Abstract
Background:
Neurocognitive deficits are common among youth with mental disorders and patterns of aberrant brain function generally cross diagnostic boundaries. This study investigated associations between functional neurocircuitry and broad transdiagnostic psychopathology dimensions in the critical preadolescent period when psychopathology is emerging.
Methods:
Participants were 9–10-year-olds from the Adolescent Brain Cognitive Development Study®. Factor scores of general psychopathology, externalizing, internalizing, and thought disorder dimensions were calculated from a higher-order model of psychopathology using confirmatory factor analysis (n=11,721) and entered as explanatory variables into linear mixed models to examine associations with resting state functional connectivity (n=9,074) and neural activation during the Emotional N-back task (n=6,146), when covarying for sex, race/ethnicity, parental education, and cognitive function.
Results:
All dimensions of psychopathology were commonly characterized by: hypoconnectivity within the dorsal attention and retrosplenial-temporal networks; hyperconnectivity between the frontoparietal and ventral attention networks and between the dorsal attention network and amygdala; and hypoactivation of the caudal middle frontal gyrus. Externalizing pathology was uniquely associated with hyperconnectivity between the salience and ventral attention networks and hyperactivation of the cingulate and striatum. Internalizing pathology was uniquely characterized by hypoconnectivity between the default mode and cingulo-opercular networks. Connectivity between the cingulo-opercular network and putamen was uniquely higher for internalizing pathology and lower for thought disorder pathology.
Conclusions:
These findings provide novel evidence that broad psychopathology dimensions are characterized by common and dissociable patterns, particularly for externalizing pathology, of functional connectivity and task-evoked activation throughout neurocognitive networks in preadolescence.
Keywords: psychopathology, preadolescence, functional magnetic resonance imaging, mental disorder, functional connectivity, neural activation
Introduction
Mental disorders often first manifest during childhood, adolescence, or young adulthood (1–3). Diagnoses based on classification systems such as the Diagnostic and Statistical Manual of Mental Disorders (DSM) tend to have heterogeneous clinical presentations with high rates of comorbidity (4, 5). This clinical comorbidity is mirrored by functional neurocircuit nonspecificity, where multiple disorders appear to have shared etiology (6–10). In the triple network of psychopathology model, aberrant functional organization of the salience, frontoparietal, and default mode neurocognitive networks and subnetworks (i.e., cingulo-opercular, cingulo-parietal, dorsal and ventral attention, retrosplenial-temporal) are theorized to underlie a wide range of psychopathologies (11). Clinical symptoms are thought to be a function of enhanced or reduced salience detection, which have cascading consequences in terms of attentional allocation of frontoparietal systems important for higher-order cognition, and the ability to balance internal mental processes with external stimulus–driven cognitive and affective processes (11).
Recent meta-analyses lend support to common underlying functional disorganization of neurocognitive networks across mental disorders. For example, Sha et al (6) reported shared alterations in functional connectivity across eight mental disorders within and between the three large-scale neurocognitive networks. Likewise, McTeague et al (7) demonstrated a common transdiagnostic pattern of disruption in the salience and ‘multi-demand’ frontoparietal network during cognitive control tasks among patients with various disorders across the lifespan, including schizophrenia, bipolar or unipolar depression, anxiety, and substance use disorders. Furthermore, Sprooten et al (9) has demonstrated common task-evoked functional patterns throughout subcortical regions subserving higher-order cognitive and emotional processes (i.e., striatum, amygdala, hippocampus) in individuals aged 18 to 65 years. These overlapping patterns of functional connectivity and task-evoked activation resonate with prior reports of common neurostructural (e.g., 10) and genetic underpinnings across mental disorders (e.g., 12, 13).
Despite growing interest in identifying common biomarkers of mental disorders, current limitations are notable. First, most studies contributing to these meta-analyses have focused on adult populations, and the limited existing work in youth has often used relatively small samples, resulting in underpowered meta-analyses to detect differences in youth. Second, studies have usually adopted a categorical case-control design, and while the incidence of psychiatric comorbidity is high, studies generally do not evaluate its impact. These limitations of traditional approaches using diagnostic categories have motivated a shift towards alternative research frameworks, such as the Research Domain Criteria (RDoC; 14) and the Hierarchical Taxonomy of Psychopathology (HiTOP; 15, 16). Accordingly, an emerging body of literature has focused on identifying biomarkers associated with an overarching general psychopathology factor (or ‘p factor’), as well as shared and unique biomarkers of lower-order broad dimensional spectra that represent latent liabilities towards externalizing (e.g., antisocial behavior, hyperactivity), internalizing (e.g., depression, anxiety), and thought disorder (e.g., disorganized thoughts, delusional beliefs, hallucinations, obsessions, compulsions) pathology (17–19). Taking a dimensional approach removes arbitrary boundaries between categorical disorders by grouping related disorders together and assigning unrelated disorders to different dimensional spectra. This approach outperforms traditional diagnostic categories in prediction of onset, chronicity, and severity of mental illness, as well as individuals’ treatment response and functional impairment (4).
