Abstract
Objective
To assess the antibody response in non-immunocompromised adults after two doses of BNT162b2.
Methods
Prospective, single-centre observational study in non-immunocompromised adults aged 18 years or more who received two doses of BNT162b2. The study contemplates analyses of serum samples collected 1.5, 3, 6, 9 and 12 months after the second dose of BNT162b2; results of the 1.5- and 3-month time-points are presented in this report. Antibodies against the receptor binding domain of the S1 subunit of the spike protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (anti-RBD antibodies) were measured using a commercial quantitative immunoassay. A threshold of 4160 AU/mL (corresponding to an ID50 of 1:250) was used as surrogate marker for serum neutralizing activity.
Results
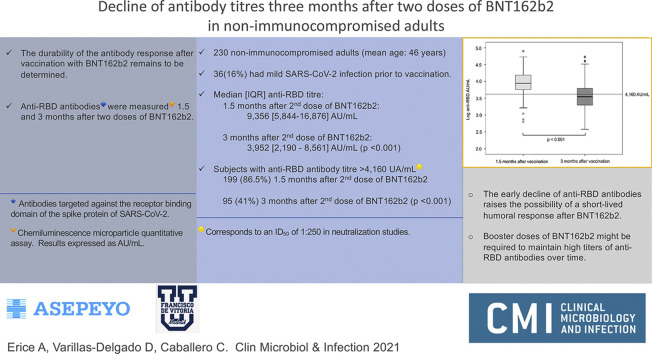
Of 273 hospital workers who received two doses of BNT162b2, 260 (95%) agreed to participate in the study; 2/260 (0.8%) were excluded because of immunocompromised conditions. At the time of this report, 230/258 (89%) participants (mean age 46.0 years (SD 11.4 years); 143/230 (62%) female; 87/230 (38%) male) had completed 3 months of follow up after the second dose of BNT162b2. Thirty-six (16%) of the 230 had documented mild SARS-CoV-2 infection before receiving the first dose of BNT162b2. Median (interquartile range (IQR)) anti-RBD titres 1.5 months after vaccination were 9356 (5844–16 876) AU/mL; 3 months after vaccination, median anti-RBD titres had declined to 3952 (2190–8561) AU/mL (p < 0.001). Of 199/230 (86.5%) participants who had anti-RBD titres above 4160 AU/mL 1.5 months after the second dose of BNT162b2, only 95/230 (41%) maintained anti-RBD titres above this level 3 months after vaccination (p < 0.001).
Conclusions
The decline of anti-RBD antibodies 3 months after the second dose of BNT162b2 is of concern because it raises the possibility of a short-lived humoral immunity after vaccination. Booster doses of BNT162b2 might be required to maintain high titres of anti-RBD antibodies over time.
Keywords: BNT162b2, COVID-19, Vaccine, Immunogenicity, SARS-CoV-2
Graphical abstract
Introduction
The mRNA vaccine BNT162b2 (Pfizer-BioNtech) encoding the receptor binding domain of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein has shown 95% efficacy in preventing symptomatic infection in clinical trials [1]. In phase I/II studies, the vaccine produced robust anti-SARS-CoV-2 antibody responses in healthy adults [2,3]. However, the durability of the antibody response after vaccination with BNT162b2 remains to be determined [4,5].
Materials and methods
We are conducting a prospective, single-centre observational study to assess the evolution of the antibody response in non-immunocompromised hospital workers aged 18 years or more who received two doses of BNT162b2 at our institution. The study contemplates collection of serum samples 1.5, 3, 6, 9 and 12 months after the second dose of BNT162b2; analysis of results obtained 1.5 and 3 months after vaccination are presented in this report. At each time-point, data on previous SARS-CoV-2 infection and the diagnostic method used were collected. The study was approved by the local ethics committee (approval number 4502). All participants provided informed consent.
