Abstract
Introduction
There is controversy regarding the best predictors of clinical deterioration in COVID-19.
Objective
This work aims to identify predictors of risk factors for deterioration in patients hospitalized due to COVID-19.
Methods design
Nested case-control study within a cohort. Setting: 13 acute care centers of the Osakidetza-Basque Health Service. Participants: patients hospitalized for COVID-19 with clinical deterioration—defined as onset of severe ARDS, ICU admission, or death—were considered cases. Two controls were matched to each case based on age. Sociodemographic data; comorbidities; baseline treatment; symptoms; date of onset; previous consultations; and clinical, analytical, and radiological variables were collected. An explanatory model of clinical deterioration was created by means of conditional logistic regression.
Results
A total of 99 cases and 198 controls were included. According to the logistic regression analysis, the independent variables associated with clinical deterioration were: emergency department O2 saturation ≤90% (OR 16.6; 95%CI 4–68), pathological chest X-ray (OR 5.6; 95%CI 1.7–18.4), CRP > 100 mg/dL (OR 3.62; 95%CI 1.62–8), thrombocytopenia with <150,000 platelets (OR 4; 95%CI 1.84–8.6); and a medical history of acute myocardial infarction (OR 15.7; 95%CI, 3.29–75.09), COPD (OR 3.05; 95%CI 1.43–6.5), or HT (OR 2.21; 95%CI 1.11–4.4). The model’s AUC was 0.86. On the univariate analysis, female sex and presence of dry cough and sore throat were associated with better clinical progress, but were not found to be significant on the multivariate analysis.
Conclusion
The variables identified could be useful in clinical practice for the detection of patients at high risk of poor outcomes.
Keywords: COVID-19, Prognostic factors, Clinical deterioration
Abstract
Introducción
Existe controversia sobre los mejores factores predictores de deterioro clínico en la COVID-19.
Objetivo
Identificar factores predictores de riesgo de deterioro en pacientes hospitalizados por COVID-19.
Métodos
Diseño: caso-control anidado dentro de una cohorte. Ámbito: 13 centros de agudos de Osakidetza-Servicio Vasco de Salud. Participantes: se consideró casos a pacientes hospitalizados por COVID-19 con deterioro clínico, definido como la aparición de síndrome de distrés respiratorio del adulto grave, ingreso en UCI o fallecimiento. Se emparejaron 2 controles por caso en función de la edad. Se recogieron variables sociodemográficas, comorbilidades, tratamientos basales, síntomas y fecha de inicio, consultas previas, así como variables clínicas, analíticas y radiológicas. Se creó un modelo explicativo del deterioro clínico mediante regresión logística condicional.
Resultados
Se incluyeron 99 casos y 198 controles. Mediante análisis de regresión logística las variables independientes asociadas con deterioro clínico fueron: saturación de O2 en Urgencias ≤90% (OR = 16,6, IC del 95%, 4–68), radiografía de tórax patológica (OR = 5,6, IC del 95%, 1,7–18,4), PCR > 100 mg/dL (OR = 3,62, IC del 95% 1,62–8) y trombocitopenia < 150.000 plaquetas (OR = 4, IC del 95%, 1,84–8,6) y, entre los antecedentes, haber padecido infarto agudo de miocardio (OR = 15,7, IC del 95%, 3,29–75,09), EPOC (OR = 3,05, IC del 95%, 1,43–6,5) o hipertensión arterial (OR = 2,21, IC del 95%1,11–4,4). El área bajo la curva alcanzado por el modelo fue 0,86. En el análisis univariado, se asociaron con mejor evolución clínica el sexo femenino, la presencia de tos seca y dolor de garganta, pero no resultaron significativas en el análisis multivariado.
Conclusión
Las variables identificadas podrían ser de utilidad en la práctica clínica para la detección de pacientes con alto riesgo de mala evolución.
Palabras clave: COVID-19, Factores pronósticos, Deterioro clínico
Introduction
COVID-19 is a disease that has challenged health systems all over the world1.
The majority of patients progress favorably. However, 20% of patients require hospitalization and their disease can progress rapidly to acute respiratory distress syndrome (ARDS), multiple organ dysfunction syndrome, or even death2. In addition, the symptoms are characterized by a significant clinical-radiological dissociation, such that clinical deterioration occurs suddenly3. Therefore, the identification of risk factors is a priority for the proper management of these patients.
The SEMI-COVID-19 Registry has revealed that mortality in Spain reached 21%, according to data from 20204. In addition, the percentage of hospitalized patients who required mechanical ventilation was as high as 65%.
The aim of this study was to identify factors of poor progress in SARS-CoV-2 infection in patients hospitalized due to COVID-19. Poor progress was defined as onset of severe ARDS during hospitalization (P/F ratio ≤100 mmHg), admission to the intensive care unit (ICU), or in-hospital death.