Working within this framework of latent dimensions, two studies have examined neurocognitive functional correlates of psychopathology in youth. One study examined patterns of functional connectivity associated with four dimensions of psychopathology (mood, fear, externalizing, psychosis) in 999 youth from the Philadelphia Neurodevelopmental Cohort, aged 8 to 22 years (20). They found that a loss of network segregation between the default mode and executive networks (salience, frontoparietal) emerged as a common feature across all dimensions. Capitalizing on data from the same cohort, Shanmugan et al (21) identified that a transdiagnostic general psychopathology factor was associated with failed activation of executive regions within the cingulo-opercular network (linked to the salience network) during the N-back working memory task. They also observed dissociable patterns of task-evoked activation for anxious-misery (internalizing), behavioral (externalizing), and psychosis-spectrum (part of thought disorder) dimensions in varying executive regions. Overall, these two studies provide early support for the notion that common and dissociable alterations in functional patterns of neurocognitive networks may underlie general and lower-order dimensions of psychopathology in youth.
These previous studies span a wide age range from childhood through to young adulthood (8–22 years; mean age 15–16 years), a developmental period characterized by marked changes in both neurobiology and psychopathology. Given psychopathology often first manifests in preadolescence, it is critical to investigate the functional neurocircuitry correlates of psychopathology in this important developmental period. Using data from the Adolescent Brain Cognitive Development (ABCD) Study®, this preregistered analysis (22) aimed to investigate how transdiagnostic dimensions of psychopathology (internalizing, externalizing, thought, general psychopathology) relate to (a) alterations in intrinsic, large-scale functional connectivity (resting state fMRI analysis), and (b) alterations in extrinsic, context-specific neural processes (task-evoked fMRI analysis) during a task of working memory.
Methods and Materials
Participants
Cross-sectional baseline data were analyzed from the ABCD Study curated annual release 2.0.1, which contains postprocessed, precomputed data from 11,875 children, aged 9–10 years (Mage=9.9±0.6; male=52.1%), born between 2005 and 2008. A probability sample was recruited through schools proximal to the 21 research sites across the United States (23). Informed consent and assent were obtained from a parent or legal guardian and the child, respectively. All procedures were approved by a central Institutional Review Board.
Indicators of psychopathology
Past and present mental disorder diagnoses were determined using parent-reported responses to the self-administered computerized Kiddie Schedule for Affective Disorders and Schizophrenia for DSM-5 (KSADS-5; 24). The computerized version of the KSADS-5 has been shown to have good psychometric properties (25). Past and present disorders were combined to provide an index of lifetime disorder status (present/absent) for each of the 14 disorders examined (see Table 1). In total, 5,831 (49.7%) youth had at least one lifetime mental disorder diagnosis, marginally higher than previous US community samples of 13–14-year-olds (~45%) (26). Comorbidity was common, with less youth meeting criteria for a single category (n=2,856) than multiple categories (n=2,975). Among those meeting criteria for more than one disorder, the mean number of mental disorders diagnosed was 3.11 (SD=1.39) (Supplement Figures 1–2).
Table 1.
Summary of demographic and clinical data. (n=11,721)a
| Total | Male | Caucasian | Age (Years) | Cognition1 | Parent University Educated | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | % | % | Mean | SD | Mean | SD | % | |
| Psychiatric disorder 2 | 5,831 | 49.7 | 56.1 | 53.1 | 9.9 | 0.6 | 46.9 | 11.2 | 52.1 |
| Typically developing | 5,890 | 50.3 | 48.4 | 51.2 | 9.9 | 0.6 | 48.6 | 11.2 | 54.8 |
| Externalizing | |||||||||
| Attention Deficit Hyperactivity | 2,428 | 20.7 | 66.4 | 51.0 | 9.9 | 0.6 | 44.7 | 11.0 | 49.0 |
| Oppositional Defiant | 1,666 | 14.2 | 62.4 | 58.0 | 9.9 | 0.6 | 46.7 | 11.3 | 52.8 |
| Conduct | 374 | 3.2 | 70.6 | 41.4 | 9.9 | 0.6 | 44.0 | 10.5 | 37.2 |
| Internalizing | |||||||||
| Major Depression | 318 | 2.7 | 56.9 | 44.0 | 10.0 | 0.6 | 45.5 | 11.3 | 50.6 |
| Generalized Anxiety | 510 | 4.4 | 52.0 | 62.7 | 10.0 | 0.6 | 46.8 | 11.7 | 55.3 |
| Panic | 32 | 0.3 | 56.3 | 46.9 | 10.1 | 0.7 | 46.9 | 13.9 | 43.8 |
| Separation Anxiety | 1,048 | 8.9 | 53.5 | 57.0 | 9.9 | 0.6 | 46.5 | 11.3 | 56.0 |
| Social Anxiety | 547 | 4.7 | 52.8 | 56.5 | 10.0 | 0.6 | 46.5 | 11.0 | 56.1 |
| Post-Traumatic Stress Disorder | 231 | 2.0 | 56.3 | 41.6 | 10.0 | 0.6 | 44.4 | 10.0 | 37.7 |
| Specific phobia | 3,130 | 26.7 | 51.2 | 51.1 | 9.9 | 0.6 | 47.5 | 11.1 | 49.5 |
| Thought | |||||||||
| Hallucinations | 55 | 0.5 | 60.0 | 54.5 | 9.9 | 0.6 | 45.9 | 11.4 | 43.6 |
| Delusions | 215 | 1.8 | 54.9 | 35.8 | 9.9 | 0.6 | 44.7 | 10.8 | 37.2 |
| Obsessive-Compulsive Disorder | 1,096 | 9.4 | 59.9 | 49.4 | 9.9 | 0.6 | 46.1 | 11.2 | 46.2 |
| Bipolar | 428 | 3.7 | 55.8 | 39.3 | 9.9 | 0.6 | 45.3 | 10.3 | 38.8 |
Comorbidity was quite common; 2,975 youth are present in multiple case-control diagnostic categories.
