3C).
Fig. 3.
Intra-basolateral amygdala (BLA) D034 modulates acoustic startle reactivity (Vmax) in female rats. A. Correct placements of intra-BLA cannulas. Each diagram corresponds to a coronal section of the rat brain according to the bregma (Paxinos and Watson, 2007). B. Representative photomicrograph of injection site in the BLA after cresyl violet staining. C. Acoustic startle response in female rats microinjected with vehicle or the diacylglycerol lipase inhibitor DO34 in the BLA. Data presented as mean ± SEM. * denotes P < .05.
3.4. Startle reactivity in female rats injected with intra-CeA URB597
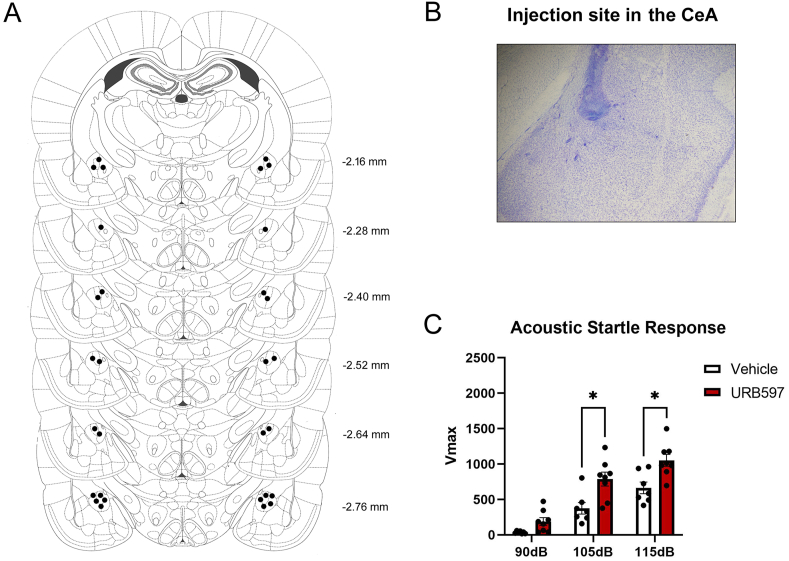
Fig. 4A shows correct placements of intra-CeA cannulas. Fig. 4B is a representative photomicrograph of the drug injection site in the CeA. A two-way RM ANOVA yielded significant main effects of drug (F (1, 13) = 12.38, P = .0038; ηp2 = 0.488) and decibel (F (2, 26) = 118.70, P < .0001; ηp2 = 0.901), as well as a drug × decibel interaction effect (F (2, 26) = 4.29, P = .024; ηp2 = 0.248) on ASR in females that received intra-CeA microinjections of URB597. Bonferroni's multiple comparisons revealed that, at 105 dB and 115 dB, URB597-injected females exhibited higher ASR in comparison with vehicle-injected females (P = .019 and .017, respectively) (Fig. 4C).
Fig. 4.
Intra-central amygdala (CeA) URB597 modulates acoustic startle reactivity (Vmax) in female rats. A. Correct placements of intra-CeA cannulas. Each diagram corresponds to a coronal section of the rat brain according to the bregma (Paxinos and Watson, 2007). B. Representative photomicrograph of injection site in the CeA after cresyl violet staining. C. Acoustic startle response in female rats microinjected with vehicle or the fatty acid amide hydrolase inhibitor URB597 in the CeA. Data presented as mean ± SEM. * denotes P < .05.
4. Discussion
A better understanding of sex-specific stress effects is critical to advance our understanding of the mechanisms involved in the etiology of stress-related disorders and improving treatments for such conditions. Female rats had lower levels of 2-AG in the BLA and higher levels of AEA in the CeA 2 days after exposure to bobcat urine, while predator odor did not affect amygdalar eCB content in males. Contrary to our hypothesis, microinjections of either the DAGL inhibitor DO34 into the BLA or the FAAH inhibitor URB597 into the CeA significantly enhanced ASR in experimentally naïve females compared to vehicle-treated rats. These findings describe sex-specific effects of predator odor stress on amygdalar eCBs, and new roles for amygdalar eCBs in regulating behavior in females.