Methods
This is a nested case-control study within the COVID-19 Osakidetza cohort (NCT04463706). In this study, cases were patients admitted to the hospitalization ward due to COVID-19 who presented with clinical deterioration (presence during the hospitalization of severe ARDS (PaO2/FiO2 ≤ 100 mmHg), admission to the ICU, or in-hospital death). The controls were patients hospitalized due to COVID-19 who did not present with severe ARDS. Cases and controls were matched according to age groups. The exclusion criteria were those who died in the emergency department, those who were admitted directly to the ICU, or those who presented with a P/F ratio ≤ 100 mmHg in the first 24 h of hospitalization. Two controls were identified per case. The inclusion period was from March 3, 2020 to April 10, 2020.
The project was approved by the Basque Country Ethics Committee (PI2020059). Simple random sampling was used.
Variables were obtained from the Osakidetza-Basque Health System electronic medical record (EMR) data processing system by means of a review of the EMRs by trained reviewers supervised by collaborating clinicians.
The following variables were gathered: sociodemographic variables (age and sex); personal medical history (comorbidities included in the Charlson Comorbidity Index); disease history (symptoms and date of onset, date of first contact with the healthcare system, persistence of symptoms, treatments indicated, contact with primary care in the previous month); current hospitalization episode (symptoms upon arrival at the emergency department); vital signs (oxygen saturation as measured by a pulse oximeter (SaO2p); clinical signs and physical examination; laboratory tests upon admission (biochemistry: renal function, albumin, liver panel; acute-phase reactants: LDH, CRP; complete blood count and blood differential; coagulation, prothrombin time, and d-dimer); pathological X-ray upon admission, defined as an X-ray which showed previously unknown infiltrates or condensations or data indicative of ARDS (presence of bilateral opacities not fully explained by effusion, lobar collapse, a collapsed lung, or lung nodules).
The lowest P/F ratio (PaO2/FiO2) was calculated at various points in time: upon arrival at the emergency department, the day the patient was transferred to the hospital ward, and through day three of the hospital stay. In addition, the lowest P/F ratio reached during hospitalization was recorded for both the cases and the controls.
A case was defined as a patient who presented with clinical deterioration during hospitalization, defined as presence of a P/F ratio ≤100 mmHg, admission to the ICU, or in-hospital death (date).
Statistical analysis
Descriptive statistics of the variables were performed. Categorical variables are shown as frequency and percentages and continuous variables are shown as means and standard deviations or medians and interquartile ranges. On the univariate analysis, variables from cases and controls were compared using conditional logistic regression models. The dependent variable was clinical deterioration and the rest were independent variables.
Laboratory tests upon admission, which were recorded as continuous variables, were also analyzed after being categorized into three groups: normal range values (defined in the tables), lower values, and higher values.
For the conditional logistic regression model, all independent variables with p < .20 on the univariate analysis were included. Effects were considered significant when p < .05.
The odds ratio (OR) and 95% confidence intervals (95% CI) were calculated. The model’s explanatory power was analyzed by calculating R2 and the area under the curve (AUC).
Differences in the P/F ratio between cases and controls were analyzed by means of a boxplot for the P/F ratio values at different points of the hospitalization (arrival at the emergency department; arrival on the ward; day one; day two; day three; and the critical day, defined as the day a patient presented with deterioration. Longitudinal data analysis was performed in order to identify differences in the P/F ratio trends.
