Abstract
The double-stranded (ds) RNA-dependent protein kinase (PKR) is a key mediator of antiviral effects of interferon (IFN) and an active player in apoptosis induced by different stimuli. The translation initiation factor eIF-2α (α subunit of eukaryotic translation initiation factor 2) and IκBα, the inhibitor of the transcription factor NF-κB, have been proposed as downstream mediators of PKR effects. To evaluate the involvement of NF-κB and eIF-2α in the induction of apoptosis by PKR, we have used vaccinia virus (VV) recombinants that inducibly express PKR concomitantly with a dominant negative mutant of eIF-2α or a repressor form of IκBα. We found that while expression of PKR by a VV vector resulted in extensive inhibition of protein synthesis and induction of apoptosis, coexpression of PKR with a dominant negative mutant of eIF-2α (Ser-51→Ala) reversed both the PKR-mediated translational block and PKR-induced apoptosis. Coexpression of PKR with a repressor form of IκBα (Ser-32,36-Ala) also leads to the inhibition of apoptosis by abolishing NF-κB induction, while translation remains blocked. Treating cells with two different proteasome inhibitors which block IκBα degradation, prevented PKR-induced apoptosis, supporting results from coexpression studies. Biochemical analysis and transient assays revealed that PKR expression by a VV vector induced NF-κB binding and transactivation. In addition, upregulation of Fas mRNA transcription occurred during PKR activation. Our findings provide direct evidence for the involvement of eIF-2α and NF-κB in the induction of apoptosis by PKR.
Apoptosis is a genetic program of cell death initiated by many different stimuli (reviewed in reference 64). One trigger is the accumulation of double-stranded RNA (dsRNA) in the cytoplasm of eukaryotic cells (28), an event that is principally thought to result from the infection of cells by viruses (25). The accumulation of dsRNA activates at least two interferon (IFN)-induced pathways (reviewed in reference 53), and each one independently drives cells to a translational block and to apoptosis (17, 33). One of these pathways, the 2-5A system, is composed of the dsRNA-activated 2-5A synthetases and a latent endoribonuclease, RNase L, that upon activation by 2-5A oligoadenylates cleaves single-stranded RNA, causing the abrogation of translation. This pathway also activates apoptosis in different systems (13, 17, 67). Another IFN-induced pathway involves the serine-threonine protein kinase activated by dsRNA (called PKR [41]; for the revision, see reference 44). PKR has two known cellular substrates: eIF-2α (α subunit of eukaryotic translation initiation factor 2 [48]), which upon phosphorylation abrogates translation initiation, and IκBα (29), the inhibitor of the transcription factor NF-κB (for a review, see reference 6). PKR conditions cellular apoptosis in response to activation by various stimuli (15) or when it is overexpressed (33). Although PKR has been implicated in the antiviral and anticellular actions of IFN, little is known relative to the mechanism of PKR-mediated induction of apoptosis (15, 35, 51).
In most cells, NF-κB heterodimers are present in the cytoplasm forming an inactive complex by interacting with the IκB family of proteins. In response to a variety of activators, the prototypic member of this family of inhibitors, IκBα, is phosphorylated at serines 32 and 36, rendering the factor susceptible to proteolysis via the ubiquitin-proteasome pathway (47). This event unmasks a nuclear localization sequence of the transactivating heterodimers, allowing NF-κB translocation to the nucleus. There, the complex binds to κB consensus motifs in the DNA, upregulating the transcription of many genes. NF-κB has been added recently to the list of apoptosis-associated transcription factors. Overexpression of one of the NF-κB subunits, c-rel, in chick bone marrow cells leads to apoptosis (1). Additionally, inhibition of NF-κB activity by different approaches abrogates virus-induced cell death in AT-3 cells (37), prevents induction of apoptosis by DNA-damaging agents (26), and blocks apoptosis caused by serum deprivation in HEK cells (21). Hence, it seems plausible that at least in some cell lines, NF-κB activation, alone or together with other events, is necessary to induce apoptosis. However, NF-κB activity also has preventive apoptotic roles in response to certain stimuli, such as when cells are exposed to tumor necrosis factor (TNF-α), radiation, or daunorubicin (7, 60, 62) or upon oncogenic Ras expression (39). As yet, there is no evidence of a role for NF-κB in the induction of apoptosis by PKR.
Many of the biological effects mediated by PKR, such as the control of virus pathogenesis, are related to its ability to inhibit protein synthesis (27, 49). PKR-induced translational control is a result of phosphorylation of serine 51 in eIF-2α (18). Phosphorylation of eIF-2α inhibits initiation events due to the lack of available eIF-2–GTP–Met-tRNAmet ternary complexes (for a review, see reference 22). Recently, eIF-2α phosphorylation by PKR and concomitant inhibition of translation have been shown to play a role in apoptosis induction upon TNF-α treatment of 3T3 cells (51).
In view of the biological importance of PKR, we have addressed the significance of PKR substrates, IκBα and eIF-2α, in PKR-induced apoptosis. We have taken advantage of vaccinia virus (VV) recombinants that express PKR under the regulation of the Escherichia coli lacI operator-repressor system. Upon treatment of the infected cells with the inducer IPTG (isopropyl-β-d-thiogalactopyranoside), PKR is activated, translation is downregulated, and the cells die by apoptosis (33). To define the role of eIF-2α and NF-κB on apoptosis mediated by PKR, we have coexpressed PKR together with a dominant negative form of the translation factor eIF-2α or a repressor mutant of the transcriptional regulatory factor IκBα. Our findings support the involvement of these translation and transcription controlling factors in PKR-mediated apoptosis.
(This work was presented at the Second Joint Meeting of the International Cytokine Society and the International Society for Interferon and Cytokine Research, Jerusalem, Israel, 25 to 30 October 1998.)
MATERIALS AND METHODS
Materials.
The proteasome inhibitors Cbz-Ile-Glu(O-t-Bu)-Ala-Leucinal (PSI; Sigma) and lactacystin (Calbiochem) were prepared in dimethyl sulfoxide (DMSO) as 6 and 4 mM stock solutions, respectively, and stored at −20°C. In experiments that compared cells treated with different concentrations of PSI or lactacystin, the amount of DMSO per well was maintained constant. Polyinosinic polycytidylic acid (pIC; Boehringer Mannheim) was prepared according to the manufacturer’s instructions as a 10-mg/ml stock solution and stored at −20°C. Human TNF-α (Sigma) was prepared as a 5-μg/ml stock solution and stored in aliquots at −80°C. All other reagents, except where indicated, were from Merck, Boehringer Mannheim, or Sigma.
Plasmids.
Plasmid pPR15 expresses the luciferase gene under the control of a constitutive VV p4b promoter and has been previously described (46). The pIκBα M, pIκBα Wt, and peIF-2α NP plasmids were derived from the VV insertional vector pPR35 designed for IPTG-inducible expression of genes (46). pIκBα M and Wt were generated by cloning a HindIII/Klenow/BamHI fragment excised from pcDNA3-IκBα M and pcDNA3-IκBα Wt, respectively (47), into SmaI/BamHI-digested pPR35. Plasmid peIF-2α NP was constructed by cloning an EcoRV/Asp718 pSP72-2α Ser-51→Ala fragment (42) into SmaI/Asp718-digested pPR35.
The long terminal repeat (LTR)-luciferase vector (2) contains the U3+R regions (−640/+78 fragment) of the human immunodeficiency virus (HIV)-LTR cloned into the pC-luc plasmid. The 3enh-κB-ConA-luc plasmid (3) carries a luciferase gene under the control of three synthetic copies of the κB consensus of the immunoglobulin κ-chain promoter cloned into the BamHI site located upstream of the conalbumin transcription start site.
Cells and viruses.
African green monkey kidney cells BSC-40 (ATCC CCL-26) were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% heat-inactivated newborn calf serum (NCS). Mouse 3T3 cells (ATCC CCL-92) were grown in DMEM supplemented with 10% fetal calf serum (FCS). Human bone osteosarcoma TK− 143B cells (ECACC 91112502) were maintained in DMEM supplemented with 10% FCS and 15 μg of 5-bromo-2-deoxyuridine (BUdR) per ml. HeLa cells (ECACC 85060701) were grown in DMEM supplemented with 10% NCS. 3T3-like cells derived from homozygous PKR knockout mice (PKR0/0) or wild-type animals with the same genetic background (PKR+/+) (both a generous gift of C. Weissmann, University of Zurich, Zurich, Switzerland) were grown in DMEM supplemented with 10% FCS. After mock inoculation or viral adsorption, cells were maintained with DMEM supplemented with 2% NCS or 2% FCS (supplemented with BUdR for 143B cells).
The recombinant VV expressing IPTG-inducible PKR (called WR 68K before, and VV PKR herein) was described previously (33). Virus vTF7-3 (herein VT7) contains the bacteriophage T7 RNA polymerase gene under the control of a virus constitutive promoter as described earlier (20). VVRL was obtained by insertion of pTM-RL into the thymidine kinase locus of wild-type VV Western reserve (WR) strain by homologous recombination (16). VV hbcl2 inducibly expresses the human oncoprotein bcl2 as previously described (35). VV abcl2 constitutively expresses the African swine fever virus (ASFV) A179L gene, a viral homologue of bcl2, as shown before (11). VV IκBα M, VV IκBα Wt, and VV eIF-2α NP were generated by homologous recombination of their respective pPR35 derived-plasmid with WR strain of VV in TK− 143B cells, as previously described (34).
Analysis of cell viability.
