Abstract
Coronary heart disease and psychological stress factors such as depression are prevalent and associated with high morbidity/mortality; they are also challenging to manage, especially when treated in isolation of each other. Recent advances support an integrated approach to their management that is built on a foundation of an extensive, multi-component network of neurological structures. In this review, we describe this extensive cardioneural network that encompasses the heart, brain, spinal cord, and ganglia throughout the body, and then discuss ambulatory and laboratory-based non-invasive measures of this network that both measure psychological stress and heart disease severity. Lastly, we discuss their potential transformative clinical and public health applications, and also possible cardioneural interventions such as exercise and biofeedback.
Introduction
Coronary heart disease (CHD) is the number one killer worldwide. Although recent progress has been made at decreasing its public health burden, specific gender and race disparities are growing and suggest new approaches are needed (1). Psychological conditions that are associated with increased CHD risk such as depression are increasing in prevalence (2), and the lack of integration of these stress factors in cardiac risk assessments may be contributing to our diminished progress in CHD prevention (3). Previous studies have largely focused on patient-reported outcomes, which may be subject to recall bias (4). Physiologic autonomic biomarkers may enable more rigorous and objective investigations between psychological stress and CHD and facilitate a breakthrough in CHD risk assessment and prevention. The use of such biomarkers in clinical settings may help inspire more aggressive psychological and behavioral interventions that not only reduce CHD risk but also sudden cardiac death (SCD), which may manifest as the first clinical manifestation of CHD (5).
Previous studies have shown that psychological stress can manifest in several forms and influence a large proportion of CHD events. The landmark INTERHEART study, an international multi-site study of nearly 25,000 individuals with and without recent myocardial infarction (MI) (6), estimated that 33% of CHD events are attributable to exposures or mood states related to psychological stress. Examples include work stress, home stress, financial stress, exposure to traumatic life events, reduced locus of control, and depressive symptoms. Unlike other traditional risk factors like diabetes, hypertension, and hyperlipidemia, clinicians lack validated clinical decision support tools to screen for and address these stress-related risk factors for CHD. Heart-brain biomarkers may help to collectively capture the effects of heterogeneous stress-related influences on CHD susceptibility or CHD outcomes in a way that avoids stigma, and then be used in certain therapies such as neuromodulation (7).
This review describes the relationship of psychological stress with CHD and SCD with an integrated cardioneural network that includes the heart, brain, and autonomic nervous system (8). We review the anatomy and physiology of this network as it relates to cardiovascular disease and psychological stress, as well as potential ways of measuring the bidirectional communications between the heart and brain through autonomic and cardiac-specific measures. We then discuss applications in the management of both psychological and cardiac conditions. Although we present evidence mostly from experimental studies of individuals with CHD, the core concepts are likely generalizable beyond this population.
Anatomical Basis for the Cardioneural Network from the Cardiac Perspective
The brain and heart are closely linked through several interdependent neurologic networks, as illustrated in figure 1 (9). Chemosensory and mechanosensory neurites in the heart communicate with the intrinsic cardiac nervous system (ICNS), a “little brain” comprised of intracardiac ganglia (10). Cardiac afferent neurologic activity also routes through synaptic connections in the dorsal root ganglia and nodose ganglia, which subsequently reach the brain through the spinal cord and brainstem, respectively (11). Disruption of this homeostatic mechanism due to an acute myocardial infarction may impair the ICNS and disrupt afferent neurological activity that is vital for homeostasis (12).