Antibodies against the receptor binding domain of the S1 subunit of the spike protein of SARS-CoV-2 (anti-RBD antibodies) were measured at each time-point using a chemiluminescent microparticle quantitative immunoassay (Architect SARS-CoV-2 IgG II Quant; Abbott Laboratories, Chicago, IL, USA). Results were reported as concentrations (AU/mL), with a cut-off of 50 AU/mL or more considered positive. To assess the correlation between anti-RBD antibody titres and neutralizing activity, we used a threshold of 4160 AU/mL as surrogate marker for serum neutralizing activity. This threshold corresponds to a 50% inhibitory dilution (ID50) of 1:250 in plaque-reduction neutralization studies [6]. Antibodies targeting the SARS-CoV-2 nucleocapsid (anti-N antibodies) were measured using a chemiluminescent microparticle immunoassay (Architect SARS-CoV-2 IgG; Abbott Laboratories, Chicago, IL, USA); results were reported as a cut-off index, with values of 1.49 and above considered positive. Anti-N antibodies were only analysed in serum samples obtained 1.5 months after the second dose of BNT162b2.
Previous SARS-CoV-2 infection was identified after review of health records by documented evidence of SARS-CoV-2 in upper respiratory tract samples by PCR or antigen test, detection of SARS-CoV-2 specific IgG and/or IgM (for IgM alone, concurrent symptoms were required), or a positive anti-N antibody result.
Statistical analysis was performed with SPSS, version 21.0 (IBM Corporation, Armonk, NY, USA) for Windows. Quantitative variables are expressed as mean and standard deviation (SD) or median and interquartile range (IQR). For comparisons between groups, χ2 tests and non-parametric Wilcoxon rank sum test were used. A two-tailed p value less than 0.05 was considered significant.
Results
Of 273 hospital workers who received two doses of BNT162b2 at our institution, 260/273 (95%) agreed to participate in the study; 2/260 (0.8%) were excluded because of immunocompromised conditions. At the time of this report, 230/258 (89%) participants (mean age 46.0 years (SD 11.4 years); 143/230 (62%) female; 87/230 (38%) male) had completed 3 months of follow up after the second dose of BNT162b2. Thirty-six (16%) of the 230 had documented mild SARS-CoV-2 infection before receiving the first dose of BNT162b2; no additional SARS-CoV-2 infections occurred in the remaining 194/230 (84%) study participants between vaccine doses or during follow up.
Serum samples were obtained a mean of 40.1 days (SD 2.8 days) and 88.8 days (SD 2.8 days) after the second dose of BNT162b2. All participants had anti-RBD antibodies at both time points; titres were higher in men, although the differences were not statistically significant. Individuals with previous SARS-CoV-2 infection had higher anti-RBD antibody titres at both time-points (p < 0.001). Also, 21- to 30-year-old participants had significantly higher anti-RBD antibody titres compared with the other age groups at both time-points (p 0.046 and p 0.023, respectively). Results are summarized in Table 1 .
Table 1.
