Abstract
BACKGROUND:
Bupropion (BUP) is a chiral antidepressant and smoking cessation aide with benefits and side effects correlated with parent and active metabolite concentrations. BUP is metabolized by CYP2B6, CYP2C19, and CYP3A4 to hydroxy-BUP (OH-BUP), and by 11β-hydroxysteroid dehydrogenase-1 and aldo-keto reductases to threohydrobupropion (Threo) and erythrohydrobupropion (Erythro), respectively. As pregnancy alters the activity of drug-metabolizing enzymes, the authors hypothesized that BUP metabolism and BUP metabolite concentrations, would be altered during pregnancy, potentially affecting the efficacy and safety of BUP in pregnant women.
METHODS:
Pregnant women (n=8) taking BUP chronically were enrolled, and steady-state plasma samples and dosing interval urine samples were collected during pregnancy and postpartum. Maternal and umbilical cord venous blood samples were collected at delivery from three subjects, and cord blood/maternal plasma concentration ratios were calculated. The concentrations of BUP stereoisomers and their metabolites were measured. Paired t-tests were used to compare pharmacokinetic parameters during pregnancy and postpartum.
RESULTS:
No significant changes were observed in the steady-state plasma concentrations, metabolite to parent ratios, formation clearances, or renal clearance of any of the compounds during pregnancy when compared to postpartum. The umbilical cord venous plasma concentrations of BUP and its metabolites were 30–60% lower than maternal plasma concentrations.
CONCLUSIONS:
This study showed that there are no clinically meaningful differences in the stereoselective disposition of BUP or its metabolites during pregnancy, indicating that dose adjustment during pregnancy may not be necessary. The results also showed that the placenta provides a partial barrier for bupropion and its metabolite distribution to the fetus, with possible placental efflux transport of bupropion and its metabolites.
Keywords: Pharmacokinetics, Pregnancy, Bupropion
Background
Nearly 12% of women experience depression during pregnancy1 and perinatal depression is associated with several adverse pregnancy outcomes, including preterm birth and small for gestational age neonates.2 Moreover, over 12% of women smoke during pregnancy,3 which is associated with multiple adverse pregnancy outcomes, including stillbirth,4 preterm premature rupture of membranes, and placental abruption.5 Bupropion (BUP) is prescribed as a smoking cessation aide and is the second most common antidepressant used by women of reproductive age in the United States.6 A 2019 meta-analysis of 14 studies assessing the safety of BUP use in pregnancy found no negative impact in terms of congenital anomalies, birth weight, or preterm birth.7 Given the lack of evidence harm of BUP in pregnancy, coupled with the high rates and known risks of both depression and smoking during pregnancy, BUP may be a useful medication to safely and effectively treat these conditions during pregnancy, thus mitigating disease-related risks.
The therapeutic effects of BUP as an antidepressant and smoking cessation aide correlate with the circulating concentrations of BUP stereoisomers and their active metabolites.8–12 Side effects such as dry mouth, insomnia, and seizures are also more likely with higher BUP and metabolite concentrations.13,14 These strong concentration-effect relationships suggest that if BUP disposition is altered during pregnancy this may lead to altered therapeutic efficacy and/or side effect profile.
BUP is administered as a racemic mixture of S- and R-bupropion, which undergo chiral inversion.15 The circulating concentrations of R-BUP are 3- to 6-fold higher than that of S-BUP.16,17 BUP metabolites, hydroxybupropion (OH-BUP), threohydrobupropion (Threo), and erythrohydrobupropion (Erythro), show plasma concentrations that exceed BUP concentrations by 3–30-fold.8,17,18 Concentrations of metabolite stereoisomers differ, with R,R-OH-BUP exposures up to 65-fold higher than S,S-OH-BUP.17 OH-BUP is formed by cytochrome P450 enzymes CYP2B6, CYP2C19, and CYP3A4, while CYP2C19 forms 4’-hydroxy-bupropion (4’OH-BUP).18–20 Threo and Erythro are formed by 11β-hydroxysteroid dehydrogenase-1 (11β-HSD-1) and aldo-keto reductase(s) (Figure 1).21–23 Threo and Erythro are further cleared by CYP2C19, which forms 4’-OH-eryhtrohydrobupropion (4’OH-erythro) and 4’-OH-threohydrobupropion (4’OH-threo), and all metabolites are further glucuronidated, with the largest fractions of glucuronidation observed for OH-metabolites.20
Figure 1.
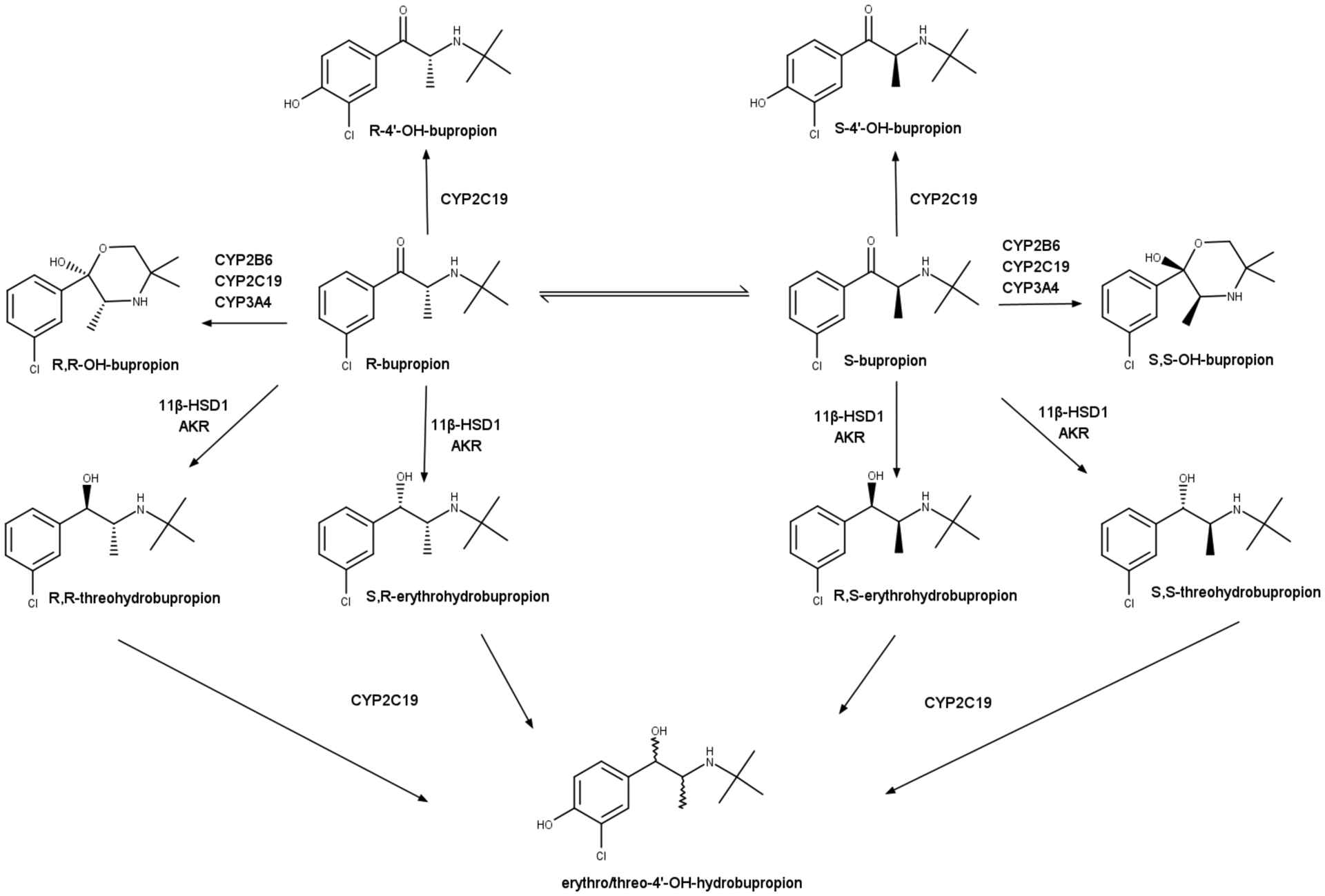
Metabolic pathways of bupropion stereoisomers. Bupropion is administered as a racemic mixture and undergoes chiral inversion and stereoselective metabolism. Furthermore, it undergoes extensive glucuronidation. AKR, Aldo-keto reductase.