Fully corrected total cognition composite t-score from the NIH toolbox.
Any lifetime psychiatric disorder endorsement.
Resting-State fMRI Connectivity
fMRI acquisition, scanning parameters, and the ABCD preprocessing pipeline are described elsewhere (27, 28) and in the Supplement. Briefly, brain data were collected on 3T scanners, including Siemens MAGNETOM Prisma, GE Discovery MR750, and Philips Achieva scanners. Participants completed four, 5-minute resting-state blood oxygen level–dependent (BOLD) scans, with their eyes open and fixated on a crosshair. Resting-state images were acquired in the axial plane using an echo-planar imaging sequence. Mean framewise displacement (mm) for participants with high quality resting-state data was 0.28 (SD=0.28). Using a functional atlas, cortical-surface regions were grouped into 12 predefined large-scaled networks (29), including 8 neurocognitive networks (cingulo-opercular, cingulo-parietal, default mode, dorsal attention, frontoparietal, retrosplenial-temporal, salience, ventral attention) and 4 sensory networks (auditory, sensorimotor-hand, sensorimotor-mouth, visual). Gordon parcellation was chosen because it comprises major cortical functional networks, covers the entire cortical surface, has been shown to exceed many other network parcellations with respect to homogeneity of BOLD signal within each parcellation (29, 30), and has been used previously in preadolescent populations (31). Resting-state functional connectivity strength indices were then calculated using the Fisher r to z transformation of the average correlation values between pairs of regions within each large-scale network (n=12), between these 12 networks (n=66), and between the networks and bilateral subcortical regions (n=108). Post-processed data were utilized in the current analyses.
Task-Evoked fMRI Activation
Data from the Emotional N-back task was utilized (27). Depending on the condition, children needed to indicate whether the stimulus was the same as 1) the one shown 2 trials earlier (2 back), or 2) the target stimulus shown at the beginning (0 back). Stimuli included houses, and emotional and neutral faces. Task-based changes in the BOLD signal were computed at the individual subject level using a general linear model (GLM) implemented in AFNI’s 3dDeconvolve (32). The GLM coefficients and t-statistics were cortically mapped and projected 1mm into cortical gray matter using Freesurfer. Mean framewise displacement (mm) for participants with high quality task data was 0.25 (SD=0.25). The present study used post-processed functional task data mapped to 34 cortical parcellations (33) and 9 subcortical segmentations (34). The left and right hemisphere mean BOLD activity levels for each region were averaged to create single bilateral values. As a measure of working memory, the contrast between 2 back vs 0 back conditions, regardless of stimulus type, was used in the current analyses.
Statistical analysis
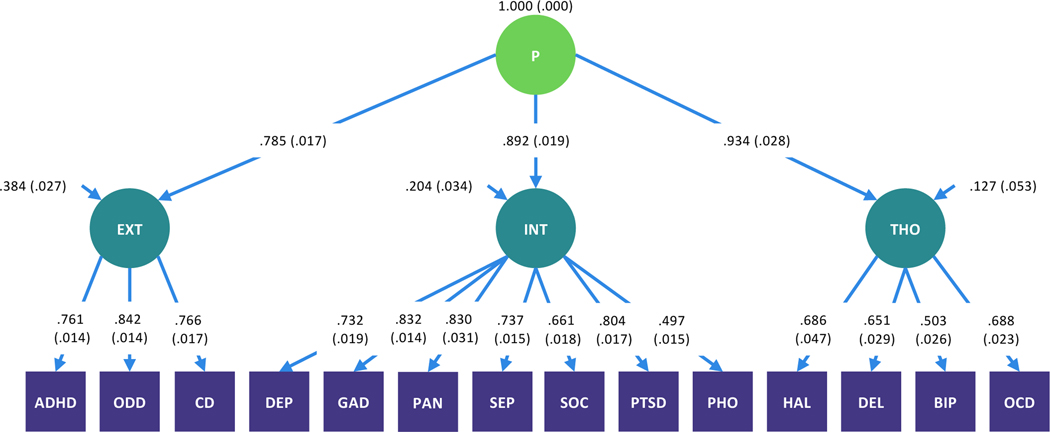
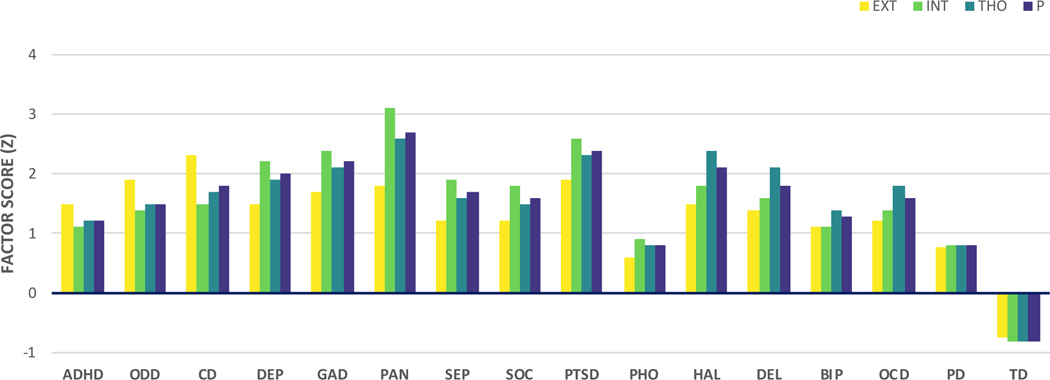
Confirmatory factor analyses of the KSADS-5 data indicated that when compared with both a one-factor and bifactor model, a higher-order model provided the best fit to the data (root mean square error of approximation [RMSEA] = 0.014, Comparative Fit Index [CFI] = 0.987, Tucker-Lewis Fit Index [TLI] = 0.984). See Supplement for further details. In accord with the literature more broadly (e.g., 1, 15, 35, 36-39), the higher order model consisted of three lower order dimensions representing externalizing (attention deficit hyperactivity disorder, oppositional defiant disorder, conduct disorder), internalizing (major depressive disorder, generalized anxiety disorder, post-traumatic stress disorder, panic disorder, separation anxiety disorder, social anxiety disorder), and thought disorder pathology (hallucinations, delusions, obsessive-compulsive disorder, bipolar disorder), as well as a single higher-order dimension representing general psychopathology that accounts for the correlations among lower-order factors (Figure 1). To produce stable and reliable factor score estimates that were representative of the population-based sample, the factor analysis was based on the whole sample that provided KSADS-5 data (n = 11,721). Measurement invariance