Anti-RBD antibody titres after two doses BNT162b2
| Anti-RBD antibody titres (AU/mL), median (IQR) |
|||||
|---|---|---|---|---|---|
| N (%) | 1.5 months after second dose of BMT162b2 | p value | 3 months after second dose of BMT162b2 | p value | |
| All | 230 (100) | 9356 (5844–16 876) |
— | 3952 (2190–8561) |
<0.001 |
| Sex | |||||
| Male | 143 (62) | 10 293 (6155–17 292) |
0.323 | 4292 (2053–11 356) |
0.454 |
| Female | 87 (38) | 8434 (5751–16 449) |
3797 (2206–7711) |
||
| Previous SARS-CoV-2 infection | |||||
| Yes | 36 (16) | 19 016 (7974–27 885) |
<0.001 | 9364 (3975–22 233) |
<0.001 |
| No | 194 (84) | 8747 (5631–15 409) |
3724 (2003–7137) |
||
| Age (years) | |||||
| 20–30 | 29 (12.6) | 15 402 (8763–21 545) |
5733 (3893–12 891) |
||
| 31–40 | 47 (20.4) | 7642 (5683–13 532) |
2949 (1981–8950) |
||
| 41–50 | 68 (29.6) | 9272 (5432–16 589) |
0.046 | 3572 (1721–6771) |
0.023 |
| 51–60 | 60 (26.1) | 9234 (6251–17 180) |
3862 (2285–7824) |
||
| 61–70 | 25 (10.9) | 9262 (4541–16 081) |
6176 (2193–14 392) |
||
| 71–80 | 1 (0.4) | 2165 | 750 | ||
Abbreviations: anti-RBD, antibody against the receptor binding domain of the S1 subunit of the spike protein of SARS-CoV-2; IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
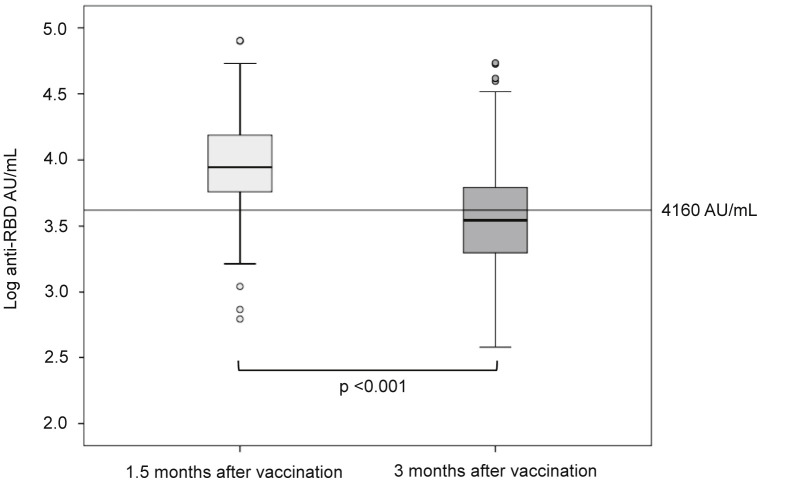
Three months after the second dose of BNT162b2, median anti-RBD antibodies had decreased by 58% in all study participants (from 9356 AU/mL to 3952 AU/mL); in individuals with previous SARS-CoV-2 infection, anti-RBD antibody titres had decreased by 51% (from 19 016 AU/mL to 9364 AU/mL). Of 199/230 (86.5%) participants who had anti-RBD antibodies above 4160 AU/mL 1.5 months after the second dose of BNT162b2, only 95/230 (41%) maintained anti-RBD antibody titres above this level 3 months after vaccination (p < 0.001) (Fig. 1 ).
Fig. 1.
Anti-RBD antibody titres 1.5 and 3 months after the second doses of BNT162b2. A log10 scale was used on the y-axis to minimize data dispersion. In each box-and-whisker plot, the horizontal line represents the median, the top and bottom of the box the interquartile range, and the whiskers the minimum and maximum values. The horizontal line indicates an anti-RBD antibody titre of 4160 AU/mL, which correlates with a 50% inhibitory dilution (ID50) of 1:250 in plaque-reduction neutralization studies. χ2 and non-parametric Wilcoxon rank sum tests were used for the following comparisons: (a) anti-RBD antibody titres in blood samples from all study participants (n = 230) measured 1.5 and 3 months after vaccination (p < 0.001); (b) participants with anti-RBD antibody titres that were above 4160 AU/mL 1.5 months after vaccination (n = 199), and 3 months after vaccination (n = 95) (p < 0.001).
Discussion
This study shows a decline of anti-RBD antibodies in non-immunocompromised adults 3 months after the second dose of BNT162b2, regardless of previous SARS-CoV-2 infection. Until recently, a fall in antibodies following vaccination with BNT162b2 has not been described in other studies with a more limited follow up [2,6]. Our results are consistent with those from recent reports showing a continuous decline of anti-RBD antibodies within 10 weeks after vaccination in individuals who had received two doses of BNT162b2 [7,8]. This early decay of anti-RBD antibodies is similar to that observed in patients with mild SARS-CoV-2 infection within 3 months after the onset of symptoms [9,10].