BSC-40 cells were seeded in 96-well plates in a total volume of 100 μl and incubated for 24 h at 37°C in 5% CO2, followed by the addition to each well of 10 μl of a stock solution of methyl thiazol tetrazolium (MTT) prepared in phosphate-buffered saline (PBS) at a concentration of 5 mg/ml. Plates were further incubated for 3 h. The formazan salts were solubilized by adding 150 μl of 10% Triton X-100 in acidified isopropanol. Plates were rocked for 10 min and the absorbances at 540 and 690 nm were measured in a spectrophotometer.
Measurement of the extent of apoptosis.
The cell death detection enzyme-linked immunosorbent assay (ELISA) kit (Boehringer Mannheim) was used according to the manufacturer’s instructions. This assay is based on the quantitative sandwich enzyme immunoassay principle and uses mouse monoclonal antibodies directed against DNA and histones to estimate the amount of cytoplasmic histone-associated DNA.
Transient luciferase reporter assays.
In order to check the translational state of cells, semiconfluent BSC-40 cells grown in 12-well plates were infected with the viruses indicated and transfected after 1 h of virus adsorption with 0.5 μg of pPR15 per well by using Lipofectamine reagent (GIBCO-BRL) according to manufacturer’s directions for an additional 5 h. Where indicated, 5 mM IPTG was added to the cultures at the time of transfection. Cells were harvested 24 h after infection and lysed, and luciferase activity was determined as previously described (10). The NF-κB reporter assay was similar, but the assay was performed with HeLa cells grown in 6-well plates, and the cells were transfected with LTR-luciferase or with 3enh-κB-ConA-luc plasmid (2 μg of DNA per well).
Metabolic labeling of proteins.
BSC-40 cells cultured in 12-well plates were infected with the viruses indicated and rinsed three times with Met-Cys-free DMEM 30 min prior to labeling. After incubation for an additional 30 min at 37°C with Met-Cys-free DMEM, the medium was removed, and 50 μCi of [35S]Met-Cys Promix (Amersham) per ml in Met-Cys-free DMEM was added for an additional hour. After three washes with PBS, cells were harvested in lysis buffer (150 mM KCl, 10% glycerol, 1 mM dithiothreitol (DTT), 5 mM magnesium acetate, 0.5% Nonidet P-40). The protein concentration was determined by using the bicinchoninic acid assay (BCA; Pierce) with bovine serum albumin (BSA) as a standard. An aliquot of the cell lysate was diluted in a 0.1-mg/ml BSA solution; the proteins were then precipitated with 5% trichloroacetic acid and collected on glass fiber filters by using a vacuum manifold instrument (Millipore). Filters were dried, and the radioactivity was counted in a scintillation counter by using liquid scintillation cocktail.
Immunoblotting.
Rabbit polyclonal antibody specific for RNase L was as previously described (16). Mouse monoclonal antibody specific for eIF-2α was a gift from César de Haro (Centro de Biologia Molecular Severo Ochoa, Madrid, Spain) and M. J. Clemens (St. George’s Hospital, London, United Kingdom). Mouse monoclonal antibody for SV5 tag was obtained from R. E. Randall (University of Glasgow, Glasgow, Scotland) through the NIBSC-MRC AIDS Reagent Project. A peptide spanning amino acids 539 to 551 of the human PKR was synthesized to produce antibodies against PKR. Polyclonal rabbit antibody specific for PKR was obtained after repeated immunizations with the purified peptide coupled to keyhole limpet hemocyanin. Rabbit antiserum directed against phosphoserine 32-IκBα that specifically recognizes the phosphorylated form of the protein (9) was purchased from New England Biolabs. Monoclonal antibody (AC-74) for mouse beta-actin was from Sigma. Secondary antibodies were purchased from Cappel.
For immunoblot analysis, total cell extracts were boiled in Laemmli sample buffer, and proteins were fractionated by 10 or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoresis, proteins were transferred to nitrocellulose paper by using a semidry blotting apparatus (Gelman Sciences). Filters were mixed with antiserum in PBS containing nonfat dry milk at 5% (BLOTTO), incubated overnight at 4°C, washed three times with PBS, and further incubated with secondary antibody coupled to horseradish peroxidase in BLOTTO. After being washed with PBS, the immunocomplexes were detected by using enhanced chemiluminescence Western blotting reagents (Amersham). Exposure of filters to Kodak X-Omat films was performed for times varying from 3 to 5 min, as needed.
Gel retardation assay.
Nuclear extracts from HeLa cells were prepared as previously described (4). First, 3 μg of nuclear extract was analyzed as described previously by using [α-32P]dCTP-labeled double-stranded synthetic wild-type HIV enhancer oligonucleotide 5′-AGCTTACAAGGGACTTTCCGCTGGGGACTTTCCAGGGA-3′ containing the two κB consensus motifs. The composition of the binding buffer included 25 mM HEPES, 1 mM EDTA, 3.5 mM spermidine, 6 mM MgCl2, 100 mM NaCl, 0.15% Nonidet P-40, 10% glycerol, 5 mM DTT, 0.5 mg of BSA per ml, and 25 μg of poly(dI-dC) per ml.
RESULTS
Coexpression of PKR and a nonphosphorylated mutant form of eIF-2α inhibits apoptosis induction by PKR.
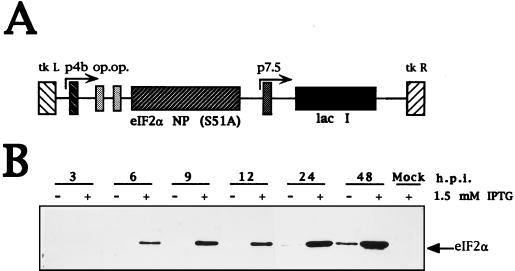
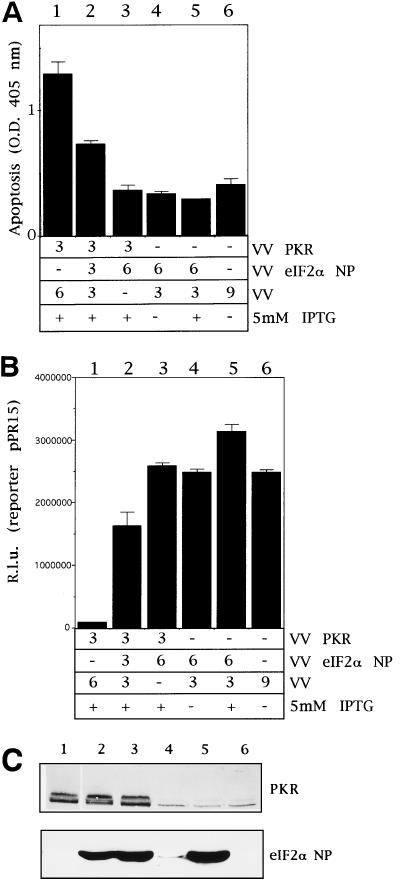
We previously described an inducible system based on VV recombinants capable of expressing the IFN-induced enzymes, PKR, 2-5A synthetase, and RNase L, and we have shown that expression of these enzymes in an activated form triggers apoptosis (17, 33). Result from this inducible virus-cell system have been confirmed by others with transfected cells and with cells derived from PKR and RNase L gene knockout mice (13, 15, 67). The VV inducible system was also used to identify apoptotic viral genes (54) and cellular and viral inhibitors of apoptosis (11, 35). Nonphosphorylatable PKR substrates were expressed in the VV-inducible system in order to evaluate their role in PKR-induced apoptosis. Expression of a 51A nonphosphorylatable form of eIF-2α has been shown to abrogate the effects of eIF-2α phosphorylation on protein synthesis (18). The construction of the VV vector and eIF-2α protein levels produced during infection, in the absence or presence of IPTG, are presented in Fig. 1. Immunoblot analysis with specific monoclonal antibody revealed that the recombinant protein was induced by 6 h postinfection (hpi) and accumulated during infection. When BSC-40 cells are infected with a VV recombinant expressing PKR, severe apoptosis is induced as defined by several criteria: DNA ladder formation, cell shrinkage, formation of apoptotic bodies, and condensation of chromatin (33). The extent of apoptosis was quantified by using a standard ELISA assay (17) which, as indicated in Fig. 2A (column 1), shows that expression of PKR induces severe apoptosis. As expected in this virus-cell system, wild-type VV does not induce apoptosis (column 6). To evaluate the interaction of eIF-2α and PKR in apoptosis, we coinfected BSC-40 cells with VV PKR and VV eIF-2α NP that expresses eIF-2α 51A. The apoptosis-induced by PKR was abrogated by coexpression of eIF-2α NP (Fig. 2A, column 2) in an eIF-2α NP concentration-dependent manner (column 3). Inhibition of apoptosis by eIF-2α NP expression correlated with its ability to reverse a translational block induced by PKR (Fig. 2B). Based on Western blotting with specific antibodies, the PKR levels in lanes 1, 2, and 3 were similar (Fig. 2C). The production of eIF-2α NP is also shown in Fig. 2C. In order to gain insight into the temporal pattern of the translational block induced by PKR, we carried out metabolic pulse-labeling experiments at different times postinfection. In support of previous data (34), upon PKR expression, at 12 hpi translation decreased 80% compared to control values. In contrast, coexpression of PKR and eIF-2α NP partially rescued the inhibition of protein synthesis (data not shown). Significantly, the expression of eIF-2α NP prolonged the ability of cells to engage in protein synthesis compared to VV PKR-infected cells. In conclusion, the expression of a mutant nonphosphorylatable form of eIF-2α overrides the PKR-induced translational block and also inhibits PKR-mediated apoptosis.