In line with previous studies, bobcat urine exposure elicited avoidance in a subset of animals (Albrechet-Souza et al., 2020; Edwards et al., 2013; Weera et al., 2020). We found that, in rats exposed to bobcat urine, 35% of males were classified as Avoiders versus 50% of females, with similar magnitude of avoidance behavior 24 h post-stress (Fig. 1). The proportion of female Avoiders was slightly higher than our previous report (37%; Albrechet-Souza et al., 2020), suggesting potential differences between cohorts (the definition of Avoider and Non-Avoider was the same across studies). These findings are interesting in light of epidemiological studies showing that women are at higher risk for development and maintenance of behaviors associated with traumatic events (Breslau, 2009; Kessler et al., 2005).
We found that predator odor stress elicits sex-specific alterations in eCB content in the amygdala (Fig. 2). Female rats showed lower levels of 2-AG in the BLA and higher levels of AEA in the CeA 2 days after exposure to bobcat urine. There were no differences in amygdalar eCB levels between female Avoider and Non-Avoider rats, suggesting a general stress-related response to bobcat urine exposure. Conversely, male rats did not exhibit any significant alterations in amygdalar eCBs 2 days after predator odor stress. While previous work has reliably found dynamic changes in amygdalar AEA, and sometimes in 2-AG as well, immediately following exposure to acute stress in males (Gray et al., 2015; Hill et al., 2009; Patel et al., 2005; Rademacher et al., 2008; Yasmin et al., 2020) the current data demonstrate that persistent changes in amygdalar eCB levels do not occur in males, at least after predator odor stress.
Following repeated exposure to stress, males have been found to have sustained reductions in 2-AG content in the amygdala (Qin et al., 2015), suggesting that males may develop similar changes as seen here in females, but only after more persistent exposure to stress. Moreover, with our current experimental design, we cannot rule out that rapid, transient changes could have occurred in amygdalar eCBs after termination of bobcat urine exposure in male rats. Future work should examine the post-stress temporal profile of eCB expression in more depth to understand if these changes are functionally relevant to sex differences in vulnerability to traumatic events.
Our findings also contrast with those of Lim et al. (2016) who have previously showed a sustained elevation in amygdalar 2-AG content in male Sprague-Dawley rats for 14 days after exposure to TMT. Discrepancies between the work of Lim et al. and our study may be due to differences in the rat strains, specificity of brain area, predator scents and experimental design. Rat strains exhibit different eCB levels in the amygdala after stress exposure (Jennings et al., 2016). Furthermore, whereas we measured eCBs in specific amygdala subnuclei 24 h after exposure to the bobcat urine-paired context (i.e., 48 h after bobcat urine exposure), Lim et al. measured 2-AG levels in the whole amygdala at various time points (30 minutes–14 days) following TMT exposure. Finally, different predator odors have been shown to induce distinct profiles of defensive behaviors and hypothalamic c-fos expression in rodents (de Oliveira Crisanto et al., 2015; Maestas-Olguin et al., 2021).
Previous studies that measured eCBs in the brains of female animals are scarce. In mice, females had higher AEA levels, but not 2-AG levels, in the whole amygdala compared to control groups immediately after a fear-potentiated startle test (Kirchhoff et al., 2019). Acute alcohol withdrawal reduced AEA content in the BLA and 2-AG content in the ventromedial prefrontal cortex of male, but not female Wistar rats (Henricks et al., 2017). Contrary to our results, that report indicated that females had significantly less AEA in the BLA at baseline compared to males. Rats in that study were exposed to intermittent alcohol vapor or to the chamber without alcohol vapor for six weeks, thus they were older at the time of euthanasia than animals in the current study. This is important to note because age-related alterations in the expression of eCBs, key enzymes and receptors involved in eCBs function have been reported in both rodent and human brains (Kwok et al., 2017).