Pregnancy may lead to number of changes that can impact the pharmacokinetics of medications, including altered absorption, distribution, metabolism, and renal clearance,24 which can alter the efficacy and maternal and fetal safety of drugs. CYP2B6, which catalyzes the formation of OH-BUP,15 is induced by estradiol in vitro.25–27 Given the elevated circulating concentrations of estradiol during pregnancy, CYP2B6 expression was predicted to increase by up to 1.9-fold during pregnancy.26 In contrast, the effect of pregnancy on CYP2C19 is currently not well defined. In vitro studies suggest downregulation of CYP2C19 expression by estrogens.28 This may impact BUP and its metabolite disposition, which are metabolized by CYP2C19, as human studies also show decreased clearance of the CYP2C19 substrate proguanil during pregnancy compared to postpartum.29 However, the concentrations of escitalopram, a CYP2C19 substrate, were not significantly altered during pregnancy compared to baseline suggesting that CYP2C19 activity may remain unaltered during pregnancy.30 Despite the potential changes and their clinical significance, few studies have investigated BUP pharmacokinetics during pregnancy.
In a study with 28 healthy, pregnant women taking BUP for therapeutic purposes, no differences were observed in the oral clearance of BUP, OH-BUP/BUP metabolic ratio, renal clearance of BUP and its metabolites during early pregnancy when compared to late pregnancy, or renal clearance of BUP in late pregnancy when compared to postpartum.31 The lack of changes in the OH-BUP/BUP metabolic ratio, which has historically been used as a measure of CYP2B6 activity, is unexpected given the changes in CYP2B6 expression previously described in vitro with exposure to estrogens. The stereoselective disposition of BUP and its metabolites was not addressed despite the known stereoselective metabolism and different contributions of CYP2B6 to the clearance of S- and R-BUP.20
In addition to understanding the pharmacokinetics of BUP during pregnancy, fetal exposure to BUP and its metabolites should be established to inform clinical decisions. An in vitro study evaluating placental metabolism of BUP showed that the placenta metabolizes BUP to OH-BUP, Threo, and Erythro, with Threo and Erythro formation several-fold higher than OH-BUP. This indicates that the primary metabolic pathway for BUP biotransformation in the placenta is via reduction.32 In a clinical study of 22 healthy, pregnant women taking BUP for therapeutic purposes, BUP, OH-BUP, and Threo were found to cross the placenta and were measured in both amniotic fluid and umbilical cord blood.33 The amniotic fluid concentrations of Threo were higher than those in the umbilical cord blood. Neither the in vitro nor in vivo studies have evaluated the stereoselective fetal disposition of BUP or its metabolites.
The goal of this study was to determine whether the circulating concentrations of BUP and its metabolite stereoisomers are altered during pregnancy when compared to postpartum in women taking BUP for therapeutic reasons, with the hypothesis that CYP2B6 mediated clearance of BUP to OH-BUP would be significantly increased during pregnancy compared to postpartum. Furthermore, the umbilical cord/maternal plasma concentration ratio was determined for BUP and its metabolites as a marker of fetal exposure.
Materials and Methods
PARTICIPANTS AND STUDY PROTOCOL
The study was approved by the University of Washington Institutional Review Board. Pregnant women chronically taking BUP for therapeutic purposes at the time of enrollment were recruited. The dosing regimen was not changed for the study, and subjects took their BUP at the same time as they had prior to entry into the study. Inclusion criteria were pregnant subjects age 18–45 years old; currently in good health with normal liver, kidney, gastrointestinal, and heart functions; taking BUP for therapeutic reasons. Exclusion criteria were subjects with a history of liver, kidney, or heart disease; taking chronic medications that are known inducers or inhibitors of CYP2B6. Subjects were consented separately for pregnancy and postpartum blood and urine sampling and for labor and delivery blood sampling and were enrolled after providing written consent.
At the time of enrollment, a buccal swab from each subject was collected using Cyto-pak cytosoft brushes (Medical Packing, Camarillo, CA) for genotyping for CYP2B6 and CYP2C19 polymorphisms. During each trimester of enrollment and at 6–12 weeks postpartum, a single steady-state blood sample and dosing interval urine were collected from each subject. For subject 6, the postpartum sample was drawn early, at 4 weeks 4 days, owing to personal life circumstances. For subjects who took their BUP in the morning, samples were collected 1.5–7 h after dosing; for subjects who took their BUP in the evening, sampling was done 11–15 h after dosing. All blood samples were collected in heparinized BD Vacutainer tubes (Becton, Dickinson and Company, Franklin Lakes, NJ) and plasma was separated by centrifugation for 10 min at 1,000 g at 4°C. For dosing interval urine, the voided samples for a given dosing interval were combined to obtain a homogenous dosing interval sample for concentration measurement. Urine output and volume were noted. Three subjects were also enrolled for the labor and delivery sample collection, which included a single maternal blood sample and umbilical cord venous blood sample. All samples were stored at −80°C until analysis.