The significance of the decline of anti-RBD antibodies we observed is unclear because the titres of anti-RBD antibodies that are protective against SARS-CoV-2 infection have not been defined. Nevertheless, this antibody decline is of concern because it raises the possibility that protection from humoral immunity after vaccination might be short-lived. Anti-RBD antibodies are a reasonable indicator of antiviral activity, and robust correlations between anti-RBD antibodies and viral neutralizing activity have been well established, with higher anti-RBD titres correlating with higher vaccine efficacy [[10], [11], [12], [13]].
Although we did not perform neutralization analyses, 3 months after the second dose of BNT162b2 most of our study participants had anti-RBD antibody titres that had fallen below a surrogate neutralization threshold [6]. Recently, breakthrough severe COVID-19 has been reported in fully vaccinated individuals a median of 39.5 days after the second dose of BNT162b2 [14]; their median anti-RBD antibody titre was 947.5 AU/mL, with lower values in those individuals with a poor outcome [14]. Although most of the patients were elderly (median age 71.1 years) with co-morbidities, the study suggests that a low anti-RBD antibody titre is one factor associated with breakthrough SARS-CoV-2 infection after complete vaccination with BNT162b2.
Additional follow up is needed to determine whether the decline of anti-RBD antibodies following vaccination will continue a downward trajectory or will plateau at a lower, steady-state level. In a recent study of convalescent patients, SARS-CoV-2 antibodies declined rapidly in the first 4 months after infection; this was followed by a more gradual descent over the ensuing months with antibodies remaining detectable 11 months after infection [15]. This antibody pattern has been attributed to a transition from an early phase of secretion of serum antibodies by short-lived plasmablasts to a later phase where anti-SARS-CoV-2 antibodies are produced by a persistent population of long-lived plasma cells residing in the bone marrow [15]. It appears therefore, that humoral immunity triggered by SARS-CoV-2 infection is long-lasting; however, it is currently unknown whether BNT162b2 produces a similar immune response. In a small study of non-infected individuals who received two doses of BNT162b2, high numbers of SARS-CoV-2 spike protein-targeting B cells were present in the germinal centres of lymph node biopsies obtained within 15 weeks of the second dose of BNT162b2 [16]. This B-cell response drives the early humoral immune response following vaccination, but its durability remains to be determined.
Anti-RBD antibodies are not the sole correlate of protection against SARS-CoV-2 infection and disease. In addition to specific antibodies and memory B cells, adaptive immunity to SARS-CoV-2 infection includes specific CD4+ T-cell and CD8+ T-cell responses. In SARS-CoV-2-infected individuals, each compartment of this complex immune response exhibits different kinetics, a marked heterogeneity among individuals, and a durability that extends beyond 6 months after onset of symptoms [17]. Although the characteristics of the cellular immune response following vaccination have not been well established, a recent study in a small group of individuals has shown that two doses of BNT162b2 induced potent SARS-CoV-2-specific CD4+ T-cell and CD8+ T-cell responses that persisted during a follow up of 9 weeks [18].
Our study has several limitations. First, blood samples were not obtained at baseline, between the first and second doses of the vaccine or immediately after the second dose; analysis of those additional time-points could have contributed to a more precise description of the kinetics of the early anti-RBD antibody response after vaccination. Second, we have not performed SARS-CoV-2 neutralization studies; therefore, we based our considerations on the correlations described in other studies between titres of binding antibodies and neutralizing capacity against SARS-CoV-2. Finally, we have not analysed the cellular immune response following vaccination.
The significance of the decline of titres of anti-RBD antibodies against SARS-CoV-2 in terms of the long-term efficacy of BNT162b2 remains to be determined. Booster doses of BNT162b2 might be necessary to maintain high antibody titres that could prevent vaccinated individuals from becoming infected with SARS-CoV-2 and transmitting the virus to others.