Figure 1:
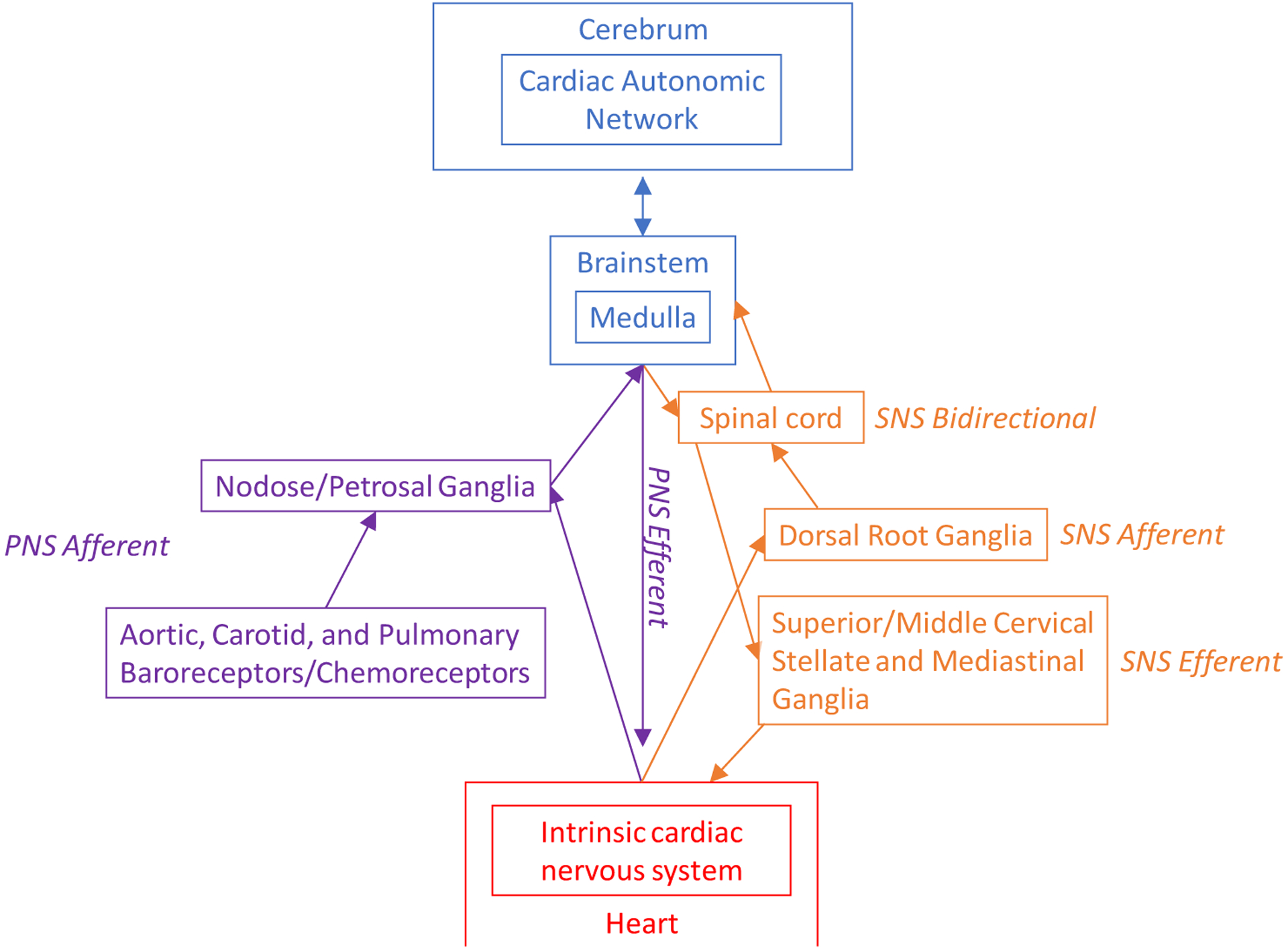
Overview of Cardioneural Network from the Perspective of the Heart. This describes in detail the cardiac and thoracic structures that relay information between the heart and brain. The role of these structures in stress perception and physiology are described in italics. Afferent, efferent, and bidirectional pathways are described.
Neurological structures in the thoracic cavity may influence the heart-brain relationship and impact both psychological and cardiovascular disease risk. Important intrathoracic extracardiac ganglia include stellate ganglion, the pulmonary stretch receptors, and the aortic/carotid baroreceptor, which regulate respiration and blood pressure through autonomic mechanisms (13). In addition, the dorsal root ganglia transmit afferent sympathetic nervous system (SNS) signals from the ICNS to the brain (13). Several other extrathoracic extracardiac ganglia also facilitate afferent communication between the cardiovascular system and the brain, including the superior and middle cervical ganglia, which transmit efferent sympathetic activity to the heart. The nodose and petrosal ganglia are extrathoracic, extracardiac ganglia that receive chemosensor and mechanosensor input from the vagus, baroreceptors, and the heart.
These anatomical relationships help to understand potential mechanisms linking incident cardiac events with heart-brain disorders. Ischemia in the anterior or posterior wall of the left ventricle may influence brain activity through afferent nerve activity traveling from the heart through the dorsal root ganglia and spinal cord, where it synapses, to the brain (14, 15). This may influence efferent outflows at the level of the heart, sympathetic thoracic ganglia, brainstem, and higher brain centers that involve the stress response (16).
This extensive network plays a vital role in emotional regulation and the perception of feelings from the body’s visceral organs, such as sensations in the chest during stress episodes. Although the psychological impact of ICNS dysregulation is not well understood, the increase in suicide rates and high depression prevalence after acute myocardial infarctions warrant further research into its effects (17). Cardiac autonomic structures may also be involved in interoception, or a heightened sensation of the visceral organs, that form the foundation for panic disorder and other psychopathologies (18). Another clinical example is idiopathic pure autonomic failure, a condition characterized by the autonomic ganglia’s selective cell death due to the accumulation of Lewy bodies (19), and can cause blunted physiologic and emotional responses to mental stress (20). The vagus nerve, which courses through the neck (21), is easily accessible by non-invasive and implanted neuromodulation therapies, and may be effective in treating depression and other stress-related conditions (22).