Our observed reductions in 2-AG levels in the BLA of stressed females is interesting in the context of prior work showing that enhanced amygdala 2-AG signaling promotes resilience to adverse effects of acute traumatic stress and facilitates adaptation to repeated stress exposure (Bluett et al., 2017). Future studies will help to identify whether lower levels of 2-AG in the BLA is related to higher risk for stress-related disorders in females. Moreover, as discussed earlier, it will be interesting to include other time points to evaluate whether differences in amygdalar eCB levels between males and females might actually reflect sex differences in temporal profile of eCB contents.
Divergent effects of AEA versus 2-AG signaling manipulations on fear memory have been recently reported in females (Morena et al., 2021). While systemic elevation of 2-AG signaling reduces freezing and facilitates extinction via activation of CB1 in female rats exposed to an auditory fear conditioning paradigm, elevated AEA signaling at TRPV1 increases freezing, fear generalization and impairs fear extinction (Morena et al., 2021). TRPV1 can be activated by high AEA levels (Bialecki et al., 2020; Di Marzo, 2008; Zygmunt et al., 1999) and, unlike CB1, its activation promotes membrane depolarization, increases neuronal firing rate and facilitates neurotransmitter release (Bialecki et al., 2020; Marinelli et al., 2003; Musella et al., 2009; Xing and Li, 2007). Thus, it is possible that the increase in AEA content observed in the CeA of female rats after exposure to predator odor can favor AEA signaling at TRPV1s. Future work is required to understand this relationship in more depth, exploring the effects of direct TRPV1 manipulations.
Exaggerated startle response is considered a hallmark symptom of PTSD (American Psychiatric Association, 2013) and is predictive of disease severity (Able and Benedek, 2019). However, investigations of startle responsivity in male and female patients with PTSD have produced mixed results (Medina et al., 2020; Morgan et al., 1996). In a recent study, we reported that female rats exhibit blunted ASR 2 days after exposure to bobcat urine, whereas predator odor stress does not affect ASR in males without a history of alcohol consumption (Albrechet-Souza et al., 2020). We tested here whether pharmacological manipulations of amygdalar eCB synthesis and degradation would mimic these effects of predator odor stress on ASR in females. We found that both acute DAGL inhibition in the BLA (Fig. 3) and acute FAAH inhibition in the CeA (Fig. 4) significantly enhanced ASR in experimentally naïve females. Although contrary to our initial hypothesis, these results are consistent with previous findings showing that, in female rats, AEA and 2-AG signaling can affect the expression of fear-related behaviors in opposite directions (Morena et al., 2021).
5. Conclusions
In conclusion, we report robust sex differences in amygdalar eCB contents after exposure to predator odor stress. Female rats had lower levels of 2-AG in the BLA and higher levels of AEA in the CeA after exposure to bobcat urine, while predator odor did not affect amygdalar eCBs in males. Contrary to our hypotheses, inhibition of 2-AG synthesis in the BLA and inhibition of AEA hydrolysis in the CeA enhanced startle reactivity in females. Our findings highlight the importance of leveraging sex differences in investigations of the neurobiological regulation of behavior, and describe a new role for amygdalar eCBs in regulating behavior in female animals.
Funding
This work was supported by the National Institutes of Health [grant numbers R01AA023305, R01AA026531], Cohen Veterans Bioscience and grants COH-0011 from Steven A. Cohen. This work was also supported in part by a Merit Review Award from the United States Department of Veterans Affairs, Biomedical Laboratory Research and Development Service [grant number I01 BX003451].
CRediT authorship contribution statement
Lucas Albrechet-Souza: Conceptualization, Investigation, Formal analysis, Visualization, Writing – original draft. Andrei S. Nastase: Investigation, Writing – review & editing. Matthew N. Hill: Methodology, Resources, Writing – review & editing. Nicholas W. Gilpin: Conceptualization, Supervision, Methodology, Resources, Writing – review & editing, Funding acquisition, All authors read and approved the final manuscript.
Declaration of competing interest
Nicholas W. Gilpin owns shares in Glauser Life Sciences, a company with interest in developing therapeutics for mental health disorders. There is no direct link between those interests and the work contained herein.
Acknowledgments
The authors thank Maria E Secci for her excellent technical support.