FIG. 1.
Inducible expression of a dominant negative eIF-2α mutant form by a VV recombinant. (A) Map of the pPR35 derived peIF-2α NP (S51A). The plasmid contains an IPTG-inducible copy of eIF-2α NP (S51A) under the regulation of a hybrid promoter consisting of the VV p4b promoter fused to two lacI operator (op) units. The plasmid also contains the lacI repressor gene under the control of the VV p7.5 promoter and was used to obtain recombinant VV by homologous recombination in TK− 143B cells. (B) Time course analysis of IPTG-dependent expression of eIF-2α NP by the VV eIF-2α NP. BSC-40 cells were infected with 4 PFU of the virus per cell and were treated with or without 1.5 mM IPTG, scraped, and collected at the indicated times postinfection. Extracts were lysed, and equal amounts of protein as determined by BCA were analyzed by SDS-PAGE in conjunction with Western blotting with an eIF-2α monoclonal antibody.
FIG. 2.
VV eIF-2α NP rescues translation inhibition and prevents PKR-induced apoptosis. (A) BSC-40 cells grown in 12-well plates were infected at a total of 9 PFU/cell with the viruses indicated in the presence of 5 mM IPTG unless stated otherwise, transfected 1 h later with 0.5 μg of pPR15, and harvested at 24 hpi for the determination of apoptosis. Mean values of triplicates of the determined absorbance at 405 nm with standard deviations are given. Columns: 1, 3 PFU of VV PKR and 6 PFU of VV per cell; 2, 3 PFU of VV PKR, 3 PFU of VV eIF-2α NP, and 3 PFU of VV per cell; 3, 3 PFU of VV PKR and 6 PFU of VV eIF-2α NP per cell; 4, 6 PFU of VV eIF-2α NP and 3 PFU of VV per cell, without adding IPTG; 5, 6 PFU of VV eIF-2α NP and 3 PFU of VV per cell, but with IPTG; 6, 9 PFU of VV per cell. (B) Samples from the same experiment were harvested and lysed in buffer for luciferase determination as described in Materials and Methods. Mean values of triplicates with the standard deviations are given of the relative luciferase units measured in equal extracts. (C) Immunoblot analysis of PKR and eIF-2α NP of the same samples used in panels A and B.
PKR mediates phosphorylation of IκBα on Ser-32 induced by pIC treatment.
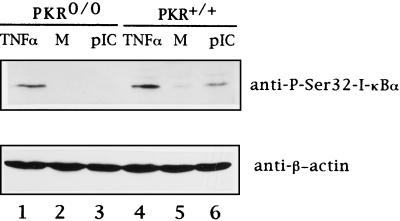
In addition to its role as a regulator of translation, PKR has been implicated in the control of transcription (29, 30, 44). PKR has been proposed to phosphorylate IκBα, the inhibitor of NF-κB-dependent transcription (29). Although PKR has been clearly shown to mediate NF-κB activation, some doubt exists regarding its role in directly phosphorylating IκBα (32). Phosphorylation of IκBα on serine residues 32 and 36 is the first step of the most common and accepted mechanism of NF-κB activation (52), but another atypical mechanism involving tyrosine phosphorylation has been proposed (24). To gain insight into the in vivo mechanism of PKR-induced NF-κB activation, it was necessary to establish that IκBα phosphorylation occurs specifically on serine 32. 3T3-like PKR knockout cells (PKR0/0) or PKR+/+ cells were treated with pIC, and the degree of IκBα phosphorylation on serine 32 was tested by using a phosphorylation state-specific antibody (9). As determined by Western blotting (Fig. 3, upper panel), treatment with pIC for 30 min induces this phosphorylation specifically in cells containing PKR but not in PKR0/0 cells (compare lanes 3 and 6). As a control, both cell lines were treated with TNF-α, a powerful activator of NF-κB that has been shown to induce this transcription factor independently of PKR (30). We observed that IκBα phosphorylation on serine 32 upon TNF-α treatment occurred both in PKR0/0 and PKR+/+ cells to a similar degree, thus supporting previous evidence of NF-κB induction in response to TNF-α independent of PKR (30). As a control to check equivalent protein loading on the gel, the membrane was blotted with a monoclonal antibody directed against mouse beta-actin (Fig. 3, lower panel).
FIG. 3.
PKR mediates pIC-induced phosphorylation of IκBα on serine 32. PKR0/0 and PKR+/+ cells grown in 6-well plates were serum starved for 3 h and then transfected with 7 μg of pIC per well with Lipofectamine or just transfected with Lipofectamine as a mock negative control (M). Cells were collected 30 min posttreatment. For the PSI–TNF-α treatment (referred to as TNF-α), cells were serum starved for 2 h, and then 60 μM PSI was added for one additional hour before treatment with 50 ng of TNF-α per ml during 3 h in the presence of 60 μM PSI in order to achieve the accumulation of the serine 32-phosphorylated form of I-κBα. At the indicated times, the cells were washed with PBS and lysed in the plates by adding 200 μl of 1× Laemmli buffer, collected, cooled on ice, and sonicated twice for 10 s. Clarified extracts were separated by SDS–12% PAGE, transferred to nitrocellulose membrane, and immunoblotted with antibodies against phosphoserine 32 IκBα (upper panel) and β-actin (lower panel).
PKR expression from a VV vector induces NF-κB binding and transactivation.
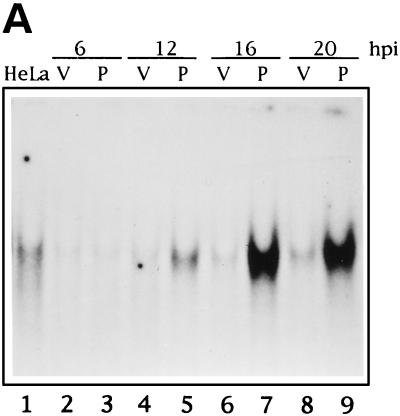
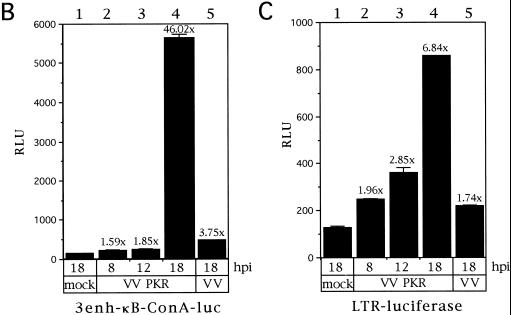
Many viruses are known to interfere with the mechanism of NF-κB activation, inhibiting it (43) or inducing it (5). Hence, it was important to check NF-κB activation levels not only by VV-mediated expression of PKR but also upon infection with VV alone. To this end, nuclear extracts from HeLa cells infected with VV or VV PKR and collected at different times postinfection were subjected to gel shift assay by using an NF-κB probe (Fig. 4A). Nuclei from mock-infected HeLa cells contain a detectable basal activity (lane 1), and after infection with VV this activity slightly diminishes (lanes 2, 4, 6, and 8). However, after PKR expression NF-κB binding activity was first detectable at 9 hpi (data not shown) and progressively increased with time (lanes 5, 7, and 9). Hence, PKR expression from a VV vector was able to induce a strong NF-κB binding activity. As a further approach we decided to check whether PKR expressed from VV was able to induce transactivation of NF-κB-driven reporter vectors. To do this, we infected HeLa cells with VV PKR or control VV and transfected them after viral adsorption with either of two different reporter plasmids, 3enh-κB-ConA-luc (3) (Fig. 4B) or LTR-luciferase (2) (Fig. 4C). With these assays we were able to detect an upregulation of levels upon expression of PKR at 18 hpi of 6.84-fold relative to unstimulated HeLa cells with the LTR-luciferase reporter (Fig. 4C, column 4). Most significantly, by using the highly NF-κB-inducible 3enh-κB-ConA-luc reporter, luciferase activity in VV PKR-infected cells (Fig. 4B, column 4) was more than 45-fold higher than in mock-infected HeLa cells and more than 12-fold higher with respect to VV-infected cells at 18 hpi. In conclusion, VV-mediated PKR expression significantly induced NF-κB activity. As NF-κB has been reported to inhibit or promote apoptosis depending on the triggering stimulus (1, 7, 21, 26, 37, 39, 60, 62), it was important to evaluate the interaction of PKR-induced NF-κB activity and apoptosis.
FIG. 4.
PKR expression from a VV vector induces NF-κB binding and transactivation. (A) Time course of NF-κB binding induced by PKR. HeLa cells were infected with 8 PFU of wild-type VV (lanes V) per cell or 4 PFU of VV PKR plus 4 PFU of VV per cell in the presence of 5 mM IPTG (lanes P) for the times indicated. As a control, HeLa cells were mock infected for 20 h. Nuclear extracts were analyzed by gel shift with a [α-32P]dCTP-labeled double-stranded probe containing two κB consensus sites. For the analysis of PKR-induced NF-κB-dependent transactivation, HeLa cells seeded in 6-well plates were mock infected or infected with 5 PFU of VV or VV PKR per cell. After 1 h of viral adsorption, 5 mM IPTG was added, and the cells were transfected with 2 μg of 3enh-ïB-ConA-luc vector (B) or LTR-luciferase plasmid (C) per well for an additional 5 h. Cells were collected at the indicated times postinfection and lysed for the luciferase determination. Duplicate experiments were performed.
Inhibition of IκBα degradation with proteasome inhibitors prevents induction of apoptosis by PKR.