Cardioneural Pathways of Stress from the Brain Vantage Point
The central autonomic network (CAN) describes a cluster of regions in the cerebral cortex and brainstem (figure 2) that jointly regulates several physiological and psychological autonomic processes implicated in CHD pathogenesis (23). External threats are processed in certain regions in the medial prefrontal cortex and the amygdala. These regions are central to the fear-based “fight or flight” adaptive response that includes sympathetic arousal and parasympathetic inhibition (24). The amygdala works closely with the insular cortex, which is involved in autonomic regulation, and has bidirectional connections with the heart and other visceral organs. The amygdala inhibits several brainstem PNS nuclei, including the nucleus ambiguous, dorsal motor nucleus, and nucleus tractus solitarius (25). The amygdala also inhibits the caudal ventrolateral medulla (CVLM), which results in increased SNS activity by activating the rostral ventrolateral medulla (RVLM) (26). The pons, which projects throughout the brain, contains the locus coeruleus and other important nuclei involved in the autonomic regulation of psychological stress, sleep, respiration, and cognition. It projects autonomic fibers throughout the brain. The amygdala also regulates the hypothalamus, which, in turn, controls temperature, thyroid function, cortisol production, circadian rhythms, and sex hormones (23).
Figure 2:
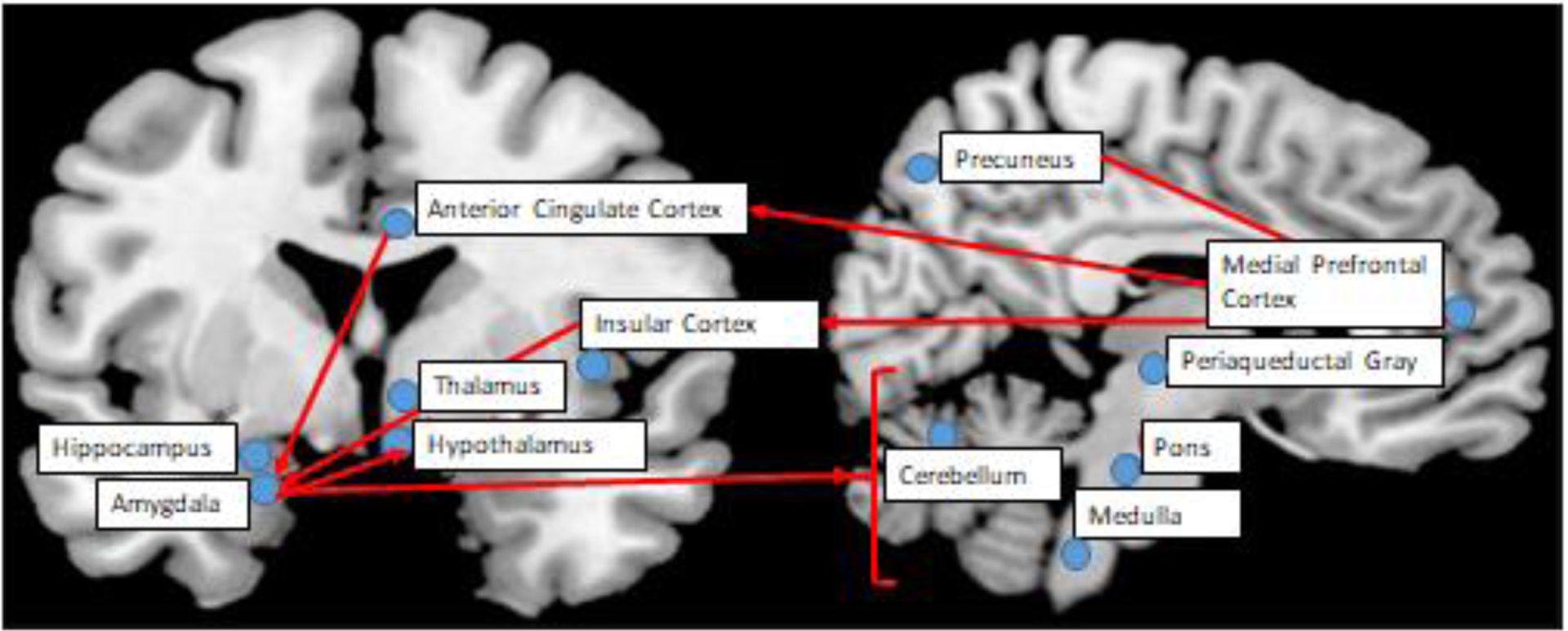
Overview of the Cardioneural Network from the Perspective of the Brain, with a Focus on the Central Autonomic Network. This figure summarizes the key brain rations in the cardiac autonomic network, and the relationship of forebrain, midbrain, and brainstem structures with the red arrows. This figure was adapted from Sklerov M. et al., Clinical Autonomic Research 2019 (29:555–566).