Data availability
Data will be made available on request.
References
- Able M.L., Benedek D.M. Severity and symptom trajectory in combat-related PTSD: a review of the literature. Curr. Psychiatr. Rep. 2019;21(7):58. doi: 10.1007/s11920-019-1042-z. [DOI] [PubMed] [Google Scholar]
- Albrechet-Souza L., Gilpin N.W. The predator odor avoidance model of post-traumatic stress disorder in rats. Behav. Pharmacol. 2019;30(2 and 3-Spec):105–114. doi: 10.1097/FBP.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrechet-Souza L., Schratz C.L., Gilpin N.W. Sex differences in traumatic stress reactivity in rats with and without a history of alcohol drinking. Biol. Sex Differ. 2020;11(1):27. doi: 10.1186/s13293-020-00303-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altemus M., Sarvaiya N., Neill Epperson C. Sex differences in anxiety and depression clinical perspectives. Front. Neuroendocrinol. 2014;35(3):320–330. doi: 10.1016/j.yfrne.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . fifth ed. American Psychiatric Publishing; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Bangasser D.A., Valentino R.J. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front. Neuroendocrinol. 2014;35(3):303–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialecki J., Werner A., Weilinger N.L., Tucker C.M., Vecchiarelli H.A., Egaña J., Mendizabal-Zubiaga J., Grandes P., Hill M.N., Thompson R.J. Suppression of presynaptic glutamate release by postsynaptic metabotropic NMDA receptor signalling to pannexin-1. J. Neurosci. 2020;40(4):729–742. doi: 10.1523/JNEUROSCI.0257-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankman J.L., Cravatt B.F. Chemical probes of endocannabinoid metabolism. Pharmacol. Rev. 2013;65(2):849–871. doi: 10.1124/pr.112.006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluett R.J., Báldi R., Haymer A., Gaulden A.D., Hartley N.D., Parrish W.P., Baechle J., Marcus D.J., Mardam-Bey R., Shonesy B.C., Uddin M.J., Marnett L.J., Mackie K., Colbran R.J., Winder D.G., Patel S. Endocannabinoid signalling modulates susceptibility to traumatic stress exposure. Nat. Commun. 2017;8:14782. doi: 10.1038/ncomms14782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N. The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma Violence Abuse. 2009;10(3):198–210. doi: 10.1177/1524838009334448. [DOI] [PubMed] [Google Scholar]
- de Oliveira Crisanto K., de Andrade W.M., de Azevedo Silva K.D., Lima R.H., de Oliveira Costa M.S., de Souza Cavalcante J., de Lima R.R., do Nascimento E.S., Jr., Cavalcante J.C. The differential mice response to cat and snake odor. Physiol. Behav. 2015;152(Pt A):272–279. doi: 10.1016/j.physbeh.2015.10.013. [DOI] [PubMed] [Google Scholar]
- Devane W.A., Hanus L., Breuer A., Pertwee R.G., Stevenson L.A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di S., Itoga C.A., Fisher M.O., Solomonow J., Roltsch E.A., Gilpin N.W., Tasker J.G. Acute stress suppresses synaptic inhibition and increases anxiety via endocannabinoid release in the basolateral amygdala. J. Neurosci. 2016;36(32):8461–8470. doi: 10.1523/JNEUROSCI.2279-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce? Nat. Rev. Drug Discov. 2008;7(5):438–455. doi: 10.1038/nrd2553. [DOI] [PubMed] [Google Scholar]