DNA EXTRACTION AND GENOTYPING
Genomic DNA was extracted from buccal swabs using a QIAamp DNA Mini Kit (Qiagen Science, Germantown, MD) in accordance with the manufacturer’s instructions. Subjects were genotyped for 3 single nucleotide polymorphisms (SNPs) that result in common CYP2C19 variant alleles: 681G>A (CYP2C19*2), 636G>A (CYP2C19*3), and −806C>T (CYP2C19*17),34 and 4 SNPs that result in common CYP2B6 variant alleles: 64C>T (CYP2B6*2), 1459C>T (CYP2B6*5), 516G>T (core SNP for CYP2B6*6 appearing in low frequency alleles including *9), and −82T>C (CYP2B6*22),35,36 using a Stepone Plus real-time PCR, Taqman primers and probes (assay IDs: C__25986767_70; C__27861809_10; C____469857_10; C___2818162_20; C__30634242_40; C___7817765_60; C__27830964_10), and Taqman reagents, in accordance with the manufacturer’s recommendations (Applied Biosystems, Foster City, CA).37
ANALYSIS OF BUP, BUP METABOLITES, AND CREATININE IN HUMAN PLASMA AND URINE
For the nonchiral and chiral plasma analyses, plasma was spiked with an internal standard mixture to a final racemic concentration of 10 μM OH-bupropion-d6, 1 μM bupropion-d9, and 2 μM threohydrobupropion-d9. Samples were vortexed, protein precipitated with a 40-fold excess of 3:1 acetonitrile:methanol, and centrifuged for 40 min at 3,000×g. The supernatant was transferred to autosampler vials for detection and quantification by liquid chromatography-tandem mass spectrometry (LC-MS/MS) as described previously.37 The details of the LC-MS method are provided in Supplemental materials and Supplemental Table 1. For the nonchiral urine analysis of OH-BUP, Threo, Erythro, and the 4’OH-metabolites, urine samples were spiked with internal standard to a final concentration of 10 μM OH-BUP-d6 (internal standard for 4’OH and OH-BUP) and 1 μM threo-d9. Samples were vortexed, protein precipitated with a 20-fold excess of 3:1 acetonitrile:methanol, and centrifuged for 40 min at 3,000×g. For the chiral analysis, urine samples were prepared as described above; however, 1μM bupropion-d9 was included in the internal standard mixture. In addition, urine samples were processed with glucuronide deconjugation using an acidification method, as previously described to determine the total amount of the metabolites excreted into urine over a dosing interval.20 A subset of samples was analyzed by LC-MS/MS in independent runs, serving as internal quality controls and all measured values (n=1–3) were averaged as a single concentration value. Urine and plasma creatinine were quantified by the Clinical Chemistry Laboratory at the University of Washington Medical Center, Department of Laboratory Medicine, Seattle, WA, using the Beckman AU System.
PHARMACOKINETIC ANALYSIS
The primary outcome was to compare the steady-state plasma concentrations of BUP and its metabolites between pregnancy and postpartum. For all calculations, a single blood draw was assumed to reflect the average steady-state concentration as subjects were taking an extended-release formulation, and BUP and metabolite half-lives are sufficiently long to minimize concentration fluctuations over the dosing interval. The concentration at steady-state (Css) in μmole/L was dose-normalized to Css in μM/g by calculating Css/Dose, where Dose is the amount of BUP in grams per dosing interval. For BUP, racemic plasma values were used as BUP undergoes chiral inversion, confounding interpretation of individual stereoisomer concentrations. For one subject, the Css of S,S-OH-BUP was not quantifiable in the postpartum sample. For this subject, the S,S-OH-BUP concentration was defined as 25% of the lowest standard curve concentration for statistical analysis. The total amount of each compound excreted unchanged (Ae) over a dosing interval was determined by multiplying the concentration of the compound in urine by the total volume of urine collected over the dosing interval. The overall amount of a given metabolite recovered in urine over a dosing interval was calculated based on urine concentrations measured after acid deconjugation. For each metabolite, the formation clearance (CLf) was calculated using the total amount of the metabolite following acid deconjugation recovered in urine, divided by the Css of the compound from which the metabolite is derived. For each compound, renal clearance (CLR) and creatinine clearance were calculated by dividing the amount of unchanged compound excreted in the urine per collection interval by the Css of the compound. The estimated filtration clearance of each compound was calculated from the unbound fraction in plasma38 and estimated glomerular filtration rate (GFR; from CLCr).
STATISTICAL ANALYSIS
A power calculation using the variability detected previously in stereoselective BUP kinetics16 was performed and determined that 7 subjects would be needed to achieve 90% power to detect a 50% decrease in BUP plasma concentrations, with an alpha of 0.05. A 50% change in circulating concentrations of any active entity was considered clinically relevant. This power calculation was conservative as it does not account for the added power of paired analysis.
Results are expressed as the geometric mean and range. The data was log-transformed for statistical analysis but reported as the non-transformed results. Paired t-tests were used to evaluate the significance of changes in pharmacokinetic parameters calculated in the second (T2) or third (T3) trimesters when compared to the postpartum values in individual subjects. Statistical analyses were performed using GraphPad Prism version 8.3 (GraphPad Software, San Diego, CA). For statistical analysis, subject 7 was excluded due to changes in her prescribed dosing regimen postpartum and concern for patient compliance. However, she was included in the analysis of samples collected during labor and delivery as she was inpatient during that time and confirmed to be taking the medication as prescribed.
Results
DEMOGRAPHICS AND GENOTYPING INFORMATION
Eight patients were enrolled in this study. Patient demographics, including BUP dosing regimens, pertinent medical conditions, co-medications, and genotyping information, are summarized in Table 1. T2 samples were collected from six subjects, T3 samples were collected from six subjects, and labor and delivery (L&D) samples were collected from three subjects. Postpartum samples were collected from all eight subjects. For patients who participated in the L&D sample collection, delivery information and time from the last dose until delivery is shown in Table 1.
Table 1.