Recent studies highlight the potential importance of these regions in cardiovascular disease pathogenesis. Tawakol et al. examined whole-body positron emission tomography scans in 293 people without CHD (mean age 55 years, 58% women). They found that higher resting amygdala activity was associated with increased arterial inflammation and risk of adverse cardiovascular events (27). Bremner et al. studied 170 individuals with known CHD in a laboratory setting and examined changes in brain perfusion with O-15 water positron emission tomography during stressful (versus neutral) tasks using a whole-brain analysis. They found an association (p<0.005) between the stress reactivity of several CAN structures (minimum size 11 voxels) and mental stress-induced myocardial ischemia (MSIMI) (28), including the insula and anterior cingulate cortex. Other important regions of stress activation that related to MSIMI were the inferior frontal gyrus and parietal cortex. In the same cohort, Moazzami et al. found that stress reactivity in the rostromedial subregion of the medial prefrontal cortex was associated with reduced high frequency heart rate variability during stress, higher interleukin-6 levels 90 minutes after stress, and increased risk of cardiovascular events or death (29). Still in the same cohort, inferior frontal lobe activation during mental stress was found to associate with angina. Most notably, this association was stronger than the association of angina with traditional myocardial ischemia (14), suggesting the need to reconsider angina as a neurocardiac symptoms, rather than strictly a condition of impaired myocardial perfusion.
The increased CHD risk due to psychological stress may involve physiologic mechanisms arising from activation in the CAN and its relationship with baroreflex sensitivity (30). For example, early life trauma can affect neurological responses to stress and associates with worsened blood pressure trajectories in children and young adults (31, 32). The cardiac effects of the CAN may be further elucidated by studies in individuals with cardiac transplantation, whose hearts are denervated from the brain. One study of 20 cardiac transplant individuals found significantly attenuated heart-rate responses to stress compared to controls age-matched to both the donor and the recipient (33). As such, it demonstrated the effects of disrupted communications from the CAN to the heart during stress provocation that may also influence their vulnerability to acute stress.
Summary of Bidirectional Communication Between the Heart and Brain
A complex network of structures supports the bidirectional communication between the heart and the brain. CHD affects neurological networks/ganglia outside of the heart in the cervical/thoracic regions and spine. It also activates several autonomic structures in the brain and brainstem that process psychological stress. From the brain perspective, psychological stress processing involves the cardiac autonomic network, which has efferent effects on the heart and can increase CHD risk through hypertension, for example. Core structures like the vagus nerve transmit bidirectional communications between the heart and brain, and may be the target for neuromodulation therapies. These connections, which are summarized in figure 3, form the anatomical basis for a new paradigm that considers ischemic heart disease and psychological conditions as interrelated, interdependent conditions.
Figure 3:
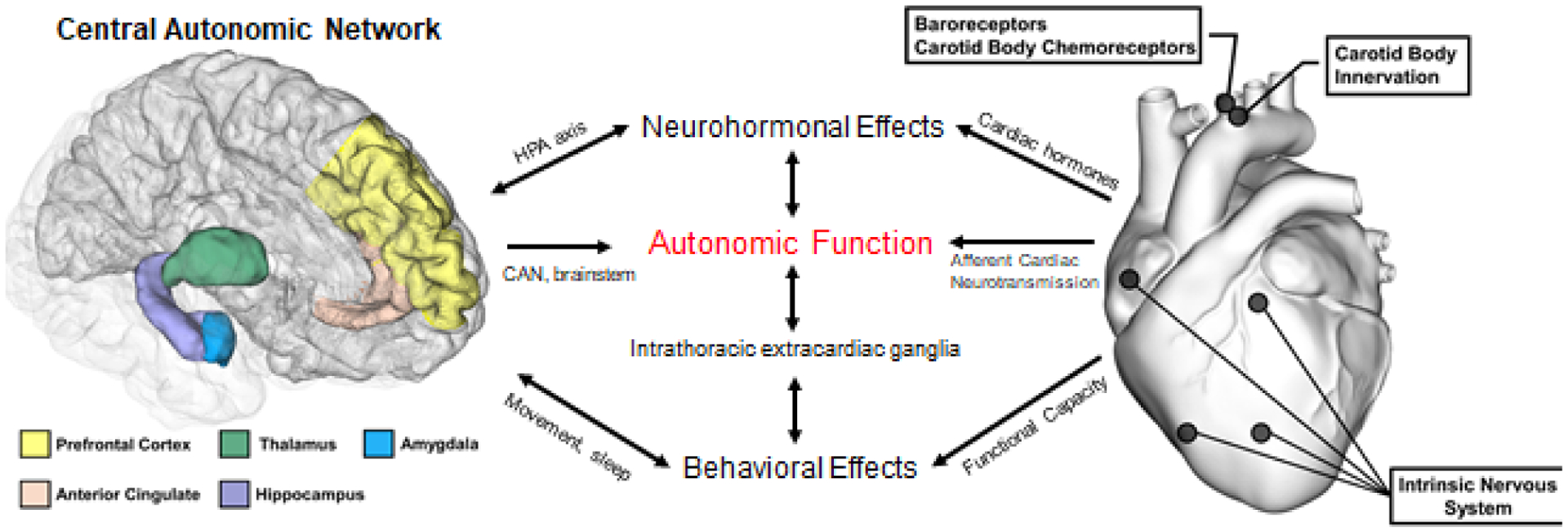
Overview of Cardioneural Network that Arise from the Brain and Heart. Several neurological structures in both the brain and heart contribute to autonomic measurements that can be measured non-invasively. The neuroendocrine and immune systems are also closely involved, both as a result of direct input from both the brain and heart, as well as the mechanisms involving the peripheral autonomic nervous system.