- Edwards S., Baynes B.B., Carmichael C.Y., Zamora-Martinez E.R., Barrus M., Koob G.F., Gilpin N.W. Traumatic stress reactivity promotes excessive alcohol drinking and alters the balance of prefrontal cortex-amygdala activity. Transl. Psychiatry. 2013;3(8):e296. doi: 10.1038/tp.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J.M., Vecchiarelli H.A., Morena M., Lee T.T., Hermanson D.J., Kim A.B., McLaughlin R.J., Hassan K.I., Kühne C., Wotjak C.T., Deussing J.M., Patel S., Hill M.N. Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J. Neurosci. 2015;35(9):3879–3892. doi: 10.1523/JNEUROSCI.2737-14.2015. 2015 Mar 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O., MacPherson K.P., Cinar R., Gamble-George J., Sugden K., Williams B., Godlewski G., Ramikie T.S., Gorka A.X., Alapafuja S.O., Nikas S.P., Makriyannis A., Poulton R., Patel S., Hariri A.R., Caspi A., Moffitt T.E., Kunos G., Holmes A. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol. Psychiatr. 2013;18(7):813–823. doi: 10.1038/mp.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henricks A.M., Berger A.L., Lugo J.M., Baxter-Potter L.N., Bieniasz K.V., Petrie G., Sticht M.A., Hill M.N., McLaughlin R.J. Sex- and hormone-dependent alterations in alcohol withdrawal-induced anxiety and corticolimbic endocannabinoid signaling. Neuropharmacology. 2017;124:121–133. doi: 10.1016/j.neuropharm.2017.05.023. [DOI] [PubMed] [Google Scholar]
- Hill M.N., McLaughlin R.J., Morrish A.C., Viau V., Floresco S.B., Hillard C.J., Gorzalka B.B. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology. 2009;34(13):2733–2745. doi: 10.1038/npp.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S.S., Mackie K. Distribution of the endocannabinoid system in the central nervous system. Handb. Exp. Pharmacol. 2015;231:59–93. doi: 10.1007/978-3-319-20825-1_3. [DOI] [PubMed] [Google Scholar]
- Itoga C.A., Roltsch Hellard E.A., Whitaker A.M., Lu Y.L., Schreiber A.L., Baynes B.B., Baiamonte B.A., Richardson H.N., Gilpin N.W. Traumatic stress promotes hyperalgesia via corticotropin-releasing factor-1 receptor (CRFR1) signaling in central amygdala. Neuropsychopharmacology. 2016;41(10):2463–2472. doi: 10.1038/npp.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings E.M., Okine B.N., Olango W.M., Roche M., Finn D.P. Repeated forced swim stress differentially affects formalin-evoked nociceptive behaviour and the endocannabinoid system in stress normo-responsive and stress hyper-responsive rat strains. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2015;64:181–189. doi: 10.1016/j.pnpbp.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatr. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. Erratum in: Arch. Gen. Psychiatry. 62 (7), 768. Merikangas, Kathleen R [added] [DOI] [PubMed] [Google Scholar]
- Kirchhoff A.M., Barker E.L., Chester J.A. Endocannabinoids and fear-related behavior in mice selectively bred for high or low alcohol preference. Brain Sci. 2019;9(10):254. doi: 10.3390/brainsci9100254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok C.H., Devonshire I.M., Imraish A., Greenspon C.M., Lockwood S., Fielden C., Cooper A., Woodhams S., Sarmad S., Ortori C.A., Barrett D.A., Kendall D., Bennett A.J., Chapman V., Hathway G.J. Age-dependent plasticity in endocannabinoid modulation of pain processing through postnatal development. Pain. 2017;158(11):2222–2232. doi: 10.1097/j.pain.0000000000001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J., Igarashi M., Jung K.M., Butini S., Campiani G., Piomelli D. Endocannabinoid modulation of predator stress-induced long-term anxiety in rats. Neuropsychopharmacology. 2016;41(5):1329–1339. doi: 10.1038/npp.2015.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestas-Olguin C.R., Parish M.M., Pentkowski N.S. Coyote urine, but not 2-phenylethylamine, induces a complete profile of unconditioned anti-predator defensive behaviors. Physiol. Behav. 2021;229:113210. doi: 10.1016/j.physbeh.2020.113210. [DOI] [PubMed] [Google Scholar]