Study participant characteristics, genotypes, and delivery information (N=8)
| Subject (Age) | BMI | Dosing Regimen | Sample Collection Times (GA**; PPA***) | CYP2B6 Genotype | CYP2C19 Genotype | Pertinent Medical Conditions | Co-medications | Time Since Last Dose Until Delivery | Delivery Information (GA**; MOD****) | Neonatal Weight(g); Placental Weight(g) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 (28) | 45.3 | 300 mg XL | T3 (34+0), PP (6+6) | *1/*1 | *1/*1 | Diabetes Smoker |
Insulin Albuterol |
|||
| 2 (42) | 42.9 | 450 mg XL | T2 (24+6), T3 (34+6), PP (6+2) | *1/*22 | *1/*1 | None | Risperidone | |||
| 3 (35) | 22.6 | 150 mg XL | T2 (27+6), T3 (35+1), PP (6+6), L&D | *1/*1 | *1/*1 | Diabetes | Insulin, Escitalopram Oxycodone |
8 h | 38+1 / VD | 3864 / 1050 |
| 4 (35) | 40.3 | 150 mg SR | T2 (28+6), PP (6+3) | *1/*1 | *1/*1 | Diabetes | Metformin | |||
| 5 (28) | 27.7 | 300 mg XL | T3 (34+0), PP (6+5) | *1/*6a | *1/*1 | None | None | |||
| 6 (38) | 27.8 | 150 mg XL | T2 (26+6), T3 (34+1), PP (4+4) | *1/*6a | *1/*17 | None | Levothyroxine | |||
| 7 (30) | 28.9 | 150 mg IR BID* | T2 (26+4), T3 (34+1), PP (12+5), L&D | *1/*2b | *1/*1 | Diabetes Smoker |
Insulin Progesterone Metoclopramide |
11 h | 34+3 / CD | 2705 / 530 |
| 8 (36) | 29.1 | 300 mg XL | T2 (26+2), PP (8+5), L&D | *2/*5b,c | *1/*1 | Diabetes Renal insufficiencyd |
Insulin Gabapentin Labetalol Fluoxetine Memantine |
17 h | 35+1 / CD | 2547 / 485 |
BMI, body mass index; T2, second trimester; T3, third trimester; L&D, labor and delivery; PP, postpartum; VD, vaginal delivery; CS, cesarean delivery.
Subject was taking IR dosing BID during her pregnancy, switched to once-daily dosing postpartum
Gestational age, expressed as weeks + days
Postpartum age, expressed as weeks + days
Mode of delivery
CYP2B6*6 was assessed through 516G>T which may also be present in low frequency (<10%) variants including *9
CYP2B6*2 core mutation 681G>A can be detected in the CYP2B6*10 haplotype which was not examined in this cohort
CYP2B6*5 variant is defined by 1459C>T mutation, which is also present in the low frequency *7 haplotype
Subject 8 presented mild renal insufficiency, with serum creatinine of 0.96 mg/dL in T2 and 1.36 mg/dL PP.
EFFECT OF PREGNANCY ON BUPROPION PHARMACOKINETICS
Dose-normalized steady-state concentrations of racemic BUP and metabolites in T2 and T3 were unchanged during pregnancy when compared to postpartum (Table 2). Considerable inter-subject variability was observed between individuals. As expected from knowledge of BUP and BUP metabolite disposition in non-pregnant subjects,8,17,18 OH-BUP concentrations were up to 28–33-fold higher than those of BUP, with R,R-OH-BUP having steady-state concentrations 16–18-fold higher than S,S-OH-BUP. Erythro concentrations were similar to BUP while Threo circulated at steady-state concentrations about 8–10-fold higher than BUP. No significant changes in the metabolite to parent steady-state concentration ratios were observed during either T2 or T3 when compared with matched postpartum values (Table 2). Similarly, no changes in the formation clearances (CLf) of OH-BUP, 4’OH-BUP, Threo or Erythro were observed (Table 2).
Table 2:
Pharmacokinetic parameters of bupropion (BUP) and its metabolites as determined in the second and third trimester of pregnancy when compared with 6–12 weeks postpartum in the same subjects
| Parameter | T2 vs Matched Postpartum (n=5) | T3 vs Matched Postpartum (n=5) | |||||
|---|---|---|---|---|---|---|---|
| T2 | PP | P-Value | T3 | PP | P-Value | ||
| Css (μM/g) | 0.33 [0.08–1.2] | 0.53 [0.11–1.3] | 0.4787 | 0.44 [0.19–1.2] | 0.41 [0.11–1.1] | 0.8056 | |
| CLR (L/h) | 2.4 [0.77–6.4] | 1.1 [0.09–18.5] | 0.4548 | 1.8 [0.26–11.8] | 1.9 [0.26–18.5] | 0.97 | |
| Css OH-BUP (μM/g) | 15.1 [10.6–18.7] | 16.3 [4.9–39.6] | 0.7839 | 16.5 [6.3–30.9] | 13.3 [4.9–39.6] | 0.6896 | |
| CLR (L/h) | 0.20 [0.11–0.36] | 0.10 [0.02–0.54] | 0.2937 | 0.08 [0.04–0.12] | 0.10 [0.03–0.46] | 0.5539 | |
| CLf (L/h) | 50.7 [9.9–400.9] | 13.4 [1.7–120.8] | 0.1619 | 24.9 [5.8–71.2] | 15.9 [3.1–120.8] | 0.228 | |
| Css (μM/g) | 0.73 [0.20–1.4] | 0.78 [0.10–2.2] | 0.7772 | 0.83 [0.36–1.7] | 0.64 [0.10–2.2] | 0.4699 | |
| CLR (L/h) | 0.93 [0.31–3.4] | 0.35 [0.09–3.5] | 0.1707 | 0.40 [0.24–0.72] | 0.47 [0.09–3.5] | 0.7751 | |
| SS-OH-BUP/ BUP | 2.2 [1.0–6.0] | 1.5 [0.7–5.9] | 0.4729 | 1.9 [0.9–8.7] | 1.6 [0.8–5.9] | 0.4943 | |
| Css (μM/g) | 15.8 [10.0–22.2] | 15.9 [5.5–35.7] | 0.9786 | 13.7 [5.9–21.2] | 14.0 [5.5–35.7] | 0.9647 | |
| CLR (L/h) | 0.15 [0.07–0.32] | 0.08 [0.02–0.45] | 0.3564 | 0.07 [0.05–0.09] | 0.09 [0.03–0.35] | 0.6659 | |
| RR-OH-BUP/ BUP | 43.8 [16.4–178.7] | 32.5 [15.8–77.2] | 0.5907 | 33.7 [13.5–99.1] | 35.6 [15.2–77.2] | 0.8544 | |
| Css (μM/g) | 3.4 [1.8–6.7] | 4.1 [1.2–10.8] | 0.4896 | 4.9 [3.1–10.7] | 4.7 [1.2–10.8] | 0.8675 | |
| CLR (L/h) | 3.9 [1.7–8.6] | 2.4 [0.55–17.6] | 0.381 | 2.0 [1.1–2.8] | 2.9 [0.59–17.6] | 0.5748 | |
| CLf (L/h) | 89.4 [20.1–275.8] | 34.5 [2.9–166.4] | 0.2477 | 56.6 [17.0–208.0] | 68.5 [15.8–166.4] | 0.5949 | |
| Threo/ BUP | 10.3 [4.8–23.1] | 7.8 [3.5–17.6] | 0.545 | 11.2 [5.6–16.6] | 11.5 [9.7–17.6] | 0.8878 | |
| Css (μM/g) | 0.49 [0.33–0.78] | 0.67 [0.25–1.7] | 0.2739 | 0.63 [0.52–0.79] | 0.80 [0.25–1.7] | 0.4832 | |
| CLR (L/h) | 2.1 [0.91–4.4] | 1.6 [0.36–9.7] | 0.5107 | 1.2 [0.74–1.7] | 1.9 [0.36–9.7] | 0.4811 | |
| CLf (L/h) | 23.8 [3.9–105.8] | 10.6 [1.3–70.7] | 0.3067 | 17.9 [4.1–112.1] | 21.1 [4.9–70.7] | 0.5992 | |
| Erythro/ BUP | 1.5 [0.4–4.2] | 1.3 [0.5–3.2] | 0.771 | 1.4 [0.6–2.7] | 1.9 [1.2–3.2] | 0.1358 | |
| Threo-OH | CLf (L/h) | 2.4 [0.4–12.4] | 1.2 [0.4–4.9] | 0.0809 | 1.7 [0.2–8.9] | 1.7 [0.4–4.9] | 0.9983 |
| Erythro-OH | CLf (L/h) | 10.8 [3.2–39.8] | 5.1 [1.7–20.8] | 0.025 | 9.4 [2.5–36.7] | 7.6 [2.6–20.8] | 0.556 |
| 4’OH-BUP | CLf (L/h) | 1.5 [0.1–28.2] | 0.7 [0.0–5.7] | 0.6186 | 0.8 [0.1–4.8] | 1.0 [0.3–5.7] | 0.3921 |
OH-BUP, hydroxybupropion; Threo, threohydrobupropion; Erythro, erythrohydrobupropion; 4’OH-BUP, 4’-hydroxy-bupropion; T2, second trimester; T3, third trimester; PP, postpartum (defined as 6–12 weeks after delivery)
Values shown above include the geometric mean and [range]. Data were log-transformed for analysis with paired t-test
Steady-state concentrations (Css) are presented as dose-normalized.