testing was conducted within a multigroup framework to ensure that the factor structure represented in Figure 1 met criteria for scalar measurement invariance1 in the sub-samples that provided valid resting state (n = 9,074) and Emotional N-back (n = 6,146) data, that passed ABCD’s extensive quality control procedure in every fMRI run (see Supplement for details). Those included and excluded in these analyses were comparable in terms of manifest clinical characteristics, although there were some differences in demographic characteristics (Supplementary Tables S4-S5). Mean factor score loadings for participants meeting criteria for each of the KSADS-5 mental disorder diagnostic categories and for typically developing participants (i.e., those who did not meet criteria for any lifetime mental disorder) were as expected (Figure 2). Increasing factor scores adequately captured the increasing number of mental disorder diagnoses per participant (Figure S2).
Figure 1.
Higher order model of the structure of psychopathology in preadolescents. N=11,721.
ADHD=attention deficit hyperactivity disorder, ODD=oppositional defiant disorder, CD=conduct disorder, DEP=major depressive disorder, GAD=generalized anxiety disorder, PAN=panic disorder, SEP=separation anxiety disorder, SOC=social anxiety disorder, PTSD=post-traumatic stress disorder, PHO=specific phobia, HAL=hallucinations, DEL=delusions, BIP=bipolar disorder, OCD=obsessive-compulsive disorder.
Figure 2.
Mean factor scores of each psychopathology dimension for each case-control diagnostic category.
ADHD=attention deficit hyperactivity disorder, ODD=oppositional defiant disorder, CD=conduct disorder, DEP=major depressive disorder, GAD=generalized anxiety disorder, PAN=panic disorder, SEP=separation anxiety disorder, SOC=social anxiety disorder, PTSD=post-traumatic stress disorder, PHO=specific phobia, HAL=hallucinations, DEL=delusions, BIP=bipolar disorder, OCD=obsessive-compulsive disorder, PD=any psychiatric disorder, TD=typically developing (i.e., those who did not meet criteria for any lifetime mental disorder).
Once the preferred confirmatory factor analysis model of psychopathology was determined, a series of linear mixed models were performed in R version 3.6.1 using lme4 (40), with family unit and MRI scanner site modelled as crossed random intercepts, and sex (female, male) and race/ethnicity (White, Black, Hispanic, Asian, Other) included as covariates. All analyses were False Discovery Rate (FDR) corrected for multiple comparisons (41). Preregistered analyses examined associations between factor scores of each dimension (entered in separate models without adjusting for the presence of the other, correlated dimensions) and 1) within-network connectivity for each of the 12 Gordon networks (12 FDR-corrected comparisons), 2) between-network connectivity (11 FDR-corrected comparisons per network), 3) subcortical connectivity (cerebellum, thalamus, caudate, putamen, pallidum, hippocampus, amygdala, nucleus accumbens, ventral diencephalon) to the Gordon networks (9 FDR-corrected comparisons per network), and 4) task-evoked fMRI activation (34 FDR-corrected comparisons for cortical parcellations, 9 FDR-corrected comparisons for subcortical segmentations). Post-hoc analyses were conducted where lower-order dimensions (i.e., externalizing, internalizing, thought disorder) were entered into models simultaneously to explicate unique associations in a multiple regression framework. Collinearity diagnostics indicated absence of troubling collinearity at the variable-set level (i.e, overall multicollinearity between dimensions), while two of the four variable pairing indices indicated possible collinearity between the internalizing and thought disorder dimensions (see Supplement). A series of preregistered sensitivity analyses were conducted to test whether results were robust to the inclusion of additional covariates, including cognitive function (as determined by the fully corrected total cognition composite t-score from the NIH toolbox®) and parental education (a proxy for socio-economic status). Similar to previous studies (20, 21, 42), results are expressed as Z-scores and effect sizes are expressed as R2 (both full and partial model with just the psychopathology dimension of interest).