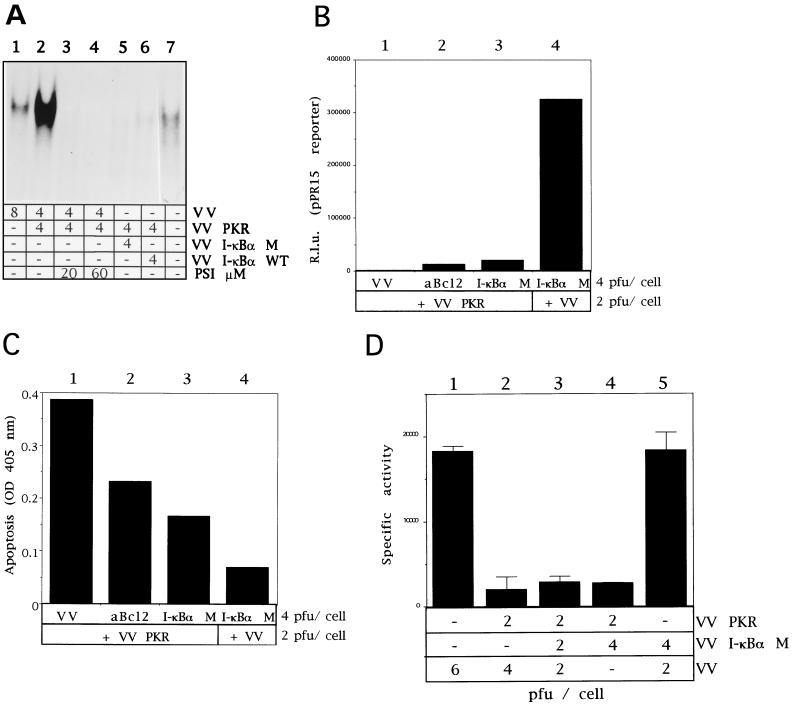
It is known that NF-κB induction is dependent upon the phosphorylation and subsequent ubiquitination of IκBα. Ubiquitin-tagged IκBα is degraded by the proteasome releasing NF-κB for migration to the nucleus. As a first approach to check the role of NF-κB in PKR-induced apoptosis, the NF-κB induction pathway was blocked by using the specific proteasome inhibitors, lactacystin and PSI. Lactacystin binds to various proteasome subunits (14), and PSI inhibits the chymotrypsin-like activity of the proteasome (19). Traenckner et al. (58) showed that PSI inhibited NF-κB induction in HeLa cells.
The viability of BSC-40 cells based upon the MTT assay and cellular apoptosis based upon the ELISA were not affected by concentrations of up to 60 μM PSI and 40 μM lactacystin. The specificity of proteasome inhibitor activity was further evaluated by plaque assays that showed VV replication was not affected by use of the inhibitors (data not shown).
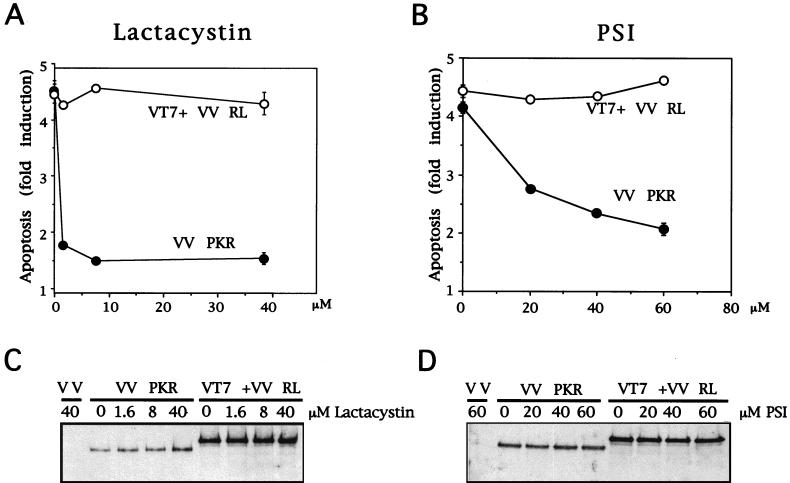
To evaluate the effect of these proteasome inhibitors on PKR-induced apoptosis, BSC-40 cells were infected with 6 PFU of VV PKR per cell and, after 1 h of virus adsorption, cells were treated with different concentrations of lactacystin or PSI, in the absence or presence of IPTG. As an additional control, BSC-40 cells were infected with VT7 and VV RL at 3 PFU/cell each, thus expressing the IFN-induced enzyme RNase L (16). Previously, RNase L has been shown to activate apoptosis by an unknown pathway not suspected to involve NF-κB activation (13, 17, 67). Lactacystin and PSI decreased PKR-induced apoptosis in a dose-dependent manner (Fig. 5A and B). However, these inhibitors did not affect apoptosis triggered by overexpression of RNase L. As determined by Western blots, the protein levels of PKR and RNase L were not affected by treatment with various doses of either proteasome inhibitor (Fig. 5C and D). These results support the concept that the NF-κB pathway is involved in the induction of apoptosis by PKR.
FIG. 5.
Lactacystin and PSI treatments prevent apoptosis of VV PKR-infected cells. (A) BSC-40 cells grown in 12-well plates were infected at a multiplicity of infection (MOI) of 6 with VV PKR in the presence of 5 mM IPTG with 3 PFU of VT7 and 3 PFU of VV RL per cell or 6 PFU of VV per cell and treated for 24 h with the indicated amounts of lactacystin. The cells were harvested at 24 hpi for the determination of apoptosis as previously described. Results are presented as the fold induction of apoptosis over that for VV-infected cells. (B) BSC-40 cells grown in 12-well plates were infected at a total MOI of 6 with VV PKR in the presence of 5 mM IPTG, with 3 PFU each of VT7 and VV RNase L per cell or with 6 PFU of VV per cell and treated for 24 h with the indicated amounts of PSI. The cells were harvested at 24 hpi for the determination of apoptosis. Results are presented as the fold induction of apoptosis over that for VV-infected cells. Extracts of the lactacystin treatment (C) and PSI treatment (D) experiments were separated by SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted with antibodies against RNase L and PKR. All of the ELISA experiments are represented as mean values of triplicate experiments.
Coexpression of PKR with a nonphosphorylatable form of IκBα inhibits the induction of apoptosis by PKR.
To evaluate the interaction of the NF-κB pathway in PKR-induced apoptosis, a repressor mutant form of IκBα was coexpressed with PKR. The nonphosphorylatable form of IκBα acts as a nondegradable mutant to repress NF-κB activation in the cytoplasm in response to different stimulating signals (36, 60).
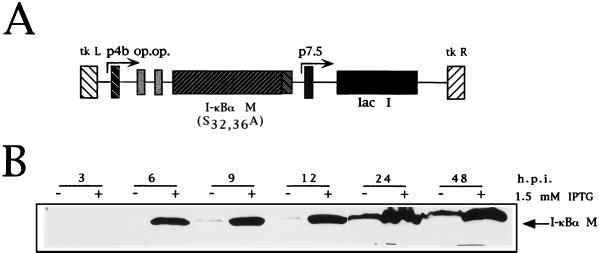
Figure 6A shows the construction of the VV insertional vector for expression of the mutant IκBα. VV IκBα M expresses, in an IPTG-dependent way, an IκBα SV5-tagged protein with serines 32 and 36 substituted by alanines (VV IκBα M). Figure 6B shows an immunoblot analysis with a monoclonal antibody against the SV5 epitope (45) fused to the IκBα M protein in its C terminus (47). Clearly, the IκBα protein was detectable at 6 hpi after IPTG addition. The leakiness of the E. coli operator-repressor pPR35 system observed at 24 hpi has been noted previously (34).
FIG. 6.
VV IκBα M expresses in an IPTG-dependent way a tagged mutant form of IκBα. (A) Map of the pPR35 derived pIκBα M (S32, 36A). The plasmid contains an IPTG-inducible copy of IκBα M (S32, 36A) fused to the SV5 tag in its C terminus under the regulation of a hybrid promoter consisting of the VV p4b promoter fused to two lacI operator (op) units. The plasmid also contains the lacI repressor gene under the control of the VV p7.5 promoter and was used to obtain recombinant VV by homologous recombination in TK− 143B cells. (B) Western blot time course analysis of IPTG-dependent expression of IκBα M by VV IκBα M. BSC-40 cells were infected at an MOI of 4 with the virus and treated with or without 1.5 mM IPTG; they were then scraped and collected at the indicated times postinfection. Extracts were lysed, and equal amounts of protein as determined by BCA were analyzed by SDS-PAGE in conjunction with Western blotting with anti-SV5 monoclonal antibody.