The average creatinine clearance (CLCr) was 153.6±49.3 mL/min for T2, 145.0±54.1mL/min for T3, and 117.7±48.4mL/min for PP. The CLCr in T2 was higher compared to PP (p=0.0436) while in T3 CLCr was not significantly greater compared to PP (p=0.5693). Despite the increased CLCr in T2, there was no significant difference in renal clearance (CLR) for BUP and its metabolites in T2 and T3 compared to PP (Table 2). There was also no difference in the ratio of renal clearance to creatinine clearance in T2 and T3 compared to PP. The observed renal clearance of bupropion and its metabolites (with the exception of Threo in T2) was lower than the estimated unbound filtration clearance, supporting effective reabsorption of BUP and metabolites in the kidney.
FETAL EXPOSURE TO BUPROPION AND ITS METABOLITES
For the L&D samples, we found that all metabolites were quantifiable within the umbilical cord venous plasma, but at lower concentrations than maternal plasma (Figure 2). RR-OH-BUP had the lowest fetal to maternal ratio (0.4±0.1), with Threo had the highest (0.7±0.1), thus suggesting differential fetal exposure to metabolites.
Figure 2.
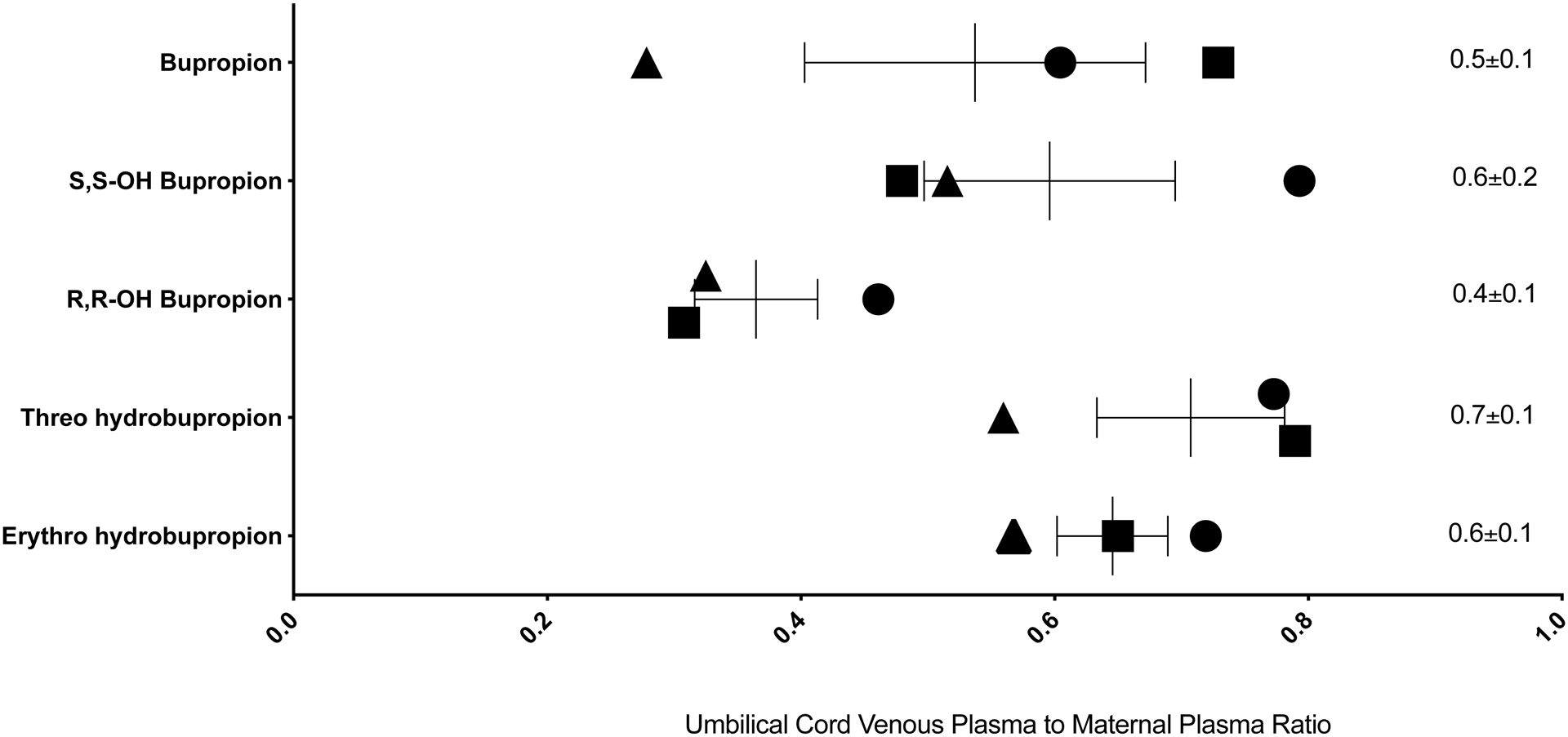
Umbilical cord venous plasma to maternal plasma concentration ratio of bupropion and its metabolites at steady-state at the time of delivery. Individual symbols represent individual subject’s value points and the mean and standard error (SE) of measurement are shown. Triangles show data for subject 1, squares data for subject 7, and circles data for subject 8, from among the subjects shown in Table 1. The mean±SE umbilical cord venous plasma steady-state concentrations (μM/g) and the maternal plasma steady-state concentrations (μM/g) were: BUP 0.2±0.1 vs. 0.5±0.2; S,S-OH-BUP 0.4±0.2 vs. 0.8±0.6; R,R-OH-BUP 6.4±3.1 vs. 19.1±12.0; Threo 3.7±4.3 vs. 4.9±5.3; Erythro 0.3±0.3 vs. 0.5±0.5.