All statistical analyses were performed using SAS for Windows, version 9.4 (SAS Institute, Cary, NC, USA) and R©, version 4.0.0
Results
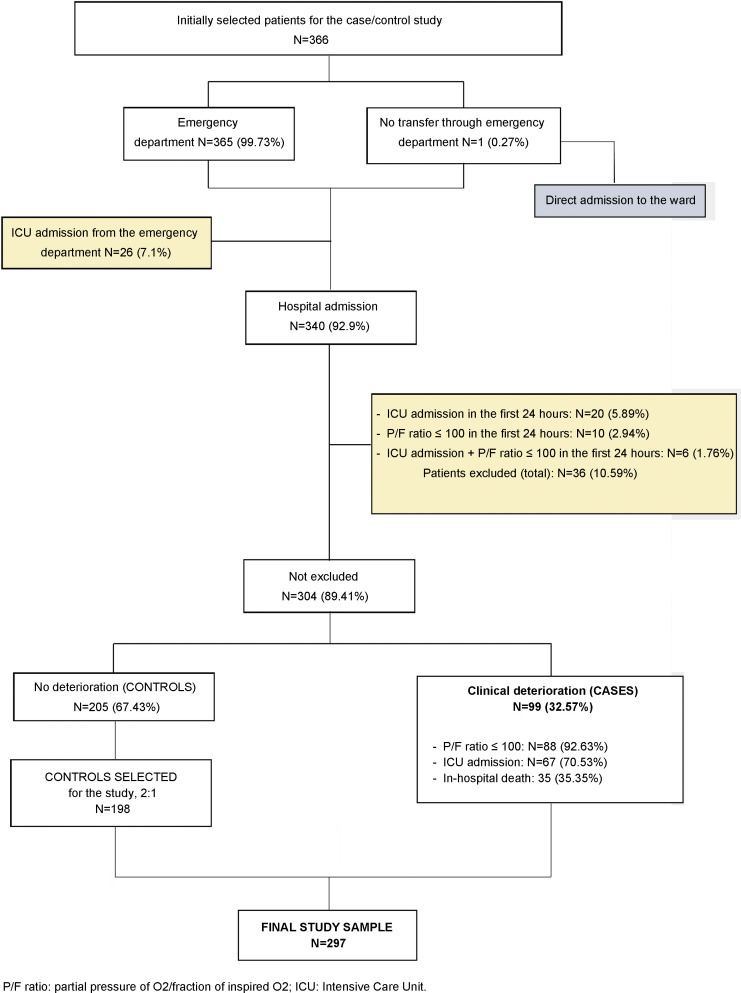
A total of 366 patients were randomly selected from the 4447 patients who were hospitalized during the study period in participating centers. Once ineligible patients were excluded, our sample comprised 99 cases and 205 controls, of which 198 eligible patients were selected in order to have a case/control ratio of 1:2 (Fig. 1 ).
Figure 1.
Flowchart.
Males were more numerous among the cases than the controls (79% vs. 65%, p = .014) as were patients who had had an acute myocardial infarction (AMI) (14% vs. 4%, p = .03), diabetes with organ damage (7% vs. 1.5%, p = .025), and hypertension (63% vs. 49.4%, p = .031). A lower frequency was observed among cases versus controls for the symptoms of dry cough (51.5% vs. 65%, p = .021) and sore throat (5% vs. 18.7%, p = .003). More cases than controls had consulted with their primary care physicians (48.5% vs. 34%, p = .016). The plain X-ray was more often pathological among the cases (91% vs. 77%, p = .004). There was an association between levels of glucose, LDH, CRP, and platelets and clinical deterioration; patients who deteriorated presented with higher figures of the first three parameters and lower platelet figures (Table 1 ). All the variables described in the methods section were considered on the univariate analysis (Table 2 ).
Table 1.
Differences according to deterioration of all patients (n = 297).
| Total | Deterioration |
pc | ||
|---|---|---|---|---|
| N (%) | Yes (cases) | No (controls) | ||
| N (%) | N (%) | |||
| Total | 297 | 99 (33.33) | 198 (66.67) | .0139 |
| Sex | ||||
| Male | 206 (69.36) | 78 (78.79) | 128 (64.65) | |
| Female | 91 (30.64) | 21 (21.21) | 70 (35.35) | |
| Prior follow-up | ||||
| Consultation in the last month for symptoms | 258 (87.16) | 81 (81.82) | 177 (89.95) | .0497 |
| Contact with primary care | 115 (38.72) | 48 (48.48) | 67 (33.84) | .0161 |
| Time between onset of symptoms and first contactb | 3 [1–5] | 2 [1–5] | 3 [1–5] | .3731 |
| Symptoms | ||||
| Syncope | 12 (4.05) | 1 (1.02) | 11 (5.56) | .0750 |
| Dry cough | 179 (60.68) | 50 (51.55) | 129 (65.15) | .0210 |
| Asthenia | 52 (17.57) | 13 (13.27) | 39 (19.7) | .1989 |
| Sore throat | 42 (14.19) | 5 (5.1) | 37 (18.69) | .0032 |
| Dyspnea | 141 (47.8) | 52 (53.61) | 89 (44.95) | .1495 |
| Pathological X-ray | 237 (81.72) | 88 (90.72) | 149 (77.2) | .0041 |
| Baseline P/F ratio | .5270 | |||
| 101–200 | 3 (1.01) | 2 (2.02) | 1 (0.51) | |
| 201–300 | 12 (4.04) | 4 (4.04) | 8 (4.04) | |
| >300 | 282 (94.95) | 93 (93.94) | 189 (95.45) | |
| Tachypnea in the emergency department | 48 (16.16) | 23 (23.23) | 25 (12.63) | .0245 |
| O2 saturation in the emergency department | <.0001 | |||
| ≤90 | 27 (9.28) | 20 (20.62) | 7 (3.61) | |
| >90 | 264 (90.72) | 77 (79.38) | 187 (96.39) | |
| Comorbidities | ||||
| AMI | 22 (7.41) | 14 (14.14) | 8 (4.04) | .0030 |
| Peripheral vascular disease | 27 (9.09) | 13 (13.13) | 14 (7.07) | .0828 |
| Dementia | 10 (3.37) | 3 (3.03) | 7 (3.54) | .8028 |
| COPD | 86 (28.96) | 34 (34.34) | 52 (26.26) | .1327 |