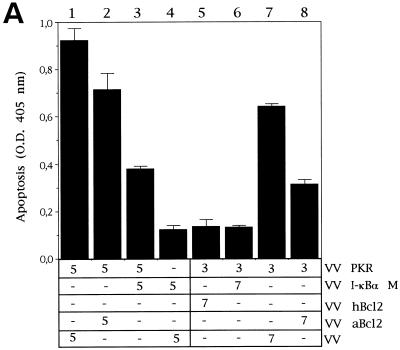
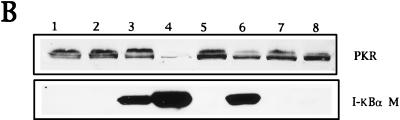
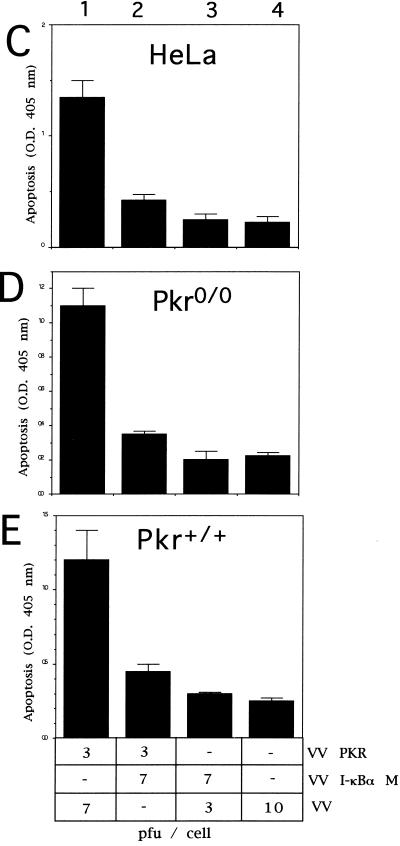
Coexpression of PKR and the nonphosphorylatable form of IκBα (IκBα M) significantly blocked PKR-mediated apoptosis (Fig. 7A, compare columns 1 and 3). Similar results were obtained with different ratios of virus multiplicities (compare columns 1 and 3 with columns 6 and 7). The apoptosis triggered by PKR was also blocked by the coexpression of PKR with the African swine fever virus Bcl-2 homologue (columns 2 and 8) and by the human Bcl-2 protein (column 5), thus confirming previous results (11, 35). The levels of expression of PKR and IκBα in the coinfected cultures are shown in the Western blot of Fig. 7B. Similarly, we were able to detect a decrease in PKR-induced apoptosis with the expression of IκBα M in other cell lines (Fig. 7C, D, and E), suggesting that the inhibition of apoptosis induced by IκBα M expression was not a particular characteristic of BSC-40 cells.
FIG. 7.
Coinfection of VV PKR with VV IκBα M inhibits PKR-induced apoptosis. (A) BSC-40 cells grown in 12-well plates were infected at an MOI of 10 with the viruses indicated in the presence of 5 mM IPTG and harvested at 24 hpi to determine the absorbance at 405 nm. The mean values of triplicate experiments with standard deviations are given. Columns: 1, 5 PFU of VV PKR and 5 PFU of VV per cell; 2, 5 PFU of VV PKR and 5 PFU of VV aBcl-2 per cell; 3, 5 PFU of VV PKR and 5 PFU of VV IκBα M per cell; 4, 5 PFU of VV and 5 PFU of VV IκBα M per cell; 5, 3 PFU of VV PKR and 7 PFU of hBcl2 per dell; 6, 3 PFU of VV PKR and 7 PFU of VV IκBα M per cell; 7, 3 PFU of VV PKR and 7 PFU of VV per cell; 8, 3 PFU of VV PKR and 7 PFU of VV aBcl-2 per cell. (B) Immunoblot analysis of PKR and IκBα M expression with the anti-SV5 tag antibody in the same extracts used to measure apoptosis. HeLa cells (C), PKR0/0 (D), or PKR+/+ (E) cells grown in 12-well plates were infected at an MOI of 10 with the viruses indicated in the presence of 5 mM IPTG and harvested at 24 hpi for the determination of apoptosis by using the cell death detection ELISA. Columns: 1, 3 PFU of VV PKR and 7 PFU of VV per cell; 2, 3 PFU of VV PKR and 7 PFU of VV IκBα M per cell; 3, 3 PFU of VV PKR and 7 PFU of VV IκBα S per cell; 4, 10 PFU of VV per cell. Triplicate experiments were performed.
The role of IκBα in preventing the PKR effect on apoptosis was further evaluated by coexpressing the wild-type IκBα (VV IκBα Wt). Lin et al. (36) demonstrated that overexpression of wild-type IκBα leads to partial inhibition of NF-κB activity in alphavirus-infected cells (36). To determine whether wild-type IκBα inhibits apoptosis in our VV model, BSC-40 cells were infected with different combinations of virus multiplicities, and apoptosis was measured at 24 hpi. The inhibition of PKR-induced apoptosis by coexpression of IκBα Wt was similar to treatments where PKR was coexpressed with IκBα M (Fig. 8A, compare columns 1 to 4 with columns 5 to 7). Since we have shown that the mechanism involving PKR activation of NF-κB involves phosphorylation of IκBα on serine 32, the most probable explanation for the similar behavior of wild-type and mutant IκBα forms in preventing PKR-induced apoptosis is the high expression levels of both proteins achieved with our system. The differences observed in the levels of IκBα when PKR was coexpressed (Fig. 8B, compare lanes 3 and 4 or lanes 6 and 7) are probably due to the translational abrogation induced by PKR. However, under those conditions there is enough IκBα protein synthesized to reverse the PKR-mediated effect on apoptosis. These results further confirm that NF-κB has a direct role in mediating PKR-induced apoptosis.
FIG. 8.
Coexpression of increasing amounts of IκBα Wt or M equally inhibit induction of apoptosis triggered by PKR. (A) BSC-40 cells grown in 12-well plates were infected at an MOI of 6 with the viruses indicated in the presence of 5 mM IPTG and harvested at 24 hpi for the determination of the absorbance at 405 nm. Mean values of triplicate experiments with standard deviations are given. Columns: 1, 2 PFU of VV PKR and 4 PFU of VV per cell; 2, 2 PFU of VV PKR, 2 PFU of VV IκBα M, and 2 PFU of VV per cell; 3, 2 PFU of VV PKR and 4 PFU of VV IκBα M per cell; 4, 4 PFU of VV IκBα M and 2 PFU of VV per cell; 5, 2 PFU of VV PKR, 2 PFU of VV IκBα Wt, and 2 PFU of VV per cell; 6, 2 PFU of VV PKR and 4 PFU of VV IκBα Wt per cell; 7, 4 PFU of VV IκBα Wt and 2 PFU of VV per cell. (B) Immunoblot analysis of PKR and IκBα M and Wt expression with the anti-SV5 tag antibody from the same extracts used to measure apoptosis.
Inhibition of PKR-induced apoptosis by a nonphosphorylatable IκBα mutant does not prevent translational abrogation and correlates with the absence of NF-κB binding activity.
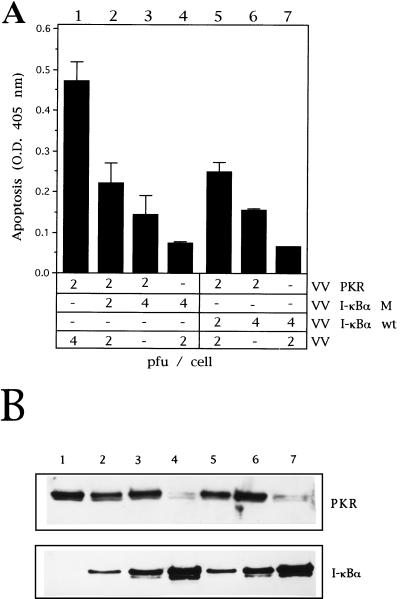
Gel shift assays were also performed to evaluate the effect of PSI and IκBα proteins directly on NF-κB binding activity. As shown in Fig. 9A, not only the expression of IκBα M or Wt (lanes 5 and 6) proteins but also the treatment with different doses of PSI (lanes 3 and 4) significantly blocked the NF-κB binding activity induced by the expression of PKR. It is important to note that the coexpression of PKR with eIF-2α NP produced no effect on PKR-induced NF-κB binding activity (data not shown). Additionally, prevention of PKR-induced apoptosis by expression of the IκBα M protein should be a specific event but a trouble intrinsic to the use of nonphosphorylated substrates could be the nonspecific inhibition of the target enzyme by the substrate analogue. Thus, we tested whether IκBα M had any effect on the PKR-induced translational block. We used the luciferase reporter gene to measure levels of protein synthesis. A similar level of translational inhibition was found in cells expressing PKR, coexpressing PKR and IκBα M (Fig. 9B, columns 1 and 3), or coexpressing PKR and the ASFV analogue of Bcl-2 (Fig. 9B, column 2). Hence, the effects on apoptosis induction (Fig. 9C) by IκBα M expression are not associated with any effect on translation. Quantitation of protein synthesis levels by using [35S]Met-Cys pulse-labeled cells similarly showed that translation was not affected by IκBα M expression (Fig. 9D) and demonstrates the functional independence of the PKR–eIF-2α and PKR–IκBα pathways.
FIG. 9.