Discussion
Given the known differences in clinical responses and side effect profiles of BUP and its metabolites, including the metabolite stereoisomers, the aim of this study was to elucidate whether the pharmacokinetics was altered during pregnancy. The current study found that the oral clearance of BUP (assessed based on Css) was not different during pregnancy when compared to 6–12 weeks postpartum, and steady-state plasma concentrations of BUP and its metabolites, including stereoisomers, were not significantly altered, suggesting that BUP dose adjustments during pregnancy may not be necessary. These findings are consistent with previous data in pregnant women, indicating that racemic BUP disposition is unaltered during pregnancy.31 Overall the concentrations of BUP and its metabolites measured postpartum were similar (means within about 40% of each other) to concentrations measured in healthy volunteers at steady-state,40 supporting the assumption of this study that single time point plasma concentrations reflect the average steady-state concentrations. Similarly, the relative exposures of the metabolites in relation to BUP were consistent with previous studies. In non-pregnant subjects, the steady-state concentrations of racemic OH-BUP and Threo were 28- and 5-fold higher than racemic BUP, respectively. Erythro concentrations were similar to BUP concentrations.17 This was also demonstrated in the current study in pregnant women where OH-BUP and Threo concentrations were 30- and 10-fold higher than BUP, while Erythro concentrations were similar. The current study also confirmed the stereoselective disposition of OH-BUP with the R,R-OH-BUP steady state concentrations 14–20-fold higher than S,S-OH-BUP. However, this difference between stereoisomers is smaller than previously reported in non-pregnant populations. Previous studies in non-pregnant subjects have found R,R-OH-BUP concentrations 40–65-fold higher than S,S-OH-BUP.16,17,40 It is possible that the smaller difference between S,S-OH-BUP and R,R-OH-BUP concentrations observed in this study reflects differences between patients and healthy volunteers or differences in formulations used and absorption kinetics and should be explored further. This is an important finding as S,S-OH-BUP has been shown to be more effective in improvement of depression, and antagonism of acute nicotine effects, in mouse models.8,9
There were no differences in metabolite to parent steady-state concentration ratios at T2 and T3 when compared to 6–12 weeks postpartum, suggesting no changes in metabolite formation and/or elimination clearance. However, both formation and elimination clearances of metabolites may be impacted, resulting in a lack of observable differences in the concentration ratio. For example, both CYP2B6 activity and glucuronidation of the metabolites could be induced during pregnancy. Nevertheless, no differences were observed in the CLf of any metabolite, although a trend toward increased OH-BUP CLf was observed (Table 2, p=0.16 and p=0.23). The lack of significance in the CLf of OH-BUP may be because multiple CYPs contribute to OH-BUP formation (Figure 1), of which some may be induced and others downregulated during pregnancy, or due to the limited power of the current study to detect a <2-fold change in CLf. As such, this study cannot overrule the potential induction of CYP2B6 during pregnancy. The lack of changes in CLf of 4’OH-BUP was surprising, as CYP2C19, which forms this metabolite, was expected to have decreased activity during pregnancy. The collected data suggest that CYP2C19 activity may not be reduced during pregnancy, a finding consistent with the lack of impact of pregnancy on escitalopram concentrations.30 Given that the metabolites have different clinical activity, as well as side effect profiles, the lack of changes in metabolite concentrations during pregnancy further supports that BUP dose adjustments are not needed in pregnancy.
The data showed the expected increase in CLCr in T2 compared to postpartum, but did not find a significant difference in T3 when compared to postpartum. This was unexpected, as generally it is considered that CLCr, an accepted method for estimation of GFR, increases for the duration of pregnancy, before decreasing back to the non-pregnant levels in the postpartum period.41 This was demonstrated in a meta-analysis of 29 studies that found that CLCr increased progressively during pregnancy until 15–21 weeks of gestation, then remained elevated when compared to pre-pregnancy values, but to a lesser extent than early pregnancy until 29–35 weeks of pregnancy, followed by a non-significant decrease at 36–41 weeks of gestation.42 In the current study, all subjects who had a T3 sample had it collected in the 34–35th week of gestation, nearing the gestational age where we might expect non-significant decrease based on the meta-analysis. A prior study specifically evaluating GFR in normal pregnancies, as well as pregnancies complicated by diabetes or gestational hypertension, has reported that the GFR remained elevated by 40% throughout pregnancy and reduced to levels similar to those in non-pregnant women within 1 month postpartum in both normal pregnancies, as well as those complicated by diabetes.41 Given that the GFR changes during pregnancy were similar to those in normal pregnancies, and those with diabetes, this suggests that, in the current study, diabetes in some subjects should not have confounded the analysis of changes in GFR during pregnancy. The data obtained in the current study, together with the previous meta-analysis, suggests that there may be some normalization of CLCR to non-pregnant baseline values that occur in T3, potentially earlier than previously demonstrated, thus rendering renal clearance in the later T3 increasingly similar to that in the non-pregnant state. This may have significant clinical implications for medication safety during pregnancy. For medications that undergo renal clearance, especially those for which therapeutic drug levels are critical for efficacy or safety, clinicians may need to initially increase dosing during pregnancy, but then begin to decrease dosing in T3, as renal clearance becomes more similar to that observed in the non-pregnant state. Further research on renal clearance changes in pregnancy are warranted to clarify these temporal changes.
Despite the significant increase in CLCR in T2 compared to postpartum, no difference was observed in renal clearances of BUP or its metabolites during T2 when compared to that postpartum, or in T3 when compared to that postpartum. Moreover, when the observed renal clearance was compared to the estimated filtration clearance of each compound, the observed renal clearance was significantly lower than filtration clearance for the metabolites during T2 and T3 (except for Threo in T2), and for R,R-OH-BUP and Erythro postpartum. This suggests that BUP metabolites undergo significant net reabsorption in the kidney. The water reabsorption processes may be altered in the kidney during pregnancy to maintain appropriate homeostasis, and this also impacts renal clearance of permeable drugs like BUP by increasing passive reabsorption processes during pregnancy. These changes in water reabsorption could, in turn, decrease the observed changes in renal clearance driven by increased GFR for highly permeable drugs.