| Mild liver disease | 26 (8.75) | 13 (13.13) | 13 (6.57) | .0718 |
| DM with organ damage | 10 (3.37) | 7 (7.07) | 3 (1.52) | .0256 |
| Kidney disease | 24 (8.08) | 11 (11.11) | 13 (6.57) | .1707 |
| HT | 160 (53.87) | 62 (62.63) | 98 (49.42) | .0306 |
| Coagulopathy | 6 (2.02) | 4 (4.04) | 2 (1.01) | .1094 |
| Gastrointestinal bleeding | 9 (3.03) | 5 (5.05) | 4 (2.02) | .1720 |
| Asthma | 43 (14.48) | 15 (15.15) | 28 (14.14) | .8137 |
| Baseline blood test | ||||
| Creatininea | 1.01 (0.64) | 1.09 (0.89) | 0.96 (0.47) | .1459 |
| Glucoseb | 109 [97−126] | 117 [102−140] | 105 [95–120] | .0009 |
| LDHb | 286.06 [229−368] | 323.21 [259−425] | 273 [217.28−330.27] | .0006 |
| CRPb | 59.37 [27.67–106.57] | 87.97 [44.7–122.12] | 48.3 [21.12–89.48] | .0008 |
| Sodiuma | 137.59 (3.53) | 137.04 (3.76) | 137.86 (3.39) | .0444 |
| Ureab | 34 [27–44] | 37 [29.98–48] | 32.5 [26.05–41.08] | .0858 |
| Hematocrita | 42.11 (5.08) | 42.73 (5.41) | 41.8 (4.89) | .1455 |
| Plateletsb | 166 [134.5–215] | 154 [125–189] | 172 [142–221] | .0084 |
DM: diabetes mellitus; COPD: chronic obstructive pulmonary disease; HT: hypertension; AMI: acute myocardial infarction; CI: confidence interval; LDH: lactate dehydrogenase; OR: odds ratio; P/F ratio: partial pressure of O2/fraction of inspired O2; CRP: C-reactive protein.
Result shown as mean (standard deviation).
Result shown as median [interquartile range].
p value of the univariate conditional logistic regression model.
Table 2.
Univariate conditional logistic regression analysis.
| β (e.e.) | OR (95% CI) | p | |
|---|---|---|---|
| Sex (men vs. women) | 0.35 (0.14) | 2.016 (1.153–3.526) | .0139 |
| Prior follow-up | |||
| Consultation in the last month for symptoms (no vs. yes) | 0.35 (0.18) | 2.026 (1.001–4.101) | .0497 |
| Contact with primary care (no vs. yes) | 0.31 (0.13) | 1.858 (1.122–3.078) | .0161 |
| Time between onset of symptoms and first contacta | −0.029 (0.032) | 0.972 (0.913–1.035) | .3731 |
| Symptoms | |||
| Syncope (yes vs. no) | −0.96 (0.54) | 0.147 (0.018–1.214) | .0750 |
| Dry cough (no vs. yes) | 0.30 (0.13) | 1.829 (1.095–3.054) | .0210 |
| Asthenia (yes vs. no) | −0.22 (0.17) | 0.639 (0.322–1.266) | .1989 |
| Sore throat (no vs. yes) | 0.79 (0.27) | 4.43 (1.709–14.295) | .0032 |
| Dyspnea (yes vs. no) | 0.19 (0.13) | 1.454 (0.874–2.418) | .1495 |
| Pathological X-ray (yes vs. no) | 0.65 (0.23) | 3.692 (1.514–9.001) | .0041 |
| Baseline P/F ratio | |||
| 101–200 vs. >300 | −0.46 (0.46) | 0.250 (0.023–2.757) | .3113 |
| 201−300 vs. >300 | −0.46 (0.58) | 0.250 (0.017–3.660) | .4235 |
| Tachypnea in the emergency department (yes vs. no) | 0.36 (0.16) | 2.041 (1.096–3.799) | .0245 |
| O2 saturation in the emergency department (≤90 vs.>90) | 0.92 (0.23) | 6.291 (2.517–15.722) | <.0001 |
| Comorbidities | |||
| AMI (yes vs. no) | 0.78 (0.26) | 4.785 (1.699–13.477) | .0030 |
| Peripheral vascular disease (yes vs. no) | 0.37 (0.22) | 2.115 (0.907–4.929) | .0828 |
| Dementia (yes vs. no) | −0.10 (0.38) | 0.826 (0.185–3.697) | .8028 |
| COPD (yes vs. no) | 0.21 (0.14) | 1.526 (0.880–2.646) | .1327 |
| Mild liver disease (yes vs. no) | 0.36 (0.20) | 2.066 (0.938–4.552) | .0718 |