Assessment of the Cardioneural Network with Non-invasive Ambulatory and Laboratory Measures
Integration of cardioneural metrics into clinical settings can help with risk stratification and monitoring of behavioral interventions that include exercise training, psychotherapy, and meditation. Many cardioneural measures are derived from the electrocardiogram (ECG), which allows for cost-effective and portable assessments in both laboratory and home settings. Additional non-invasive cardiovascular assessment strategies include impedance cardiography and photoplethysmography, which can measure the effects of mental stress on myocardial contractility and vasomotor tone, respectively. Long-term ambulatory monitoring can potentially help assess everyday stress physiology and behavior in the home setting, although more data are needed to support this approach. On the other hand, laboratory testing can be useful for more rigorous, controlled assessments of stress reactivity with mental stress challenges (28).
One of the most commonly used cardioneural metrics is heart rate variability (HRV), an ambulatory, ECG-based measure of the dynamic heart rate changes that are attributable primarily to fluctuations in ANS activity. It can be useful in detecting dysfunction in the CAN, ICNS, or specific autonomic reflex ganglia and can be influenced by acute psychological stress and myocardial ischemia (8). There are different ways to index HRV; among these, frequency domains are commonly used, followed by time domain and non-linear methods. Frequency domain HRV measures heart rate oscillations coupled with other rhythmic physiologic processes at different frequency bands. Psychosocial stress and CHD both reduce the amplitudes of these oscillations to varying degrees. High frequency (HF) HRV (0.15 – 0.40 Hz) describes parasympathetic activity modulation with respiration but also reflects activity levels and connectivity within the CAN, and is lower during exposure to stress and in persons with CHD (34). Low frequency (LF) HRV (0.04 – 0.15 Hz) reflects a combination of sympathetic and parasympathetic function that is mostly controlled by the baroreflex and the anterior cingulate region of the CAN, and is reduced by nearly 50% in posttraumatic stress disorder (PTSD) (35). HRV can also predict the future risk of depression and PTSD in trauma-exposed individuals (36). Non-linear HRV metrics, such as multiscale entropy, measure the amount of disorder in the system and indicate a breakdown in homeostasis due to either cardiovascular disease or psychological and mental health disturbances. Non-linear metrics from the Poincare plot may uniquely capture irregularities caused by periodic, disordered ANS activity bursts that occur with myocardial ischemia (37).
In addition to HRV, repolarization and myocardial contractility measurements can help evaluate the effects of psychological stress on ventricular sympathetic activation (38). T-peak to T-end interval reflects SNS stimulation through stellate activation (figure 1) and predicts ventricular tachyarrhythmias (39). Pre-ejection period estimates cardiac inotropic effort from the interval between ventricular depolarization and the mechanical ejection of blood. This metric is a useful SNS measure in laboratory settings to assess the beta-1 effects of acute mental stress challenges (40).
Baroreceptor sensitivity (BRS), which measures the ability to regulate blood pressure through PNS/SNS modulation, is another cardioneural measure that helps understand the connections between stress and CHD because of the brainstem influence on BRS (figure 1) (26, 41). Low BRS predicts sudden cardiac death after myocardial infarction and is decreased in both depression and posttraumatic stress disorder; it may also exacerbate hemodynamic reactivity to acute stress (42–44). Heart rate turbulence is another PNS measure of heart rate changes after premature ventricular contractions. It reflects baroreflex dysfunction, decreases after acute myocardial infarction, and predicts adverse cardiovascular events (45).