Results
Functional connectivity patterns in large-scale networks
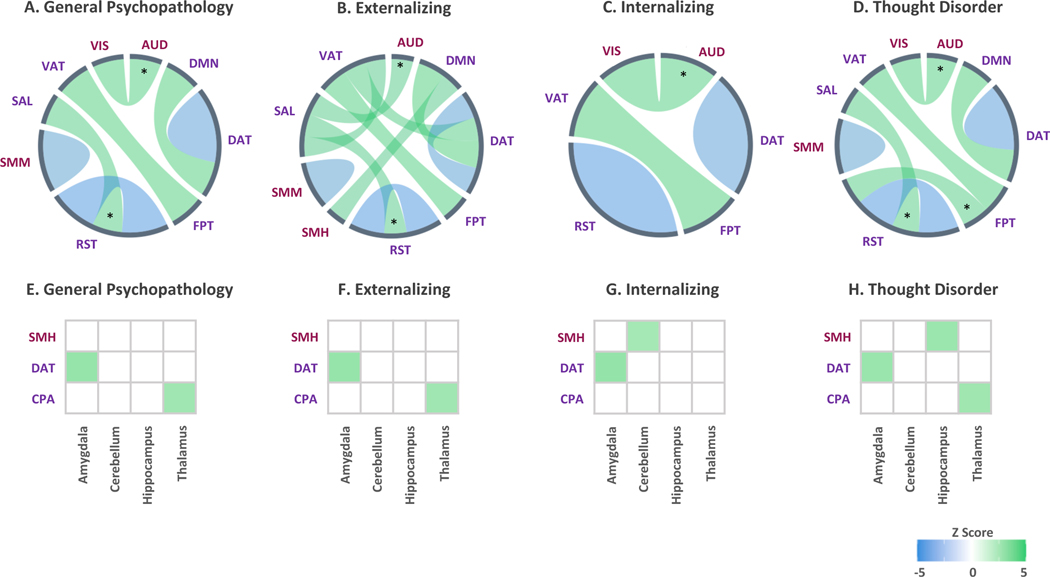
Common patterns of altered network-level connectivity across higher- and lower-order dimensions of psychopathology included lower connectivity within the neurocognitive dorsal attention (full-model R2s=.14, partial R2s≥.001) and retrosplenial-temporal networks (full-model R2s=.14, partial R2s≥.002), and greater connectivity between the neurocognitive frontoparietal and ventral attention networks (full-model R2s=.15, partial R2s=.001), after adjusting for sex and race/ethnicity (Figure 3 a-d). When examining associations between network and subcortical connectivity, all dimensions of psychopathology were associated with heightened connectivity between the dorsal attention network and the amygdala (full-model R2s≥.05, partial R2s=.001) (Figure 3 e-h), after adjusting for sex and race/ethnicity.
Figure 3.
A-D illustrate alterations in connectivity patterns within and between large-scale networks, and E-H illustrate alterations in connectivity between networks and bilateral subcortical regions, when adjusting for sex and race/ethnicity. Associations marked with an asterisk (*) or hash (#) were no longer significant when adjusting for cognitive function or parental education, respectively. Line thickness reflects relative strength of associations within each dimension. N=9,074.
AUD = auditory, COP = cingulo-opercular, CPA = cingulo-parietal, DMN = default mode, DAT = dorsal attention, FPT = frontoparietal, RST = retrosplenial-temporal, SMH = sensorimotor-hand, SMM = sensorimotor-mouth, SAL = salience, VAT = ventral attention, VIS = visual.
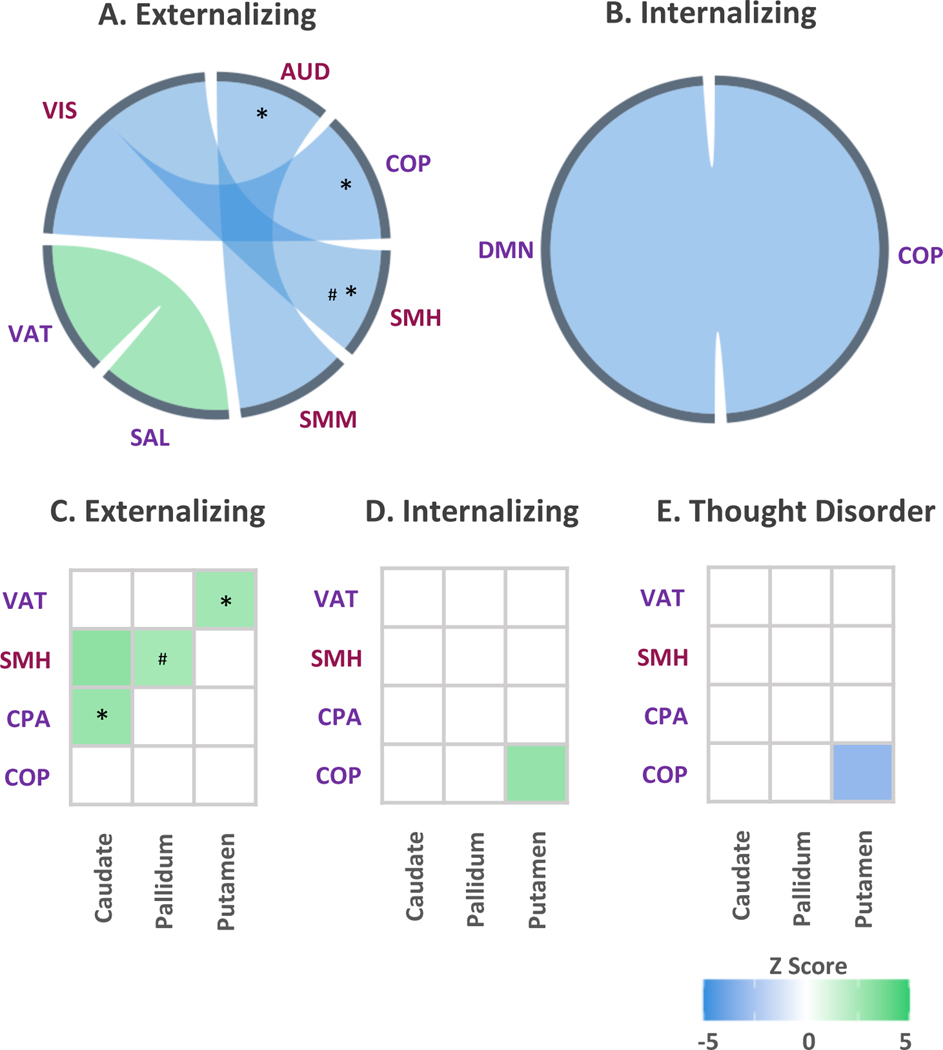
When accounting for overlap among dimensions in these analyses (i.e., lower-order dimensions entered simultaneously into models), externalizing pathology was uniquely characterized by heightened connectivity between the neurocognitive salience and ventral attention networks (full-model R2=.12, partial R2=.001), and lower connectivity between the sensorimotor mouth and auditory networks (full-model R2=.12, partial R2=.001), sensorimotor hand and visual networks (full-model R2=.21, partial