| DM with organ damage (yes vs. no) | 0.77 (0.35) | 4.667 (1.207–18.046) | .0256 |
| Kidney disease (yes vs. no) | 0.31 (0.22) | 1.846 (0.768–4.436) | .1707 |
| HT (yes vs. no) | 0.28 (0.13) | 1.755 (1.054–2.923) | .0306 |
| Coagulopathy (yes vs. no) | 0.69 (0.43) | 4.000 (0.733−21.838) | .1094 |
| Gastrointestinal bleeding (yes vs. no) | 0.46 (0.34) | 2.500 (0.671−9.310) | .1720 |
| Asthma (yes vs. no) | 0.04 (0.18) | 1.086 (0.546−2.160) | .8137 |
| Baseline blood test | |||
| Creatininea | 0.300 (0.21) | 1.349 (0.901–2.018) | .1459 |
| Glucosea | 0.011 (0.003) | 1.011 (1.005–1.018) | .0009 |
| LDHa | 0.005 (0.001) | 1.005 (1.002–1.007) | .0006 |
| CRPa | 0.006 (0.002) | 1.006 (1.003–1.010) | .0008 |
| Sodiuma | −0.077 (0.038) | 0.926 (0.859–0.998) | .0444 |
| Ureaa | 0.010 (0.006) | 1.010 (0.999–1.022) | .0858 |
| Hematocrita | 0.035 (0.024) | 1.036 (0.988–1.086) | .1455 |
| Plateletsa | −0.005 (0.002) | 0.995 (0.991–0.999) | .0084 |
β (e.e.): estimation (standard error); DM: diabetes mellitus; COPD: chronic obstructive pulmonary disease; HT: hypertension; AMI: acute myocardial infarction; CI: confidence interval; LDH: lactate dehydrogenase; P/F ratio: partial pressure of O2/fraction of inspired O2; CRP: C-reactive protein; OR: odds ratio.
Estimation per each unit increase.
When these variables were combined on the multivariate model, patients who presented with an O2 saturation in the emergency department of ≤90% had an increased risk of clinical deterioration (OR = 16.57, 95% CI 4–68, p < .001) as well as those who had had AMI (OR = 15.72, 95% CI 3.3–75, p = .0006), hypertension (OR = 2.21, 95% CI 1.1–4.4, p = .024), and COPD (OR = 3.05, 95% CI 1.4–6.5, p = .004). In addition, a pathological X-ray entailed a risk that was 5.6 times greater. CRP levels >100 were positively related to possibility of deterioration, as were blood glucose levels >200. Patients with platelet levels less than 15 × 103/µL presented with a risk of deterioration that was four times higher than those who had platelet levels within normal or elevated ranges (Table 3 ).
Table 3.
Explanatory multivariate model of clinical deterioration.
| Variables | β (e.e.) | OR (95% CI) | p |
|---|---|---|---|
| AMI (yes vs. no) | 2.76 (0.79) | 15.721 (3.291–75.088) | .0006 |
| COPD (yes vs. no) | 1.12 (0.39) | 3.052 (1.431–6.509) | .0039 |
| HT (yes vs. no) | 0.79 (0.35) | 2.211 (1.112–4.397) | .0237 |
| O2 saturation ≤90% in the emergency department (yes vs. no) | 2.81 (0.72) | 16.571 (4.030–68.130) | <.0001 |
| Pathological X-ray (yes vs. no) | 1.73 (0.60) | 5.633 (1.725–18.393) | .0042 |
| CRP > 100 mg/dL (yes vs. no) | 1.29 (0.41) | 3.624 (1.621–8.099) | .0017 |
| Hyperglycemiaa (yes vs. no) | 1.80 (0.81) | 6.066 (1.239–29.703) | .0261 |
| Thrombocytopeniab (yes vs. no) | 1.38 (0.39) | 3.972 (1.837–8.585) | .0005 |
| R2/AUC (95% CI) | 0.2525/0.862 (0.818–0.906) | ||
β (e.e.): estimation (standard error); COPD: chronic obstructive pulmonary disease; HT: hypertension; AMI: acute myocardial infarction; CI: confidence interval; OR: odds ratio; CRP: C-reactive protein.
Hyperglycemia defined as a blood glucose level >200 mg/dL.
Thrombocytopenia defined as levels lower than 150,000 platelets per microliter of circulating blood.