- Marinelli S., Di Marzo V., Berretta N., Matias I., Maccarrone M., Bernardi G., Mercuri N.B. Presynaptic facilitation of glutamatergic synapses to dopaminergic neurons of the rat substantia nigra by endogenous stimulation of vanilloid receptors. J. Neurosci. 2003;23(8):3136–3144. doi: 10.1523/JNEUROSCI.23-08-03136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G., Wotjak C.T., Azad S.C., Bisogno T., Rammes G., Cascio M.G., Hermann H., Tang J., Hofmann C., Zieglgänsberger W., Di Marzo V., Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418(6897):530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Matsuda L.A., Lolait S.J., Brownstein M.J., Young A.C., Bonner T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346(6284):561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McReynolds J.R., Doncheck E.M., Li Y., Vranjkovic O., Graf E.N., Ogasawara D., Cravatt B.F., Baker D.A., Liu Q.S., Hillard C.J., Mantsch J.R. Stress promotes drug seeking through glucocorticoid-dependent endocannabinoid mobilization in the prelimbic cortex. Biol. Psychiatr. 2018;84(2):85–94. doi: 10.1016/j.biopsych.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina A.M., Mejia V.Y., Schell A.M., Dawson M.E., Margolin G. Startle reactivity and PTSD symptoms in a community sample of women. Psychiatr. Res. 2001;101(2):157–169. doi: 10.1016/s0165-1781(01)00221-9. [DOI] [PubMed] [Google Scholar]
- Micale V., Drago F. Endocannabinoid system, stress and HPA axis. Eur. J. Pharmacol. 2018;834:230–239. doi: 10.1016/j.ejphar.2018.07.039. [DOI] [PubMed] [Google Scholar]
- Morena M., Roozendaal B., Trezza V., Ratano P., Peloso A., Hauer D., Atsak P., Trabace L., Cuomo V., McGaugh J.L., Schelling G., Campolongo P. Endogenous cannabinoid release within prefrontal-limbic pathways affects memory consolidation of emotional training. Proc. Natl. Acad. Sci. U.S.A. 2014;111(51):18333–18338. doi: 10.1073/pnas.1420285111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena M., De Castro V., Gray J.M., Palmery M., Trezza V., Roozendaal B., Hill M.N., Campolongo P. Training-associated emotional arousal shapes endocannabinoid modulation of spatial memory retrieval in rats. J. Neurosci. 2015;35(41):13962–13974. doi: 10.1523/JNEUROSCI.1983-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena M., Leitl K.D., Vecchiarelli H.A., Gray J.M., Campolongo P., Hill M.N. Emotional arousal state influences the ability of amygdalar endocannabinoid signaling to modulate anxiety. Neuropharmacology. 2016;111:59–69. doi: 10.1016/j.neuropharm.2016.08.020. [DOI] [PubMed] [Google Scholar]
- Morena M., Aukema R.J., Leitl K.D., Rashid A.J., Vecchiarelli H.A., Josselyn S.A., Hill M.N. Upregulation of anandamide hydrolysis in the basolateral complex of amygdala reduces fear memory expression and indices of stress and anxiety. J. Neurosci. 2019;39(7):1275–1292. doi: 10.1523/JNEUROSCI.2251-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena M., Nastase A.S., Santori A., Cravatt B.F., Shansky R.M., Hill M.N. Sex-dependent effects of endocannabinoid modulation of conditioned fear extinction in rats. Br. J. Pharmacol. 2021;178(4):983–996. doi: 10.1111/bph.15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C.A., 3rd, Grillon C., Southwick S.M., Davis M., Charney D.S. Exaggerated acoustic startle reflex in Gulf War veterans with posttraumatic stress disorder. Am. J. Psychiatr. 1996;153(1):64–68. doi: 10.1176/ajp.153.1.64. [DOI] [PubMed] [Google Scholar]
- Munro S., Thomas K.L., Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365(6441):61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Musella, A., De Chiara, V., Rossi, S., Prosperetti, C., Bernardi, G., Maccarrone, M., Centonze, D. TRPV1 channels facilitate glutamate transmission in the striatum. Mol. Cell. Neurosci.ci. 40 (1), 89-97. 10.1016/j.mcn.2008.09.001. [DOI] [PubMed]
- Patel S., Roelke C.T., Rademacher D.J., Hillard C.J. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur. J. Neurosci. 2005;21(4):1057–1069. doi: 10.1111/j.1460-9568.2005.03916.x. [DOI] [PubMed] [Google Scholar]