Several other laboratory measures can be important indicators of disruption in cardioneural networks, which can be important in risk prediction and provide a deeper understanding of cardioneural mechanisms underlying CHD pathogenesis. MSIMI, for example, is a phenomenon due, at least in part, to microvascular dysfunction during acute laboratory mental stress challenge. It has been associated with over a 2-fold increased risk of adverse events (46). SNS stimulation during mental stress causes the initiation of the inflammatory cascade, which results in the release of cytokines, including interleukin-6, monocyte chemoattractant protein-1, and matrix metallopeptidase 9 (47). SNS activation during acute mental stress also causes peripheral microvascular vasoconstriction and reduced brachial artery flow-mediated dilation (48, 49). These represent relatively low-cost methods that may index SNS activation and are also predictive of future CHD events (49, 50).
Applications of Cardioneural Measures in Clinical Diagnosis and Treatment
Further research into the measurement of the cardioneural network can help set the stage for a new clinical paradigm of behavioral preventive cardiology that includes an increased focus on autonomic health and intensive behavioral interventions. While ambulatory ECG-based assessment strategies are currently the most well-studied and easy to translate into clinical practice using Holter monitors or ECG monitoring patches, other methods that involve laboratory-based mental stress challenges provide more rigorous assessments of stress physiology. Individuals with high CHD risk who may be amenable to cardioneural assessments and interventions may include those with psychiatric diagnoses such as depression and PTSD, those reporting high levels of general stress, and potentially also those with abnormal stress reactivity- for example, patients with white coat hypertension. More research in this area is needed however, to develop and test clinical decision-making programs in order to translate research findings into preventive action. Randomized controlled trials of behavioral and neuromodulation therapies in high-risk individuals, with cardioneural outcomes to assess treatment response, are especially needed. Examples include behavioral and stress management programs during cardiac rehabilitation and sleep hygiene/education. Therapies such as HRV biofeedback, yoga, and vagal nerve stimulation target specific autonomic mechanisms, including the baroreceptor and PNS (51). In these cases, cardioneural outcomes may be vital for monitoring treatment efficacy (7).
Overall, CHD and mental health problems are the largest contributors to morbidity and mortality in the world. Their collective high prevalence underscores the need for future research on the cardioneural network as a common mechanism in both conditions that could shed light on synergistic treatment pathways. Metrics such as HRV and vascular and ischemic responses to mental stress may someday become incorporated with other traditional measures for the risk assessment of CHD patients. These metrics may facilitate identifying individual patients who would benefit the most from biobehavioral and neuromodulatory interventions based on their autonomic activations at home or during laboratory stress. More research is needed on large and diverse populations to create robust clinical standards for cardioneural metrics and accompanying mind-body and neurmodulating interventions.
Funding:
NIH K23 HL127251, R03 HL146879, P01 HL101398, R01 HL088726, K24 MH076955, T32MH067547, R01 MH56120, K24 HL077506, R01 HL068630, R01 HL109413, R01 HL125246, ULTR002378, and S10 RR16917
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None for all authors
References
- 1.Wilmot KA, O’Flaherty M, Capewell S, Ford ES, Vaccarino V. Coronary Heart Disease Mortality Declines in the United States From 1979 Through 2011CLINICAL PERSPECTIVE. Circulation. 2015;132(11):997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malhi GS, Mann JJ. Depression. Lancet (London, England). 2018;392(10161):2299–312. [DOI] [PubMed] [Google Scholar]
- 3.Muntner P, Colantonio LD, Cushman M, Goff DC Jr., Howard G, Howard VJ, et al. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. JAMA. 2014;311(14):1406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmier JK, Halpern MT. Patient recall and recall bias of health state and health status. Expert Review of Pharmacoeconomics & Outcomes Research. 2004;4(2):159–63. [DOI] [PubMed] [Google Scholar]
- 5.Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98(21):2334–51. [DOI] [PubMed] [Google Scholar]
- 6.Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):953–62. [DOI] [PubMed] [Google Scholar]
- 7.Chen S, Sun P, Wang S, Lin G, Wang T. Effects of heart rate variability biofeedback on cardiovascular responses and autonomic sympathovagal modulation following stressor tasks in prehypertensives. Journal of Human Hypertension. 2016;30:105–11. [DOI] [PubMed] [Google Scholar]