The variability explained by the model measured by R2 was 25% and the predictive power as measured by AUC was 0.86.
The P/F ratio was lower among the cases at the various moments it was analyzed (p < .001 for all). The difference in the P/F ratio trend between cases and controls was also statistically significant overall (p < .001), but can be divided into two intervals: the difference is not significant in measurements upon arrival at the emergency department and hospitalization day one; furthermore, the trends are practically parallel during this period (p = .064). However, after hospitalization day one, the cases continued on a downward trend whereas controls stabilized more (p < .001). On the second day of hospitalization, 75% of the cases had values lower than 253.6 whereas 75% of the controls had values higher than 271.4. The decline in the third day of hospitalization until the day a patient presented with deterioration or the critical day was more marked among the cases (p < .001) (Fig. 2 ).
Figure 2.
Evolution of P/F ratio during the hospitalization.
Discussion and conclusions
This study sheds light on the main clinical characteristics of patients hospitalized due to COVID-19 who present with poor progress, defined as PaO2/FiO2 ≤100, admission to the ICU, or death. In this work, it was possible to determine that presence of SaO2 ≤90%, hyperglycemia, elevated CRP levels, thrombocytopenia, a pathological X-ray upon admission, and a previous associated disease (AMI, COPD, or hypertension) were associated with clinical deterioration.
Numerous studies coincide on highlighting age, male sex, and presence of dyspnea as predictive factors of mortality or ICU admission in COVID-19 patients5, 6. As this study paired cases and controls by age, we were not able to evaluate this variable’s effect on outcomes. In regard to sex, 78.79% of patients who developed clinical deterioration in this study were men.
A meta-analysis by Jain and Yuan7 evaluated symptoms and comorbidities that were predictors of severity and ICU admission in patients with COVID-19 and found that dyspnea was the only predictive symptom of both outcomes. Unlike other published works, dyspnea was not significantly more frequent among the cases in our study. This is probably related to the exclusion of patients who required mechanical ventilation in the first 24 h, given that patients who were admitted to the ICU in that time period were transferred due to presence of severe ARDS.
It is important to note that our main objective was to identify patients who, hospitalized in the pulmonary phase of the disease as per the phases proposed by Siddiqi and Mehra8, presented with clinical deterioration during hospitalization. Thus, the cases more frequently had an SaO2p that was lower than the controls upon arrival to the emergency department. Despite the fact that the oxygen values were practically within normal ranges, there was an objective clinical repercussion given that in addition, the cases also presented with tachypnea more often, which is undoubtedly a determining factor of poor progress in pneumonia and is included on COVID-19 severity scales9.
Fu et al.’s10 study on 200 patients observed that both age and comorbidities such as low oxygenation were associated with greater mortality. Therefore, it seems that lower oxygenation values upon admission may predict later deterioration. Beyond this, as Fig. 2 shows, there is a point of inflection in oxygenation, which has been termed the “critical day” for patients. Therefore, proper identification of the clinical characteristics of patients at high risk of worsening is crucial. Bilateral lung involvement and ground-glass opacities have been demonstrated to be the most frequent radiological form of presentation in the literature11, 12. However, in our study, we could not determine the type of radiological involvement; it was only known that patients who developed clinical worsening during the course of the disease presented with some type of initial radiological abnormality versus the controls, who had normal X-rays.
In regard to associated diseases, it seems logical that patients with chronic diseases such as COPD who presented with chronic lung parenchyma inflammation, limitation of expiratory flow, and greater susceptibility to infections would be more vulnerable to the disease12. Likewise, in line with other Spanish cohorts13, a greater association between COPD and clinical deterioration has been observed, which is likely related to presence of a greater number of comorbidities than COVID-19 patients usually have14.
Hypertension and AMI are significantly related to clinical deterioration15. Zhao et al.16 identified that age >60 years, cardiovascular disease, hypertension, and diabetes were independent factors of mortality due to COVID-19. Another two recent studies indicated that these patients more often had myocardial damage and arrhythmias during the hospitalization, which are associated with greater mortality17, 18. Direct viral involvement as well as inflammation secondary to the cytokine storm in SARS-CoV-2 infection could be behind this myocardial damage19. The potential impact this damage may have in the long-term is still to be determined.
Lastly, elevated glucose and CRP levels were predictors of clinical deterioration. Both parameters have been widely studied in pneumonia. Hyperglycemia that is either secondary to the stress of the infection or due to diabetes or an underlying metabolic syndrome could increase mortality in COVID-19 patients20, 21, 22.