Coexpression of IκBα M with PKR inhibits NF-κB activity without affecting translational abrogation. (A) HeLa cells grown in 100-mm-diameter plates were mock infected or infected with the viruses indicated in the presence of 5 mM IPTG. When noted, 20 or 60 μM PSI was added to cells after 1 h of viral adsorption. Cells were collected at 20 hpi, and nuclear extracts were prepared and analyzed by gel shift assay as described above. Lanes: 1, 8 PFU of VV per cell; 2 to 4, 4 PFU of VV and 4 PFU of VV PKR per cell in the absence of PSI (lane 2) or with 20 or 60 μM (lanes 3 and 4, respectively); 5, 4 PFU of VV PKR and 4 PFU of VV IκBα M per cell; 6, 4 PFU of VV PKR and 4 PFU of VV IκBα WT per cell; 7, mock infected. (B) BSC-40 cells grown in 12-well plates were infected at an MOI of 6 with the viruses indicated in the presence of 5 mM IPTG, transfected 1 h later with 0.5 μg of pPR15, and harvested at 24 hpi; half of the samples were lysed in buffer for luciferase determination as described in Materials and Methods. Mean values of relative luciferase units (n = 3) measured in equal extracts, along with the standard deviations, are given. Columns: 1, 2 PFU of VV PKR and 4 PFU of VV per cell; 2, 2 PFU of VV PKR and 4 PFU of VV aBcl-2 per cell; 3, 2 PFU of VV PKR and 4 PFU of VV IκBα M per cell; 4, 4 PFU of VV IκBα M and 2 PFU of VV per cell. (C) The other half of the samples were processed according to the manufacturer’s instructions, and the absorbance at 405 nm was determined as a measure of apoptosis. Mean values of triplicate experiments with standard deviations are given. (D) BSC-40 cells were infected with the indicated viruses at the indicated concentrations, and the [35S]Met-Cys incorporation during a 1-h pulse was measured beginning at 12 hpi as described in Materials and Methods. Specific activities (in counts per minute per microgram of protein) are represented as the mean value from two experiments, along with the standard deviation.
DISCUSSION
Although previous studies by us and others have demonstrated that activation of PKR leads to induction of apoptosis (15, 33), the molecular mechanism by which PKR activates the apoptotic pathway remains to be defined. Since eIF-2α and IκBα have been proposed as downstream mediators of PKR effects (29, 48), it is important to establish the contribution of both pathways, if any, in the process of apoptosis. Additionally, in view of the biological importance of PKR as a controlling factor of viral infections (27, 49), it is of interest to know the contribution of both substrates to apoptosis induced by PKR within the context of virus infection. Hence, in this investigation we describe a virus-cell system in which activation of eIF-2α-dependent or NF-κB-dependent pathways by PKR can be selectively blocked. Our model system is ideally suited for the sensitive dissection of PKR-mediated effects in vivo. Although the coexpression of PKR and its substrates is within the context of VV infection in a background of normal cell constituents, the regulated expression allows a direct interaction without regard to temporal or localization issues that may complicate assays in other in vivo model systems. It should be pointed out that conclusions obtained to date with this system have been validated by different approaches, such as the use of cells derived from PKR and RNase L knockout mice (15, 67), or with different expression systems (13, 51).
The overriding issue of this study was to evaluate the contribution of the phosphorylation of eIF-2α and the activation of NF-κB to PKR induced apoptosis. Coexpressing a dominant negative mutant form of eIF-2α with PKR caused a significant reversion of the translational block conditioned by eIF-2α phosphorylation concomitantly with inhibiting the induction of apoptosis. These results are in agreement with previous transient-expression studies (51) and suggest that translational control is important in the induction of apoptosis by PKR. The finding that the NF-κB pathway is involved in the transcription of death genes (31, 57, 65, 66) and that PKR has a regulatory impact on that pathway (29, 30) also suggest a link of PKR-induced NF-κB activation and apoptosis.
The use of two different proteasome inhibitors that block NF-κB activation and also inhibit apoptosis induced by PKR in our system supports an interaction between this transcription factor and PKR-induced apoptosis. Although several parameters were unaffected in our virus-cell system after treatment with both drugs and, additionally, the use of other IFN-induced apoptosis-triggering enzymes, such as RNase L, served as a control, the cellular functions other than NF-κB activity (58) could be altered by inhibiting the proteasome (14). However, these data provided a basis for further studies to elucidate the role of NF-κB on PKR-induced apoptosis. The key step in the activation of NF-κB-dependent transcription is the inducible degradation of the NF-κB inhibitor IκBα, which in turn makes possible NF-κB migration to the nucleus. IκBα phosphorylation of serines 32 and 36 is the signal that induces the degradation of this inhibitor (47). Thus, a mutant form which cannot be phosphorylated on these residues acts as a repressor (60, 62, 63). In addition, we have shown that the role of PKR in the activation of NF-κB involves IκBα phosphorylation on serine 32, one of the two residues responsible for phosphorylation-mediated NF-κB activation. The expression of nonphosphorylatable forms of IκBα has been widely used to inhibit NF-κB activation in other systems (36, 60). In our system, we observed a significant decrease in apoptosis induction when the IκBα mutant was coexpressed with PKR. In addition, the expression of not only a nonphosphorylatable mutant form of IκBα but also of wild-type IκBα abrogated PKR-induced apoptosis. Lin et al. (36) expressed IκBα Wt while checking the effect of IκBα on alphavirus-induced apoptosis and observed an inhibitory effect on NF-κB activity. The high levels of expression of the IκBα Wt protein likely explains its inhibitory effect on PKR-induced cell death. However, we cannot exclude some peculiarity occurring in the PKR-mediated mechanism of NF-κB activation that could account for this result. The specificity of the effects caused by expression of IκBα proteins was confirmed by checking the translational state of the cells. Luciferase reporter assays and metabolic labeling studies showed that in cells coexpressing PKR and IκBα M, despite observing a rescue of apoptosis induction that was correlated with the absence of NF-κB binding activity, translation was significantly inhibited. This is the first evidence of a transcriptional pathway and not only a translational block in the commitment of apoptosis by PKR.
The identities of putative NF-κB genes that are upregulated and induce cell death upon PKR expression are not known. Transcription of several death-inducing genes is regulated by NF-κB. One of these genes is the FasL gene, which has consensus κB-binding sites in its promoter (55). Its NF-κB-dependent upregulation has been described as the mechanism of apoptosis induced by DNA-damaging agents in T lymphocytes (26). Alternatively, Fas mRNA levels also increase upon influenza virus infection, with the possible involvement of PKR (56). Upregulation of Fas mRNA levels in PKR+/+ but not in PKR0/0 mouse embryo fibroblasts after treatment with different apoptotic stimuli has also been observed (15). Although analysis of the human Fas promoter region (8) reveals the existence of two putative NF-κB consensus sequences, NF-IL6 has been proposed as the transcription factor mediating Fas induction in the influenza virus infection model (61). In the PKR-inducible VV system described here, we found that basal transcription of FasL mRNA was not increased upon PKR expression. However, we observed that Fas mRNA levels are upregulated after PKR activation at 6 hpi (unpublished observations). A more detailed analysis of mRNA levels upon PKR expression must be completed. Other candidate mRNAs that are NF-κB-induced death genes that have been previously characterized include several transcription factors, such as IRF-1 (30, 57) c-Myc (31), and p53 (65, 66), and caspases, such as caspase-1 (12).
Using different approaches, we have shown the involvement of NF-κB and eIF-2α in cell death induced upon PKR expression. However, a global explanation of the role of both PKR substrates in apoptosis is needed. One possible explanation is a different timing of events triggered by PKR phosphorylation of IκBα and eIF-2α. Hence, the amount of IκBα phosphorylated required for the activation of NF-κB-dependent transcription is surpassed sooner than the threshold of phosphorylated eIF-2α to a degree sufficient to lead to protein synthesis inhibition. Thus, a window of time exists for the translation of death genes. It is proposed that the inhibition of apoptosis in cells coexpressing PKR and the S51A mutant of eIF-2α is due to the translation at later times of selected mRNAs, such as inhibitors of death gene products, as observed with the antiapoptotic mechanism triggered by other signals of apoptosis such as TNF-α (63). Thus, the inhibition of protein synthesis mediated by eIF-2α phosphorylation is required for cell commitment to apoptosis. This could be interpreted as a security mechanism of the cell that regulates entry into the cell death program depending on the dsRNA levels (probably as a sensor of the status of viral infection). An alternative explanation for the observed results is that PKR acts as a selective translation inhibitor. Hence, it is possible that a certain class of mRNAs with several AUGs upstream of the initiation codon could be translated despite high levels of eIF-2α phosphorylation, as has been observed with GCN4 mRNA in yeast cells upon GCN2 activation (23). This could account for the inhibition of apoptosis both when NF-κB activation is blocked and when eIF-2α NP is expressed. Additionally, this hypothesis also explains the fact that the only expression of an S51D eIF-2α mutant that mimics the phosphorylated eIF-2α induced apoptosis in 3T3 cells (51).
A recent report (51) has demonstrated that 3T3 cells expressing a noncatalytic K296P mutant of the PKR are resistant to apoptosis upon serum starvation, whereas control cells are not. In addition, Grimm et al. (21) found that apoptosis induced by serum starvation in human embryonic kidney (HEK) 293 cells was dependent on NF-κB activity. Our work provides a clue to linking these two independent observations and suggests a role for NF-κB activity induced by PKR as a signal mediating apoptosis induction in serum-deprived cells. Blocking PKR action and inhibition of apoptosis are two common strategies used by different viruses to circumvent the clearance of cells (27, 40, 49, 59). Given the fact that apoptosis induced by such viruses as dengue virus (38) or alphavirus (37) is dependent on NF-κB activation, it is conceivable that PKR may have a key role in the induction of cell death upon infection by certain viruses. Hence, PKR would have a dual role in viral clearance, acting not only by inhibiting virus replication at the translational level but also by inducing apoptosis and thus preventing the production and spread of nascent viral particles that would escape immune surveillance.
In conclusion, the results presented here provide the first evidence for a PKR–NF-κB interaction mediating the apoptosis pathway induced by PKR. Moreover, our findings define the role of eIF-2α in apoptosis in the course of a virus infection.