- 8.Smith R, Thayer JF, Khalsa SS, Lane RD. The hierarchical basis of neurovisceral integration. Neurosci Biobehav Rev. 2017;75:274–96. [DOI] [PubMed] [Google Scholar]
- 9.Lyra V, Parissis J, Kallergi M, Rizos E, Filippatos G, Kremastinos D, et al. (18) F-FDG PET/CT brain glucose metabolism as a marker of different types of depression comorbidity in chronic heart failure patients with impaired systolic function. Eur J Heart Fail. 2020. [DOI] [PubMed] [Google Scholar]
- 10.Armour JA, Murphy DA, Yuan BX, Macdonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. The Anatomical record. 1997;247(2):289–98. [DOI] [PubMed] [Google Scholar]
- 11.Pearsall P, Schwartz GE, Russek LG. Changes in heart transplant recipients that parallel the personalities of their donors. Integr Med. 2000;2(2):65–72. [DOI] [PubMed] [Google Scholar]
- 12.Rajendran PS, Nakamura K, Ajijola OA, Vaseghi M, Armour JA, Ardell JL, et al. Myocardial infarction induces structural and functional remodelling of the intrinsic cardiac nervous system. J Physiol. 2016;594(2):321–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armour JA. Functional anatomy of intrathoracic neurons innervating the atria and ventricles. Heart rhythm : the official journal of the Heart Rhythm Society. 2010;7:994–6. [DOI] [PubMed] [Google Scholar]
- 14.Moazzami K, Wittbrodt MT, Alkhalaf M, Lima BB, Nye JA, Mehta PK, et al. Association Between Mental Stress-Induced Inferior Frontal Cortex Activation and Angina in Coronary Artery Disease. Circ Cardiovasc Imaging. 2020;13(8):e010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armour JA. Myocardial ischaemia and the cardiac nervous system. European heart journal. 1999;16:1751–2. [DOI] [PubMed] [Google Scholar]
- 16.Ardell JL, Andresen MC, Armour JA, Billman GE, Chen PS, Foreman RD, et al. Translational neurocardiology: preclinical models and cardioneural integrative aspects. Journal of Physiology. 2016;594:3877–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu CH, Yeh MK, Wang JH, Weng SC, Bai MY, Chang JC. Acute Coronary Syndrome and Suicide: A Case-Referent Study. J Am Heart Assoc. 2016;5(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khalsa SS, Adolphs R, Cameron OG, Critchley HD, Davenport PW, Feinstein JS, et al. Interoception and Mental Health: A Roadmap. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2018;3(6):501–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arai K, Kato N, Kashiwado K-I, Hattori T. Pure autonomic failure in association with human α-synucleinopathy. Neuroscience Letters. 2000;296(2–3):171–3. [DOI] [PubMed] [Google Scholar]
- 20.Critchley HD, Mathias CJ, Dolan RJ. Neuroanatomical basis for first- and second-order representations of bodily states. Nature neuroscience. 2001;4(2):207–12. [DOI] [PubMed] [Google Scholar]
- 21.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85(1–3):1–17. [DOI] [PubMed] [Google Scholar]
- 22.Bremner JD, Gurel NZ, Wittbrodt MT, Shandhi MH, Rapaport MH, Nye JA, et al. Application of Noninvasive Vagal Nerve Stimulation to Stress-Related Psychiatric Disorders. Journal of Personalized Medicine. 2020;10(3):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benarroch EE. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc. 1993;68(10):988–1001. [DOI] [PubMed] [Google Scholar]
- 24.Davis M. The Role of the Amygdala in Fear and Anxiety. Annual Review of Neuroscience. 1992;15(1):353–75. [DOI] [PubMed] [Google Scholar]
- 25.Porges SW. The polyvagal theory: new insights into adaptive reactions of the autonomic nervous system. Cleveland Clinic journal of medicine. 2009;76 Suppl 2:S86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev. 2009;33(2):81–8. [DOI] [PubMed] [Google Scholar]
- 27.Tawakol A, Ishai A, Takx RA, Figueroa AL, Ali A, Kaiser Y, et al. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet. 2017;389(10071):834–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bremner JD, Campanella C, Khan Z, Shah M, Hammadah M, Wilmot K, et al. Brain Correlates of Mental Stress-Induced Myocardial Ischemia. Psychosom Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moazzami K, Wittbrodt MT, Lima BB, Nye JA, Mehta PK, Pearce BD, et al. Higher Activation of the Rostromedial Prefrontal Cortex during Mental Stress Predicts Major Cardiovascular Disease Events in Individuals with Coronary Artery Disease. Circulation. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding K, Tarumi T, Wang C, Vernino S, Zhang R, Zhu DC. Central autonomic network functional connectivity: correlation with baroreflex function and cardiovascular variability in older adults. Brain Struct Funct. 2020;225(5):1575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su S, Wang X, Pollock JS, Treiber FA, Xu X, Snieder H, et al. Adverse childhood experiences and blood pressure trajectories from childhood to young adulthood: the Georgia stress and Heart study. Circulation. 2015;131(19):1674–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wittbrodt MT, Moazzami K, Lima BB, Alam ZS, Corry D, Hammadah M, et al. Early childhood trauma alters neurological responses to mental stress in patients with coronary artery disease. Journal of affective disorders. 2019;254:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shapiro PA, Sloan RP, Bigger JT Jr., Bagiella E, Gorman JM. Cardiac denervation and cardiovascular reactivity to psychological stress. The American journal of psychiatry. 1994;151(8):1140–7. [DOI] [PubMed] [Google Scholar]