R2=.001), and cingulo-opercular and visual networks (full-model R2=.07, partial R2=.00002), after adjusting for sex and race/ethnicity (Figure 4 a). Internalizing pathology was uniquely associated with lower connectivity between the neurocognitive default mode and cingulo-opercular networks (full-model R2=.18, partial R2=.0002) (Figure 4 b). No statistically significant differences in within- or between-network connectivity were found for the thought disorder dimension, after adjusting for sex and race/ethnicity and in the context of the other dimensions. When examining associations between network and subcortical connectivity, the externalizing dimension was uniquely associated with heightened connectivity between the cingulo-parietal network and caudate (full-model R2=.10, partial R2=.0001), the sensorimotor hand network and caudate (full-model R2=.18, partial R2=.001), the sensorimotor hand network and pallidum (full-model R2=.04, partial R2=.001), and the ventral attention network and putamen (full-model R2=.03, partial R2=.001) (Figure 4 c). The internalizing and thought disorder dimensions exhibited unique divergent patterns between the cingulo-opercular network and putamen, where the internalizing dimension was associated with higher connectivity (full-model R2=.12, partial R2=.001), and the thought disorder dimension was associated with lower connectivity (full-model R2=.12, partial R2=.0003) (Figure 4 d-e).
Figure 4.
A-B illustrate alterations in connectivity patterns within and between large-scale networks, and C-E alterations in connectivity between networks and bilateral subcortical regions, when adjusting for sex and race/ethnicity. Associations marked with an asterisk (*) or hash (#) were no longer significant when adjusting for cognitive function or parental education, respectively. Line thickness reflects relative strength of associations within each dimension. N=9,074.
AUD = auditory, COP = cingulo-opercular, CPA = cingulo-parietal, DMN = default mode, DAT = dorsal attention, FPT = frontoparietal, RST = retrosplenial-temporal, SMH = sensorimotor-hand, SMM = sensorimotor-mouth, SAL = salience, VAT = ventral attention, VIS = visual.