Author contributions
AE and CC conceived and designed the study, and acquired the data. DVD analysed the data. AE, CC and DVD interpreted the data. AE drafted the manuscript; all authors critically revised the manuscript for its intellectual content and approved the submitted version. All authors had full access to all the data in the study and agree to be accountable for all aspects of the work.
Transparency declaration
The authors declare that they have no conflicts of interest.
Funding
This work did not receive any funding from public, private or commercial agencies.
Acknowledgements
We are indebted to the nursing team and the laboratory technicians of the hospital for their contributions to the study. We are grateful to all study participants.
Editor: A. Huttner
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.08.023.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L.M., Vormehr M., et al. COVID-19 vaccine BNT162b1 elicits human antibody and Th1 cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 3.Walsh E.E., Frenck R.W., Jr., Falsey A.R., Kitchin N., Absalon J., Gurtman A., et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Favresse J., Bayart J.L., Mullier F., Dogné J.M., Closset M., Douxfils J., et al. Early antibody response in health-care professionals after two doses of SARS-CoV-2 mRNA vaccine (BNT162b2) Clin Microbiol Infect. 2021;27(9):1351.e5–1351.e7. doi: 10.1016/j.cmi.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.del Rio C., Malani P. COVID-19 in 2021—continuing uncertainty. JAMA. 2021;325:1389–1390. doi: 10.1001/jama.2021.3760. [DOI] [PubMed] [Google Scholar]
- 6.Ebinger J.E., Fert-Bober J., Printsev I., Wu M., Sun N., Prostko J.C., et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med. 2021;27:981–984. doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Favresse J., Bayart J.L., Mullier F., Elsen M., Eucher C., Van Eeckhoudt, et al. Antibody titers decline 3-month post-vaccination with BNT162b2. Emerg Microb Infect. 2021;10:1495–1498. doi: 10.1080/22221751.2021.1953403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shrotri M., Navaratnam A., Nguyen V., Byrne T., Geismar C., Fragaszy E., et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet. 2021;398:385–386. doi: 10.1016/S0140-6736(21)01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seow J., Graham C., Merrick B., Acors S., Pickering S., Steel K.J., et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020;5:1598–1607. doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ibarrondo F.J., Fulcher J.A., Goodman-Meza D., Elliot J., Hofmann C., Hausner M.A., et al. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild COVID-19. N Engl J Med. 2020;383:1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J., et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 12.Wajnberg A., Amanat F., Firpo A., Altman D.R., Bailey M.J., Mansour M., et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Earle K.A., Ambrosino D.M., Fiore-Gartland A., Goldbaltt D., Gilbert P.B., Siber G.R., et al. Evidence for antibody as a protective correlate for COVID-19 vaccine. Vaccine. 2021;39:4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brosh-Nissimov T., Orenbuch-Harroch E., Chowers M., Elbaz M., Nesher L., Stein M., et al. BNT162b2 vaccine breakthough: clinical characteristics of 152 fully vaccinated hospitalized COVID-129 patients in Israel. Clin Microbiol Infect. 2021;27(11):1652–1657. doi: 10.1016/j.cmi.2021.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner J.S., Kim W., Kalaidina E., Goss C.W., Rauseo A.M., Schmitz A.J., et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature. 2021;595:421–425. doi: 10.1038/s41586-021-03647-4. [DOI] [PubMed] [Google Scholar]
- 16.Turner J.S., O´Halloran J.A., Kalaidina E., Kim W., Schmitz A.J., Zhou J.Q., et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2020;596:109–113. doi: 10.1038/s41586-021-03738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dan J.M., Mateus J., Kato Y., Hastie K.M., Dawen Yu E., Faliti C.E., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371 doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahin U., Muik A., Vogler I., Derhovanessian E., Kranz L.M., Vormehr M., et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595:572–577. doi: 10.1038/s41586-021-03653-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.