- 34.Thayer JF, Ahs F, Fredrikson M, Sollers JJ 3rd, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 2012;36(2):747–56. [DOI] [PubMed] [Google Scholar]
- 35.Shah AJ, Lampert R, Goldberg J, Veledar E, Bremner JD, Vaccarino V. Posttraumatic stress disorder and impaired autonomic modulation in male twins. Biological psychiatry. 2013;73:1103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minassian A, Maihofer AX, Baker DG, Nievergelt CM, Geyer MA, Risbrough VB. Association of Predeployment Heart Rate Variability With Risk of Postdeployment Posttraumatic Stress Disorder in Active-Duty Marines. JAMA psychiatry. 2015;72(10):979–86. [DOI] [PubMed] [Google Scholar]
- 37.Shah AS, Lampert R, Goldberg J, Bremner JD, Li L, Thames MD, et al. Alterations in heart rate variability are associated with abnormal myocardial perfusion. Int J Cardiol. 2020;305:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park J, Marvar PJ, Liao P, Kankam ML, Norrholm SD, Downey RM, et al. Baroreflex dysfunction and augmented sympathetic nerve responses during mental stress in veterans with post-traumatic stress disorder. The Journal of physiology. 2017;595(14):4893–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yagishita D, Chui RW, Yamakawa K, Rajendran PS, Ajijola OA, Nakamura K, et al. Sympathetic Nerve Stimulation, Not Circulating Norepinephrine, Modulates T-Peak to T-End Interval by Increasing Global Dispersion of Repolarization. Circulation: Arrhythmia and Electrophysiology. 2015;8(1):174–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gurel NZ, Carek AM, Inan OT, Levantsevych O, Abdelhadi N, Hammadah M, et al. Comparison of autonomic stress reactivity in young healthy versus aging subjects with heart disease. PLoS One. 2019;14(5):e0216278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Ferrari GM, Sanzo A, Bertoletti A, Specchia G, Vanoli E, Schwartz PJ. Baroreflex Sensitivity Predicts Long-Term Cardiovascular Mortality After Myocardial Infarction Even in Patients With Preserved Left Ventricular Function. Journal of the American College of Cardiology. 2007;50:2285–90. [DOI] [PubMed] [Google Scholar]
- 42.Davydov DM, Shapiro D, Cook IA, Goldstein I. Baroreflex mechanisms in major depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2007;31:164–77. [DOI] [PubMed] [Google Scholar]
- 43.Park J, Marvar PJ, Liao P, Kankam ML, Norrholm SD, Downey RM, et al. Baroreflex dysfunction and augmented sympathetic nerve responses during mental stress in veterans with post-traumatic stress disorder. Journal of Physiology. 2017;595:4893–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hohnloser SH, Klingenheben T, Bloomfield D, Dabbous O, Cohen RJ. Usefulness of microvolt T-wave alternans for prediction of ventricular tachyarrhythmic events in patients with dilated cardiomyopathy: results from a prospective observational study. Journal of the American College of Cardiology. 2003;41(12):2220–4. [DOI] [PubMed] [Google Scholar]
- 45.Carney RM, Howells WB, Blumenthal JA, Freedland KE, Stein PK, Berkman LF, et al. Heart Rate Turbulence, Depression, and Survival After Acute Myocardial Infarction. Psychosomatic medicine. 2007;69(1):4–9. [DOI] [PubMed] [Google Scholar]
- 46.Wei J, Rooks C, Ramadan R, Shah AJ, Bremner JD, Quyyumi AA, et al. Meta-analysis of mental stress-induced myocardial ischemia and subsequent cardiac events in patients with coronary artery disease. Am J Cardiol. 2014;114(2):187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hammadah M, Sullivan S, Pearce B, Al Mheid I, Wilmot K, Ramadan R, et al. Inflammatory response to mental stress and mental stress induced myocardial ischemia. Brain, behavior, and immunity. 2018;68:90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bruno RM, Ghiadoni L, Seravalle G, Dell’oro R, Taddei S, Grassi G. Sympathetic regulation of vascular function in health and disease. Frontiers in physiology. 2012;3:284-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lima BB, Hammadah M, Kim JH, Uphoff I, Shah A, Levantsevych O, et al. Association of Transient Endothelial Dysfunction Induced by Mental Stress With Major Adverse Cardiovascular Events in Men and Women With Coronary Artery Disease. JAMA cardiology. 2019;4(10):988–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim JH, Almuwaqqat Z, Hammadah M, Liu C, Ko YA, Lima B, et al. Peripheral Vasoconstriction During Mental Stress and Adverse Cardiovascular Outcomes in Patients With Coronary Artery Disease. Circulation research. 2019;125(10):874–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown RP, Gerbarg PL. Sudarshan Kriya yogic breathing in the treatment of stress, anxiety, and depression: part I-neurophysiologic model. J Altern Complement Med. 2005;11(1):189–201. [DOI] [PubMed] [Google Scholar]