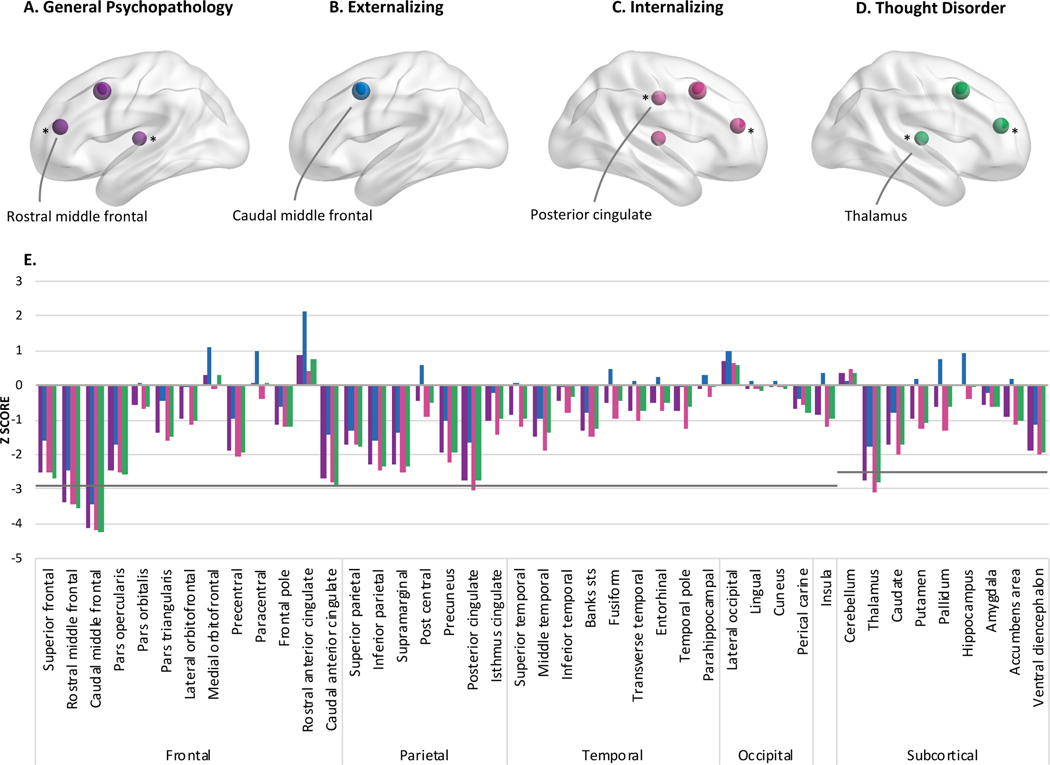
Task-evoked activation during working memory
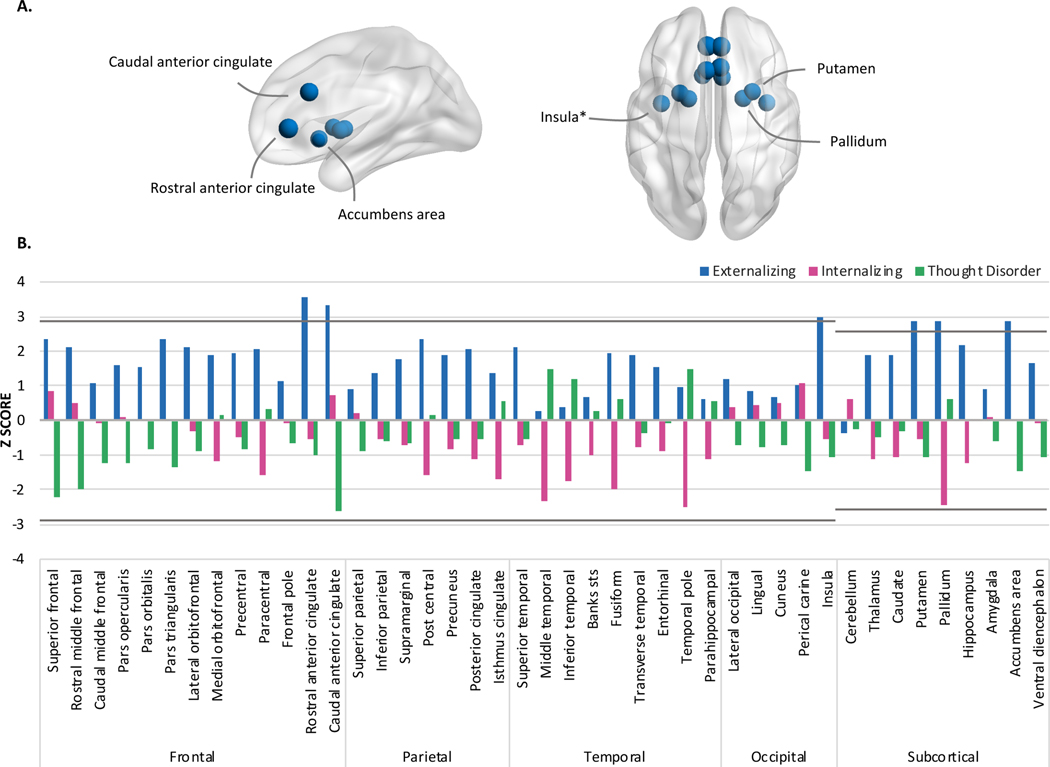
All psychopathology dimensions, when entered into separate models, were associated with lower activation in the caudal middle frontal gyrus during the Emotional N-Back task, when accounting for the effects of sex and race/ethnicity (full-model R2s≥.004, partial R2s=.001) (Figure 5). When accounting for overlap among dimensions in multiple regression analyses, externalizing pathology was uniquely characterized by greater activation in the rostral (full-model R2=.02, partial R2=.0004) and caudal anterior cingulate (full-model R2=.01, partial R2=.0001), insula (full-model R2=.02, partial R2=.0001), nucleus accumbens (full-model R2=.004, partial R2=.0001), putamen (full-model R2=.05, partial R2=.00005), and pallidum (full-model R2=.004, partial R2=.00004) after adjusting for sex and race/ethnicity (Figure 6). No unique associations for internalizing or thought disorder dimensions passed FDR correction.
Figure 5.
Lower task-evoked activation during the Emotional N-back task (2 vs 0 contrast) was observed for dimensions of psychopathology in the middle frontal gyrus (A,C,D), posterior cingulate (C), and thalamus (A,C,D). Associations in A-D marked with an asterisk (*) or hash (#) were no longer significant when adjusting for cognitive function or parental education, respectively. E illustrates the Z-scores for all parcellations and segmentations examined when entered as predictors into mixed models separately. N=6,146.
The nodes on the brain figures represent the location where activation differed for youth with higher psychopathology factor scores and the size corresponds to the z-score.
Figure 6.
A illustrates the regions exhibiting heightened connectivity which is uniquely associated with externalizing pathology during the Emotional N-back task (2 vs 0 contrast). Associations marked with an asterisk (*) or hash (#) were no longer significant when adjusting for cognitive function or parental education, respectively. B illustrates the Z-scores for all parcellations and segmentations when examined in multiple regression framework. N=6,146.