Diabetes is one of the most frequent comorbidities in COVID-19 and can worsen its prognosis23. An inappropriate immune response along with exacerbation of the proinflammatory storm could explain this association24. Undoubtedly, proper management and control of glucose levels must be a key part of COVID-19 treatment. In regard to CRP, its elevation has been associated with disease severity and, in addition, it seems to have surpassed other biomarkers more commonly used in sepsis25.
On the other hand, the cases presented with lower platelet levels than the controls. A recent retrospective study which included 1476 patients observed that 20.7% of the sample presented with thrombocytopenia, which was significantly associated with in-hospital mortality26. It has been observed that disseminated intravascular coagulation is frequent among those who have died due to SARS-CoV-2 infection, which could explain the increase in platelet consumption27.
This study has certain limitations inherent to its case-control design. In addition, it was not possible to identify the specific radiological involvement, there were some missing analytical variables, and the results are not able to be extrapolated to patients excluded from the study due to deterioration in the first 24 h. In regard to the study period, in the initial phases of the pandemic, it was less likely that patients with mild-moderate disease would have been admitted to the hospital; the majority were severe patients. In addition, variables that were later determined to be predictors of poor progress in COVID-19 patients were not included because at the time of recruitment, they were not routinely requested (for example, ferritin).
New studies are needed that help identify patients at risk of clinical deterioration so that healthcare professionals can make appropriate decisions about their destination and treatment.
Funding
This project has been funded by the Carlos III Institute of Health in the extraordinary call for research projects on SARS-CoV-2 and COVID-19 disease, file number COV20/0459, and the Network for Research in Chronic Disease Healthcare Departments (REDISSEC, for its initials in Spanish).
Conflicts of interest
The authors declare that they do not have any conflicts of interest.
Footnotes
Please cite this article as: Uranga A, Villanueva A, Lafuente I, González N, Legarreta MJ, Aguirre U, et al. Factores de riesgo de deterioro clínico en pacientes ingresados por COVID-19: estudio caso-control. Rev Clin Esp. 2022;222:22–30.
Appendix A.
The COVID-Osakidetza working group includes the following investigators: Susana García-Gutiérrez, Miren Orive, Nerea González, Iratxe Lafuente, Ane Antón, Ane Villanueva, Josune Martín, Cristina Muñoz, María José Legarreta, Urko Aquirre, and José María Quintana (Research Unit, Galdakao-Usansolo Hospital); Pedro Pablo España Yandiola, Ane Uranga, Mikel Egurrola, Amaia Aramburu, Amaia Artaraz, Leire Chasco, Olaia Bronte, Patricia García, Ana Jodar, Virginia Fernández, and Cristóbal Esteban (Respiratory Unit, Galdakao-Usansolo Hospital); Naia Mas (ICU, Galdakao-Usansolo Hospital); Esther Pulido (Emergency Department, Galdakao-Usansolo Hospital); Itxaso Bengoetxea (At-Home Hospitalization, Galdakao-Barrualde Healthcare Organization); Antonio Escobar Martínez, Amaia Bilbao, and Iñigo Gorostiza (Research Unit, Basurto University Hospital); Iñaki Arriaga (Respiratory Unit, Basurto University Hospital); José Joaquín Portu Zapiarain (Internal Medicine, BioAraba Institute); Naiara Parraza (Research Unit, BioAraba Institute); Milagros Iriberri, Rafael Zalacaín, Luis Alberto Ruiz, and Leyre Serrano (Respiratory Unit, Cruces University Hospital); Adriana Couto and Oier Ateka (Internal Medicine, Donostia University Hospital); Arantza Cano (Respiratory Unit, Santa Marina Hospital); Maria Olatz Ibarra (Pharmacy, Urduliz Hospital); Eduardo Millán, Mayte Bacigalupe, Jon Letona, and Andoni Arcelay (Osakidetza Central Services); and Iñaki Berraondo (Health Department, Government of the Basque Country).