- Patel S., Hill M.N., Cheer J.F., Wotjak C.T., Holmes A. The endocannabinoid system as a target for novel anxiolytic drugs. Neurosci. Biobehav. Rev. 2017;76(PtA):56–66. doi: 10.1016/j.neubiorev.2016.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus M.P., Stein M.B., Simmons A.N., Risbrough V.B., Halter R., Chaplan S.R. The effects of FAAH inhibition on the neural basis of anxiety-related processing in healthy male subjects: a randomized clinical trial. Neuropsychopharmacology. 2021;46(5):1011–1019. doi: 10.1038/s41386-020-00936-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., Watson C. sixth ed. Academic Press; San Diego: 2007. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- Qi M., Morena M., Vecchiarelli H.A., Hill M.N., Schriemer D.C. A robust capillary liquid chromatography/tandem mass spectrometry method for quantitation of neuromodulatory endocannabinoids. Rapid Commun. Mass Spectrom. 2015;29(20):1889–1897. doi: 10.1002/rcm.7277. [DOI] [PubMed] [Google Scholar]
- Qin Z., Zhou X., Pandey N.R., Vecchiarelli H.A., Stewart C.A., Zhang X., Lagace D.C., Brunel J.M., Béïque J.C., Stewart A.F., Hill M.N., Chen H.H. Chronic stress induces anxiety via an amygdalar intracellular cascade that impairs endocannabinoid signaling. Neuron. 2015;85(6):1319–1331. doi: 10.1016/j.neuron.2015.02.015. [DOI] [PubMed] [Google Scholar]
- Rademacher D.J., Meier S.E., Shi L., Ho W.S., Jarrahian A., Hillard C.J. Effects of acute and repeated restraint stress on endocannabinoid content in the amygdala, ventral striatum, and medial prefrontal cortex in mice. Neuropharmacology. 2007;54(1):108–116. doi: 10.1016/j.neuropharm.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Sugiura T., Kondo S., Sukagawa A., Nakane S., Shinoda A., Itoh K., Yamashita A., Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995;215(1):89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Weera M.M., Schreiber A.L., Avegno E.M., Gilpin N.W. The role of central amygdala corticotropin-releasing factor in predator odor stress-induced avoidance behavior and escalated alcohol drinking in rats. Neuropharmacology. 2020;166:107979. doi: 10.1016/j.neuropharm.2020.107979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker A.M., Gilpin N.W. Blunted hypothalamo-pituitary adrenal axis response to predator odor predicts high stress reactivity. Physiol. Behav. 2015;147:16–22. doi: 10.1016/j.physbeh.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J., Li J. TRPV1 receptor mediates glutamatergic synaptic input to dorsolateral periaqueductal gray (dl-PAG) neurons. J. Neurophysiol. 2007;97(1):503–511. doi: 10.1152/jn.01023.2006. [DOI] [PubMed] [Google Scholar]
- Yasmin F., Colangeli R., Morena M., Filipski S., van der Stelt M., Pittman Q.J., Hillard C.J., Teskey G.C., McEwen B.S., Hill M.N., Chattarji S. Stress-induced modulation of endocannabinoid signaling leads to delayed strengthening of synaptic connectivity in the amygdala. Proc. Natl. Acad. Sci. U.S.A. 2020;117(1):650–655. doi: 10.1073/pnas.1910322116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L.J., Pfaff D.W. Sex differences in neurological and psychiatric disorders. Front. Neuroendocrinol. 2014;35(3):253–254. doi: 10.1016/j.yfrne.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Zhang W.H., Zhang J.Y., Holmes A., Pan B.X. Amygdala circuit substrates for stress adaptation and adversity. Biol. Psychiatr. 2021;89(9):847–856. doi: 10.1016/j.biopsych.2020.12.026. [DOI] [PubMed] [Google Scholar]
- Zygmunt P.M., Petersson J., Andersson D.A., Chuang H., Sørgård M., Di Marzo V., Julius D., Högestätt E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400(6743):452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.