ACKNOWLEDGMENTS
We thank R. E. Randall (University of Glasgow, Glasgow, Scotland) for the SV5 monoclonal antibody (from the NIBSC-MRC AIDS Reagent Project), J. Hershey (University of California) for the eIF-2α plasmid, M. J. Clemens (St. George’s Hospital, London, United Kingdom) and César de Haro (CBMSO) for the eIF-2α monoclonal antibody, and Fernando Arenzana (Institut Pasteur, Paris, France) for providing the NF-κB reporter plasmids. We also thank C. Weissman (University of Zurich, Zurich, Switzerland) for the generous gift of the 3T3-like PKR0/0 and PKR+/+ cells. We especially thank Juan Pablo Albar from the Department of Immunology and Oncology, Centro Nacional de Biotecnologia, for the production of the PKR synthetic peptide. We thank Manuel Collado, Carmen Rivas, Don Roth, and Isabel Vázquez for their critical reading of the manuscript and helpful suggestions and Victoria Jiménez and Laura Giménez for expert technical assistance.
This investigation was supported by grants SAF 95 0022 and PM98-0112 from the Comision Interministerial de Ciencia y Tecnologia of Spain (to M.E.) and grants from Fundación Caja de Madrid and Ministerio de Educación y Ciencia MEC (SAF 96/186) (to J.A.). J.G. was the recipient of an FPI fellowship from the Spanish MEC.
REFERENCES
- 1.Abbaddie C N, Kabrun F, Bouali F, Vandenbunder B, Enrietto P. High levels of c-rel expression are associated with programmed cell death in the developing avian embryo and in bone marrow cells in vitro. Cell. 1993;75:899–912. doi: 10.1016/0092-8674(93)90534-w. [DOI] [PubMed] [Google Scholar]
- 2.Alcamí J, Lain de Lera T, Folgueira L, Pedraza M A, Jacqué J M, Bachelerie F, Noriega A R, Hay R T, Harrich D, Gaynor R B, Virelizier J L, Arenzana-Seisdedos F. Absolute dependence on κB responsive elements for initiation and Tat-mediated amplification of HIV transcription in blood CD4 T lymphocytes. EMBO J. 1995;14:1552–1560. doi: 10.1002/j.1460-2075.1995.tb07141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arenzana-Seisdedos F, Fernandez B, Dominguez J, Jaqué M, Thompson D, Díaz-Meco M T, Moscat J, Virelizier J L. Phosphatidylcholine hydrolysis activates NF-κB and increases human immunodeficiency virus replication in human monocytes and T lymphocytes. J Virol. 1993;67:6596–6604. doi: 10.1128/jvi.67.11.6596-6604.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arenzana-Seisdedos F, Thompson J, Rodriguez M S, Bachelerie F, Thomas D, Hay R T. Inducible nuclear expression of newly synthesized IκBα negatively regulates DNA-binding and transcriptional activities of NF-κB. Mol Cell Biol. 1995;15:2689–2696. doi: 10.1128/mcb.15.5.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baeuerle P A, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin A S., Jr The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 7.Beg A, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 8.Behrmann I, Walczac H, Krammer P H. Structure of the human APO-1 gene. Eur J Immunol. 1994;24:3057–3062. doi: 10.1002/eji.1830241221. [DOI] [PubMed] [Google Scholar]
- 9.Belich M P, Salmeron A, Johnston L H, Ley S C. TPL-2 kinase regulates the proteolysis of the NF-κB-inhibitory protein NF-κB1 p105. Nature. 1999;397:363–368. doi: 10.1038/16946. [DOI] [PubMed] [Google Scholar]
- 10.Braiser A R, Tate J E, Habener J F. Optimized use of the firefly luciferase assay as a reporter gene in mammalian cell lines. BioTechniques. 1989;7:1116–1122. [PubMed] [Google Scholar]
- 11.Brun A, Rivas C, Esteban M, Escribano J M, Alonso C. African swine fever virus gene A179L, a viral homologue of bcl-2 protects cells from programmed cell death. Virology. 1996;225:227–230. doi: 10.1006/viro.1996.0592. [DOI] [PubMed] [Google Scholar]
- 12.Casano F J, Rolando A M, Mudgett U S, Molineaux S M. The structure and complete nucleotide sequence of the murine gene encoding interleukin-1 beta converting enzyme (ICE) Genomics. 1994;20:474–481. doi: 10.1006/geno.1994.1203. [DOI] [PubMed] [Google Scholar]
- 13.Castelli J A, Hassel B A, Wood K A, Li X-L, Amemiya K, Dalakas M C, Torrence P, Youle R J. A study of the interferon antiviral mechanism: apoptosis activation of the 2-5A system. J Exp Med. 1997;186:967–972. doi: 10.1084/jem.186.6.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craiu A, Gaczynska M, Akopian T, Gramm C F, Fenteany G, Goldberg A L, Rock K L. Lactacystin and clasto-lactacystin beta-lactone modify multiple proteasome beta-subunits and inhibit intracellular protein degradation and major histocompatibility complex class I antigen presentation. J Biol Chem. 1997;272:13437–13445. doi: 10.1074/jbc.272.20.13437. [DOI] [PubMed] [Google Scholar]
- 15.Der S D, Yang Y, Weissmann C, Williams B R G. A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:3279–3283. doi: 10.1073/pnas.94.7.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Díaz-Guerra M, Rivas C, Esteban M. Inducible expression of the 2-5A-synthetase/RNase L system results in inhibition of vaccinia virus replication. Virology. 1997;227:220–228. doi: 10.1006/viro.1996.8294. [DOI] [PubMed] [Google Scholar]
- 17.Díaz-Guerra M, Rivas C, Esteban M. Activation of the IFN-inducible enzyme RNase L causes apoptosis of animal cells. Virology. 1997;236:354–363. doi: 10.1006/viro.1997.8719. [DOI] [PubMed] [Google Scholar]
- 18.Donzé O, Jagus R, Koromilas A E, Hershey J W B, Sonenberg N. Abrogation of translation initiation factor eIF2 phosphorylation causes malignant transformation of NIH 3T3 cells. EMBO J. 1995;14:3828–3834. doi: 10.1002/j.1460-2075.1995.tb00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Figueiredo-Pereira M E, Berg K A, Wilk S. A new inhibitor of the chymotrypsin-like activity of the multicatalytic proteinase complex (20S proteasome) induces accumulation of ubiquitin-protein conjugates in a neuronal cell. J Neurochem. 1994;63:1578–1581. doi: 10.1046/j.1471-4159.1994.63041578.x. [DOI] [PubMed] [Google Scholar]
- 20.Fuerst T R, Niles E G, Studier F W, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimm S, Bauer M K A, Bauerle P A, Schulze-Osthoff K. Bcl-2 down-regulates the activity of transcription factor NF-κB induced upon apoptosis. J Cell Biol. 1996;134:13–23. doi: 10.1083/jcb.134.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hershey J W B. Translational control in mammalian cells. Annu Rev Biochem. 1991;60:715–754. doi: 10.1146/annurev.bi.60.070191.003441. [DOI] [PubMed] [Google Scholar]
- 23.Hinnebusch A G. Gene-specific translational control of the yeast GCN4 gene by phosphorylation of eukaryotic initiation factor 2. Mol Microbiol. 1993;10:215–223. doi: 10.1111/j.1365-2958.1993.tb01947.x. [DOI] [PubMed] [Google Scholar]
- 24.Imbert V, Rupec R A, Livolsi A, Pahl H L, Traenckner E B M, Mueller-Dieckmann C, Farahifar D, Rossi B, Auberger P, Bauerle P A, Peyron J F. Tyrosine phosphorylation of I-κBα activates NF-κB without proteolytic degradation of I-κBα. Cell. 1996;86:787–798. doi: 10.1016/s0092-8674(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs B L, Langland J O. When two strands are better than one: the mediators and modulators of the cellular responses to double-stranded RNA. Virology. 1996;219:339–349. doi: 10.1006/viro.1996.0259. [DOI] [PubMed] [Google Scholar]
- 26.Kasibhatla S, Brunner T, Genestier L, Echeverri F, Mahboubi A, Green D R. DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-κB and AP-1. Mol Cell. 1998;1:543–551. doi: 10.1016/s1097-2765(00)80054-4. [DOI] [PubMed] [Google Scholar]
- 27.Katze M G. Games viruses play: a strategic initiative against the IFN-induced, ds-RNA activated protein kinase. Semin Virol. 1993;4:259–268. [Google Scholar]
- 28.Kibler K V, Shors T, Perkins K B, Zeman C C, Banaszak M P, Biesterfeldt J, Langland J O, Jacobs B L. Double-stranded RNA is a trigger for apoptosis in vaccinia virus-infected cells. J Virol. 1997;71:1992–2003. doi: 10.1128/jvi.71.3.1992-2003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar A, Haque J, Lacoste J, Hiscott J, Williams B R G. Double-stranded RNA-dependent protein kinase activates transcription factor NF-κB by phosphorylating IκB. Proc Natl Acad Sci USA. 1994;91:6288–6292. doi: 10.1073/pnas.91.14.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar A, Yang Y, Flati V, Der S, Kadereit S, Deb A, Haque J, Reis L, Weissmann C, Williams B R G. Deficient cytokine signalling in mouse embryo fibroblasts with a targeted deletion in the PKR gene: role of IRF-1 and NF-κB. EMBO J. 1997;16:406–416. doi: 10.1093/emboj/16.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.La Rosa F A, Pierce J W, Sorenshein G E. Differential regulation of the c-myc oncogene promoter by the NF-κB Rel family of transcription factors. Mol Cell Biol. 1994;14:1039–1044. doi: 10.1128/mcb.14.2.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leaman D W, Salvekar A, Patel R, Sen C S, Stark G R. A mutant cell line defective in response to double-stranded RNA and in regulating basal expression of interferon-stimulated genes. Proc Natl Acad Sci USA. 1998;95:9442–9447. doi: 10.1073/pnas.95.16.9442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee S B, Esteban M. The interferon-induced double-stranded RNA-activated protein kinase induces apoptosis. Virology. 1994;199:491–496. doi: 10.1006/viro.1994.1151. [DOI] [PubMed] [Google Scholar]