References
- 1.García-Basteiro A., Alvarez-Dardet C., Arenas A., Bengoa R., Borrell C., del Val M., et al. The need for an independent evaluation of the COVID-19 response in Spain. Lancet. 2020;396(10250):529–530. doi: 10.1016/S0140-6736(20)31713-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk factors associated with acute respiratory distress síndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casas-Rojo J.M., Antón-Santos J.M., Millán-Núñez-Cortés J., Lumbreras-Bermejo C., Ramos-Rincón J.M., Roy-Vallejo E., et al. Características clínicas de los pacientes hospitalizados con COVID-19 en España: resultados del Registro SEMI-COVID-19. Rev Clin Esp. 2020;220:480–494. doi: 10.1016/j.rce.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y., et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma C., Gu J., Hou P., Zhang L., Bai Y, Guo Z., et al. Incidence, clinical characteristics and prognostic factor of patients with COVID-19: a systematic review and meta-analysis. medRxiv. 2020 doi: 10.1101/2020.03.17.20037572. 03.17.20037572. [DOI] [Google Scholar]
- 7.Jain V., Yuan J.-M. Predictive symptoms and comorbidities for severe COVID-19 and intensive care unit admission: a systematic review and meta-analysis. Int J Public Health. 2020;65:533–546. doi: 10.1007/s00038-020-01390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta R.K., Harrison E.M., Ho A., Docherty A.B., Knight S.R., van Smeden M., et al. ISARIC4C Investigators. Development and validation of the ISARIC 4C Deterioration model for adults hospitalised with COVID-19: a prospective cohort study. Lancet Respir Med. 2021;9:349–359. doi: 10.1016/S2213-2600(20)30559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu L., Fei J., Xiang H.-X., Xiang Y., Tan Z.-X., Li M.-D., et al. Influence factors of death risk among COVID-19 patients in Wuhan, China: a hospital-based case-cohort study. medRxiv. 2020;30:5702–5708. [Google Scholar]
- 11.Liu J., Chen T., Yang H., Cai Y., Yu Q., Chen J., et al. Clinical and radiological changes of hospitalised patients with COVID-19 pneumonia from disease onset to acute exacerbation: a multicentre paired cohort study. Eur Radiol. 2020;30:5702–5708. doi: 10.1007/s00330-020-06916-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang W., Liang H., Ou L., Chen B., Chen A., Li C., et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180:1081–1089. doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gómez Antúnez M., Muiño Míguez A., Bendala Estrada A.D., Maestro de la Calle G., Monge Monge D., Boixeda R., et al. Clinical characteristics and prognosis of COPD patients hospitalized with SARS-CoV-2. Int J Chron Obstruct Pulmon Dis. 2020;15:3433–3445. doi: 10.2147/COPD.S276692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almagro P., Cabrera F.J., Díez-Manglano J., Boixeda R., Recio J., Mercade J., et al. Working Group on COPD, Spanish Society of Internal Medicine. Comorbidome and short-term prognosis in hospitalised COPD patients: the ESMI study. Eur Respir J. 2015;46:850–853. doi: 10.1183/09031936.00008015. [DOI] [PubMed] [Google Scholar]
- 15.Rodilla E., Saura A., Jiménez I., Mendizábal A., Pineda-Cantero A., Lorenzo-Hernández E., et al. Association of Hypertension with All-Cause Mortality among Hospitalized Patients with COVID-19. J Clin Med. 2020;9:3136. doi: 10.3390/jcm9103136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao M., Wang M., Zhang J., Ye J., Xu Y., Wang Z., et al. Advances in the relationship between coronavirus infection and cardiovascular diseases. Biomed Pharmacother. 2020;127 doi: 10.1016/j.biopha.2020.110230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tao G., Yongzhen F., Ming C., Xiaoyan W., Lin Z., Tao H., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaobo S., Mu Q., Bo S., Yuli C., Tao L., Fan Y., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao M., Wang M., Zhang J., Ye J., Xu Y., Wang Z., et al. Advances in the relationship between coronavirus infection and cardiovascular diseases. Biomed Pharmacother. 2020;127:110230. doi: 10.1016/j.biopha.2020.110230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu L., She Z.G., Cheng X., Quin J.-J., Zhang X.-J., Cai J., et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31 doi: 10.1016/j.cmet.2020.04.021. 1068–1077, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stefan N., Birkenfeld A.L., Schulze M.B., Ludwig D.S. Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol. 2020;16:341–342. doi: 10.1038/s41574-020-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh A.K., Singh R. Does poor glucose control increase the severity and mortality in patients with diabetes and COVID-19? Diabetes Metab Syndr. 2020;14:725–727. doi: 10.1016/j.dsx.2020.05.037. [Published online ahead of print, 27 May 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carrasco-Sánchez F.J., López-Carmona M.D., Martínez-Marcos F.J., Pérez-Belmonte L.M., Hidalgo-Jiménez A., Buonaiuto V., et al. SEMI-COVID-19 Network. Admission hyperglycaemia as a predictor of mortality in patients hospitalized with COVID-19 regardless of diabetes status: data from the Spanish SEMI-COVID-19 Registry. Ann Med. 2021;53:103–116. doi: 10.1080/07853890.2020.1836566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheen A.J., Marre M., Thivolet C. Prognostic factors in patients with diabetes hospitalized for COVID-19: findings from the CORONADO study and other recent reports. Diabetes Metab. 2020;46:265–271. doi: 10.1016/j.diabet.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu F., Li L., Xu M., Wu J., Luo D., Zhu Y., et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang X., Yang Q., Wang Y., Wu Y., Xu J., Yu Y., et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1469–1472. doi: 10.1111/jth.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]