The current study found that BUP and its metabolites cross the placenta, with lower concentrations observed in the umbilical cord venous plasma than the maternal plasma for all metabolites. This has been seen in prior ex vivo43,44 and in vitro studies.33 We found that concentrations of Erythro were similar to those of BUP in the umbilical cord venous plasma, while concentrations of OH-BUP and Threo were higher than those of BUP. This is consistent with a prior study, which reported that OH-BUP and Threo concentrations in the umbilical cord plasma are higher than BUP.33 Furthermore, this parallels what is observed in terms of the steady-state concentrations of BUP and metabolites in the maternal plasma. However, when directly comparing the fetal to maternal steady-state concentration ratios, R,R-OH-BUP showed the lowest ratio, while Threo showed the highest. This suggests possible differences in the metabolism of BUP to Threo in the placenta or efficient efflux transport of R,R-OH-BUP within the placenta. The specific formation of Threo in the placenta is supported by a prior in vitro study reporting that BUP is metabolized to OH-BUP, Erythro, and Threo in human placentas, with rates of formation of Erythro and Threo exceeding that for OH-BUP by several-fold.32 Collectively, this suggests greater fetal exposure to the metabolites than the parent drug and further indicates that metabolism and/or transport processes in the placenta impact fetal exposure.32,43–45 This is important for clinicians and patients to consider as studies have shown that the metabolites are both clinically active and also related to side effect profiles. Therefore, elucidating the fetal exposure not only to the parent drug but also to the metabolites will help better understand the safety and potential exposures and risks to the fetus. It also highlights the need for future studies specifically examining placental-mediated metabolism and transport mechanisms, to best understand how the placenta impacts fetal exposures to the parent drug and metabolites, and how this could be impacted by other medications with potential drug-drug interactions.
Conclusion
Overall, no significant differences were observed in pharmacokinetic parameters of BUP or its metabolites during pregnancy when compared to postpartum. Specifically, there was no difference in steady-state concentrations, parent to metabolite ratios, or CLf, suggesting that dose adjustments are not required in pregnancy. Interestingly, renal clearance did not increase as expected during T3 when compared to postpartum. BUP and its metabolites were all found to cross the placenta but were present at lower concentrations in fetal circulation than in maternal circulation, indicating that the placenta provides a partial barrier against BUP and metabolite distribution in the fetus, with possible differences in placental metabolism and/or transport.
Supplementary Material
Conflicts of Interest and Source of Funding:
NI reports consultancy agreements with Boehringer-Ingelheim, and Xenon Pharmaceuticals; honoraria from the National Institutes of Health and Associate Editor of Clinical and Translational Science, Drug Metabolism and Disposition and Pharmacology & therapeutics.The other authors declare no conflicts of interest. This work was funded by the National Institutes of Health grants T32 GM007750 and National Institutes of Drug Abuse P01DA032507.
Footnotes
Ethics statement:
This study was approved by the University of Washington Institutional Review Board and adhered to the Helsinki Declaration. All subjects provided written informed consent to participate in the study.
References
- 1.Woody CA, Ferrari AJ, Siskind DJ, Whiteford HA, Harris MG. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J Affect Disord. 2017;219:86–92. [DOI] [PubMed] [Google Scholar]
- 2.Szegda K, Markenson G, Bertone-Johnson ER, Chasan-Taber L. Depression during pregnancy: a risk factor for adverse neonatal outcomes? A critical review of the literature. J Matern Fetal Neonatal Med. 2014;27(9):960–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tong VT, Dietz PM, Morrow B, et al. Trends in smoking before, during and after pregnancy—Pregnancy Risk Assessment Monitoring System, United States, 40 sites, 2000–2010. MMWR Surveill Summ. 2013;62(6):1–19. [PubMed] [Google Scholar]
- 4.Pineles BL, Hsu S, Park E, Samet JM. Systematic review and meta-analyses of perinatal death and maternal exposure to tobacco smoke during pregnancy. Am J Epidemiol. 2016;184(2):87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castles A, Adams EK, Melvin CL, Kelsch C, Boulton ML. Effects of smoking during pregnancy. Five meta-analyses. Am J Prev Med. 1999;16(3):208–215. [DOI] [PubMed] [Google Scholar]
- 6.Dawson AL, Ailes EC, Gilboa SM, et al. Antidepressant prescription claims among reproductive-aged women with private employer-sponsored insurance- United States 2008–2013. MMWR Morb Mortal Wkly Rep. 2016;65(3):41–46. [DOI] [PubMed] [Google Scholar]
- 7.Turner E, Jones M, Vaz LR, Coleman T. Systematic review and meta-analysis to assess the safety of bupropion and varenicline in pregnancy. Nicotine Tob Res. 2019;21(8):1001–1010. [DOI] [PubMed] [Google Scholar]
- 8.Damaj MI, Carroll FI, Eaton JB, et al. Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Mol Pharmacol. 2004;66(3):675–682. [DOI] [PubMed] [Google Scholar]
- 9.Damaj MI, Grabus SD, Navarro HA, et al. Effects of hydroxymetabolites of bupropion on nicotine dependence behavior in mice. J Pharmacol Exp Ther. 2010;334(3):1087–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golden RN, De Vane CL, Laizure SC, Rudorfer MV, Sherer MA, Potter WZ. Bupropion in depression. II. The role of metabolites in clinical outcome. Arch Gen Psychiatry. 1988;45(2):145–149. [DOI] [PubMed] [Google Scholar]
- 11.Preskorn SH. Antidepressant response and plasma concentrations of bupropion. J Clin Psychiatry. 1983;44(5 Pt 2):137–139. [PubMed] [Google Scholar]
- 12.Zhu AZX, Cox LS, Nollen N, et al. CYP2B6 and bupropion’s smoking-cessation pharmacology: the role of hydroxybupropion. Clin Pharmacol Ther. 2012;92(6):771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston JA, Fiedler-Kelly J, Glover ED, Sachs DP, Grasela TH, DeVeaugh-Geiss J. Relationship between drug exposure and the efficacy and safety of bupropion sustained release for smoking cessation. Nicotine Tob Res. 2001;3(2):131–140. [DOI] [PubMed] [Google Scholar]
- 14.Silverstone PH, Williams R, McMahon L, Fleming R, Fogarty S. Convulsive liability of bupropion hydrochloride metabolites in Swiss albino mice. Ann Gen Psychiatry. 2008;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coles R, Kharasch ED. Stereoselective metabolism of bupropion by cytochrome P4502B6 (CYP2B6) and human liver microsomes. Pharm Res. 2008;25(6):1405–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karasch ED, Mitchell D, Coles R. Stereoselective bupropion hydroxylation as an in vivo phenotypic probe for cytochrome P4502B6 (CYP2B6) activity. J Clin Pharmacol. 2008;48(4):464–474. [DOI] [PubMed] [Google Scholar]