- 34.Lee S B, Melkova Z, Yan W, Williams B R G, Hovanessian A G, Esteban M. The interferon-induced double-stranded RNA-activated human p68 protein kinase potently inhibits protein synthesis in cultured cells. Virology. 1993;192:380–385. doi: 10.1006/viro.1993.1048. [DOI] [PubMed] [Google Scholar]
- 35.Lee S B, Rodriguez D, Rodriguez J R, Esteban M. The apoptosis pathway triggered by the IFN-induced dsRNA activated protein kinase PKR requires the third basic domain, initiates upstream of Bcl-2 and involves ICE-like proteases. Virology. 1997;231:81–88. doi: 10.1006/viro.1997.8494. [DOI] [PubMed] [Google Scholar]
- 36.Lin K, DiDonato J A, Hoffmann A, Hardwick J M, Ratan R R. Suppression of steady-state, but not stimulus-induced NF-κB activity inhibits alphavirus-induced apoptosis. J Cell Biol. 1998;141:1479–1487. doi: 10.1083/jcb.141.7.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin K, Lee S, Narayanan R, Barbarian J M, Hardwick J M, Ratan R R. Thiol agents and Bcl-2 identify an alphavirus-induced apoptotic pathway that requires activation of the transcription factor NF-kappa B. J Cell Biol. 1995;131:1149–1161. doi: 10.1083/jcb.131.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marianneau P, Cardona A, Edelman L, Deubel V, Després P. Dengue virus replication in human hepatoma cells activates NF-κB which in turn induces apoptotic cell death. J Virol. 1997;71:3244–3249. doi: 10.1128/jvi.71.4.3244-3249.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayo M W, Wang C, Cogswell P C, Rogers-Graham K S, Lowe S L, Der C J, Baldwin A S., Jr Requirement of NF-κB activation to suppress p53-independent apoptosis induced by oncogenic ras. Science. 1997;278:1812–1815. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]
- 40.McFadden G. Even viruses can learn to cope with stress. Science. 1998;279:40–41. doi: 10.1126/science.279.5347.40. [DOI] [PubMed] [Google Scholar]
- 41.Meurs E F, Chong K, Galabru J, Thomas N S, Kerr I M, Williams B R, Hovanessian A G. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990;62:379–390. doi: 10.1016/0092-8674(90)90374-n. [DOI] [PubMed] [Google Scholar]
- 42.Pathak V K, Schindler D, Hershey J W B. Generation of a mutant form of protein synthesis initiation factor eIF-2 lacking the site of phosphorylation by eIF-2 kinases. Mol Cell Biol. 1988;8:993–995. doi: 10.1128/mcb.8.2.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Powel P P, Dixon L K, Parkhouse R M E. An I-κB homolog encoded by african swine fever virus provides a novel mechanism for downregulation of proinflammatory cytokine responses in host macrophages. J Virol. 1996;70:8527–8533. doi: 10.1128/jvi.70.12.8527-8533.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Proud C G. PKR: a new name and new roles. Trends Biochem Sci. 1995;20:241–246. doi: 10.1016/s0968-0004(00)89025-8. [DOI] [PubMed] [Google Scholar]
- 45.Randall R E, Young D F, Goswami K K A, Russell W C. Isolation and characterization of monoclonal antibodies to simian virus 5 and their use in revealing antigenic differences between human, canine and simian isolates. J Gen Virol. 1987;68:2769–2780. doi: 10.1099/0022-1317-68-11-2769. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez J F, Smith G L. Inducible gene expression from vaccinia virus vectors. Virology. 1990;177:239–250. doi: 10.1016/0042-6822(90)90477-9. [DOI] [PubMed] [Google Scholar]
- 47.Roff M, Thompson J, Rodriguez M S, Jaque J M, Baleux F, Arenzana-Seisdedos F, Hay R T. Role of I-κBα ubiquitination in signal-induced activation of NF-κB in vivo. J Biol Chem. 1996;271:7844–7850. doi: 10.1074/jbc.271.13.7844. [DOI] [PubMed] [Google Scholar]
- 48.Rowlands A G, Panniers R, Henshaw E C. The catalytic mechanism of guanine nucleotide exchange factor action and competitive inhibition by phosphorylated eukaryotic initiation factor 2. J Biol Chem. 1988;263:5526–5533. [PubMed] [Google Scholar]
- 49.Samuel C E. Antiviral actions of interferon. Interferon-regulated cellular proteins and their surprisingly selective antiviral activities. Virology. 1991;183:1–11. doi: 10.1016/0042-6822(91)90112-o. [DOI] [PubMed] [Google Scholar]
- 50.Schouten G J, Vertegaal A C O, Whiteside S T, Israel A, Toebes M, Dorsman J C, van der Eb A J, Zantema A. I-κBα is a target for the mitogen-activated 90 kDa ribosomal S6 kinase. EMBO J. 1997;16:3133–3144. doi: 10.1093/emboj/16.11.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Srivastava S P, Kumar K U, Kaufman R J. Phosphorylation of eukaryotic translation initiation factor 2 mediates apoptosis in response to activation of the double-stranded RNA-dependent protein kinase. J Biol Chem. 1998;273:2416–2423. doi: 10.1074/jbc.273.4.2416. [DOI] [PubMed] [Google Scholar]
- 52.Stancovski I, Baltimore D. NF-κB activation: the I-κBα kinase revealed? Cell. 1997;91:299–302. doi: 10.1016/s0092-8674(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 53.Stark G R, Kerr I M, Williams B R G, Silverman R H, Schreiber R D. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 54.Suarez P, Díaz-Guerra M, Prieto C, Esteban M, Castro J M, Nieto A, Ortín J. Open reading frame 5 of porcine reproductive and respiratory syndrome virus as a cause of virus-induced apoptosis. J Virol. 1996;70:2876–2882. doi: 10.1128/jvi.70.5.2876-2882.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takahashi T, Tanaka M, Inazawa J, Abe T, Suda T, Nagata S. Human Fas ligand: gene structure, chromosomal localization and species specificity. Int Immunol. 1994;6:1567–1574. doi: 10.1093/intimm/6.10.1567. [DOI] [PubMed] [Google Scholar]
- 56.Takizawa T, Ohashi K, Nakanishi Y. Possible involvement of double-stranded RNA-activated protein kinase in cell death by influenza virus infection. J Virol. 1996;70:8128–8132. doi: 10.1128/jvi.70.11.8128-8132.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tanaka N, Ishihara M, Kitagawa M, Harada H, Kimura T, Matsuyama T, Lamphier M S, Aizawa S, Mak T W, Taniguchi T. Cellular commitment to oncogene-induced transformation or apoptosis is dependent of the transcription factor IRF-1. Cell. 1994;77:829–839. doi: 10.1016/0092-8674(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 58.Traenckner E B M, Wilk S, Baeuerle P A. A proteasome inhibitor prevents activation of NF-κB and stabilizes a newly phosphorylated form of I-κBα that is still bound to NF-κB. EMBO J. 1994;13:5433–5441. doi: 10.1002/j.1460-2075.1994.tb06878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tschopp J, Thome M, Hofmann K, Mein E. The fight of viruses against apoptosis. Curr Opin Genet Dev. 1998;8:82–87. doi: 10.1016/s0959-437x(98)80066-x. [DOI] [PubMed] [Google Scholar]
- 60.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 61.Wada N, Matsumura M, Ohba Y, Kobayashi N, Takizawa T, Nakanishi Y. Transcription stimulation of the Fas-encoding gene by nuclear factor for interleukin-6 expression upon influenza virus infection. J Biol Chem. 1995;270:18007–18012. doi: 10.1074/jbc.270.30.18007. [DOI] [PubMed] [Google Scholar]
- 62.Wang C, Mayo M W, Baldwin A S., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 63.Wang C, Mayo M W, Korneluk R G, Goeddel D V, Baldwin A S., Jr NF-κB antiapoptosis: Induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 64.White E. Life, death, and the pursuit of apoptosis. Genes Dev. 1996;10:1–15. doi: 10.1101/gad.10.1.1. [DOI] [PubMed] [Google Scholar]
- 65.Wu H, Lozano G. NF-κB activation of p53. A potential mechanism for suppressing cell growth in response to stress. J Biol Chem. 1994;269:20067–20074. [PubMed] [Google Scholar]
- 66.Yeung M C, Lau A S. Tumor suppressor p53 as a component of the tumor necrosis factor-induced, protein kinase PKR-mediated apoptotic pathway in human promonocytic U937 cells. J Biol Chem. 1998;273:25198–25202. doi: 10.1074/jbc.273.39.25198. [DOI] [PubMed] [Google Scholar]
- 67.Zhou A, Paranjape J, Brown T L, Nie H, Naik S, Dong B, Chang A, Trapp B, Fairchild R, Colmenares C, Silverman R H. Interferon action and apoptosis are defective in mice devoid of 2′,5′-oligoadenylate-dependent RNase L. EMBO J. 1997;16:6355–6363. doi: 10.1093/emboj/16.21.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]