- 17.Masters AR, Gufford BT, Lu JB, Metzger IF, Jones DR, Desta Z. Chiral plasma pharmacokinetics and urinary excretion of bupropion and metabolites in healthy volunteers. J Pharmacol Exp Ther. 2016;358(2):230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benowitz NL, Zhu AZX, Tyndale RF, Dempsey D, Jacob P. Influence of CYP2B6 genetic variances on plasma and urine concentrations of bupropion and metabolites at steady state. Pharmacogenet Genomics. 2013;23(3):135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faucette SR., Hawke RL, Lecluyse EL, Shord SS, Yan B, Laethem RM, Lindley CM. Validation of bupropion hydroxylation as a selective marker of human cytochrome P450 2B6 catalytic activity. Drug Metab Dispos. 2000;28(10):1222–1230. [PubMed] [Google Scholar]
- 20.Sager JE, Price LSL, Isoherranen N. Stereoselective metabolism of bupropion to OH-bupropion, threohydrobupropion, erythrohydrobupropion, and 4’-OH-bupropion in vitro. Drug Metab Dispos. 2016;44(10):1709–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connarn JN, Zhang X, Babiskin A, Sun D. Metabolism of bupropion by carbonyl reductases in liver and intestine. Drug Metab Dispos. 2015,43)7):1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer A, Vuorinen A, Zielinska AE, et al. Formation of threohydrobupropion from bupropion is dependent on 11β-hydroxysteroid dehydrogenase 1. Drug Metab Dispos. 2013;41(9):1671–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skarydova L, Tomanova R, Havlikova L, Stambergova H, Solich P, Wsol V. Deeper insight into the reducing biotransformation of bupropion in the human liver. Drug Metab Pharmacokinet. 2014;29(2):177–184. [DOI] [PubMed] [Google Scholar]
- 24.Koren G, Pariente G. Pregnancy-associated changes in pharmacokinetics and their clinical implications. Pharm Res. 2018;35(3):61. [DOI] [PubMed] [Google Scholar]
- 25.Choi SY, Koh KH, Jeong H. Isoform-specific regulation of cytochromes P450 expression by estradiol and progesterone. Drug Metab Dispos. 2013;41(2):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dickmann LJ, Isoherranen N. Quantitative prediction of CYP2B6 induction by estradiol during pregnancy: potential explanation for increased methadone clearance during pregnancy. Drug Metab Dispos. 2013;41(2):270–274. [DOI] [PubMed] [Google Scholar]
- 27.Koh KH, Jurkovic S, Yang K, et al. Estradiol induces cytochrome P450 2B6 expression at high concentrations: Implication in estrogen-mediated gene regulation in pregnancy. Biochem Pharmacol. 2012;84(1):93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mwinyi J, Cavaco I, Steen Pedersen R, et al. Regulations of CYP2C19 expression by estrogen receptor α: Implications for estrogen-dependent inhibition of drug metabolism. Mol Pharmacol. 2010;78(5):886–894. [DOI] [PubMed] [Google Scholar]
- 29.McGready R, Stepniewska K, Seaton E, et al. Pregnancy and use of oral contraceptives reduces the biotransformation of proguanil to cycloguanil. Eur J Clin Pharmacol. 2003;59:553–557. [DOI] [PubMed] [Google Scholar]
- 30.Westin AA, Brekke M, Molden E, Skogvoll E, Spigset O. Selective serotonin reuptake inhibitors and venlafaxine in pregnancy: Changes in drug deposition. PLoS One. 2017;12(7):e0181082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fokina VM, Xu M, Rytting E, et al. Pharmacokinetics of bupropion and its pharmacologically active metabolites in pregnancy. Drug Metab Dispos. 2016;44(11):1832–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Abdelrahman DR, Zharikova OL, et al. Bupropion metabolism by human placenta. Biochem Pharmacol. 2010;79(11):1684–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fokina VM, West H, Oncken C, et al. Bupropion therapy during pregnancy: the drug and its major metabolites in umbilical cord plasma and amniotic fluid. Am J Obstet Gynecol. 2016;215(4):497.e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pratt VM, Del Tredici AL, Hachad H, et al. Recommendations for clinical CYP2C19 genotyping allele selection. A report of the Association for Molecular Pathology. J Mol Diagn. 2018;20:269–276. [DOI] [PubMed] [Google Scholar]
- 35.Kirchheiner J, Klein C, Meineke I, et al. Bupropion and 4-OH-bupropion pharmacokinetics in relation to gene polymorphisms in CYP2B6. Pharmacogenetics. 2003;13(10):619–626. [DOI] [PubMed] [Google Scholar]
- 36.Rotger M, Tugude H, Colombo S, et al. Predictive value of known and novel alleles of CYP2B6 for efavirenz plasma concentrations in HIV-infected individuals. Clin Pharmacol Ther. 2007;81(4):557–566. [DOI] [PubMed] [Google Scholar]
- 37.Sager JE, Lutz JD, Foti RS, Davis C, Kunze KL, Isoherranen N. Fluoxetine- and norfluoxetine-mediated complex drug–drug interactions: in vitro to in vivo correlation of effects on CYP2D6, CYP2C19, and CYP3A4. Clin Pharmacol Ther. 2014;95(6):653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sager JE, Choiniere JR, Chang J, Stephenson-Famy A, Nelson WL, Isoherranen N. Identification and structural characterization of three new metabolites of bupropion in humans. ACS Med Chem Lett. 2016;7(8):791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sager JE, Tripathy S, Price LSL, et al. In vitro to in vivo extrapolation of the complex drug-drug interaction of bupropion and its metabolites with CYP2D6; simultaneous reversible inhibition and CYP2D6 downregulation. Biochem Pharmacol. 2017;123:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kharasch ED, Neiner A, Kraus K, et al. Stereoselective steady-state disposition and bioequivalence of brand and generic bupropion in adults. Clin Pharmacol Ther. 2020;108(5):1036–1048. [DOI] [PubMed] [Google Scholar]
- 41.Krutzén E, Olofsson P, Bäck SE, Nilsson-Ehle P. Glomerular filtration rate in pregnancy: a study in normal subjects and in patients with hypertension, preeclampsia and diabetes. Scand J Clin Lab Invest. 1992;52(5):387–392. [DOI] [PubMed] [Google Scholar]
- 42.Lopes van Balen VA, van Gansewinkel TAG, de Haas S, et al. Maternal kidney function during pregnancy: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2019;54(3):297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Earhart AD, Patrikeeva S, Wang X, et al. Transplacental transfer and metabolism of bupropion. J Matern Fetal Neonatal Med. 2010;23(5):409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hemauer SJ, Patrikeeva SL, Wang X, et al. Role of transporter-mediated efflux in the placental biodisposition of bupropion and its metabolite, OH-bupropion. Biochem Pharmacol. 2010;80(7):1080–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han LW, Gao C, Zhang Y, Wang J, Mao Q. Transport of bupropion and its metabolites by the model CHO and KEH293 cell lines. Drug Metab Lett. 2019;13(1):25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