The nodes on the brain figures represent the location where activation differed for youth with higher externalizing pathology factor scores and the size corresponds to the z-score.
Sensitivity Analyses
All observed associations remained after adjusting for parental education, excluding connectivity between the sensorimotor hand and visual networks, and sensorimotor hand network and pallidum for externalizing pathology (Figure 4). Several associations no longer passed FDR correction after adjusting for cognitive function, indicated by an asterisk (*) in Figures 3–6. Table 2 summarizes the common and dissociable connectivity and task-evoked activation patterns across dimensions of psychopathology, with and without adjustment for these additional covariates.
Table 2.
Summary of aberrant functional connectivity and neural activation during a working memory task associated with latent dimensions of psychopathology among 9–10 year olds, when adjusting for sex and race/ethnicity.
| Common biomarkers | Dissociable biomarkers | |
|---|---|---|
|
| ||
| Functional connectivity | ||
|
| ||
| Within networks | ↓ Dorsal attention | - |
| ↓ Retrosplenial-temporal | ||
|
| ||
| Between networks | ↑ Frontoparietal – ventral attention | Externalizing |
| ↑ Salience – ventral attention | ||
| ↓ Sensorimotor mouth – auditory* | ||
| ↓ Sensorimotor hand – visual*# | ||
| ↓ Cingulo-opercular – visual* | ||
| Internalizing | ||
| ↓ Default mode – cingulo-opercular | ||
|
| ||
| Network-subcortical | ↑ Dorsal attention – amygdala | Externalizing |
| ↑ Sensorimotor hand – caudate | ||
| ↑ Cingulo-parietal – caudate* | ||
| ↑ Sensorimotor hand – pallidum# | ||
| ↑ Ventral attention – putamen* | ||
| Internalizing | ||
| ↑ Cingulo-opercular – putamen | ||
| Thought disorder | ||
| ↓ Cingulo-opercular – putamen | ||
|
| ||
| Neural activation during working memory | ||
|
| ||
| Cortical | ↓ Caudal middle frontal | Externalizing |
| ↑ Caudal, rostral anterior cingulate cortex | ||
|
| ||
| Subcortical | - | Externalizing |
| ↑ Nucleus accumbens | ||
| ↑ Putamen | ||
| ↑ Pallidum | ||
| ↑ Insula* | ||
Associations marked with an asterisk (*) or hash (#) were no longer significant when adjusting for cognitive function or parental education, respectively.
Discussion
In a large sample of preadolescents, the current study examined associations between diverse psychopathology, resting state functional connectivity, and neural activation during a task of working memory. All dimensions of psychopathology were characterized by common patterns of aberrant functional connectivity and task-evoked activation throughout neurocognitive networks. While unique associations were observed for all lower-order dimensions, a dissociable neurocircuitry pattern was most evident for externalizing pathology.
Common alterations across dimensions included hypoconnectivity within the dorsal attention and retrosplenial-temporal networks, hyperconnectivity between the frontoparietal and ventral attention networks and between the dorsal attention network and amygdala, and hypoactivation in the caudal middle frontal gyrus during the Emotional N-back task. Externalizing pathology was uniquely characterized by hyperconnectivity between the salience and ventral attention networks, hypoconnectivity between sensory networks, heightened network-subcortical connectivity, and hyperactivation of the cingulate, striatum, and insula during working memory, although alterations involving the sensory network and insula were no longer significant when accounting for variance in cognitive function. In contrast, internalizing pathology was characterized by hypoconnectivity between the default mode and cingulo-parietal networks and hyperconnectivity between the cingulo-opercular network and putamen, where the reverse pattern was observed for thought disorder pathology. Taken together, the results suggest that psychopathology is associated with aberrant functional patterns throughout neurocognitive networks and regions during preadolescence.
Common functional connectivity alterations associated with psychopathology
Findings across studies converge to suggest that altered connectivity within and between neurocognitive networks may be a common biomarker underlying vulnerability to a wide range of mental disorders (6, 20). Prior studies have identified more widespread alterations across neurocognitive networks than observed in the current study, including a previous study of adolescents (20) and a meta-analysis of adults (6). These inconsistencies may be due to the relatively early developmental period under study (preadolescence), perhaps indicating that neurobiological underpinnings of psychopathology become more pervasive throughout the life course.
This study was the first to examine associations between psychopathology and network-subcortical connectivity. Hyperconnectivity between the dorsal attention network and the amygdala received the highest loadings for all dimensions of psychopathology. The amygdala has long been the focus of disordered emotional processing (43, 44), and is a prominent subcortical structure of the neurocognitive salience network (11). In accord with the present findings, a recent meta-analysis identified a common pattern of aberrant neural activation during emotional processing in the amygdala and regions of the executive network (inclusive of the dorsal attention network) across psychiatric disorders (8).
Interestingly, these transdiagnostic functional impairments in neurocognitive networks parallel behavioral and structural evidence of common disruptions in neurocircuitry underlying cognitive control capacity and emotional processing (10, 45, 46). Overall, this study adds to a convergent body of literature that shows highly coordinated networks and subcortical regions that are sensitive to demands on cognitive control and emotional processing underlie complex and wide-ranging psychiatric symptoms across different age groups.
Dissociable patterns of connectivity associated with lower-order dimensions of psychopathology
Lower-order dimensions of psychopathology were characterized by some unique functional connectivity patterns in networks or subcortical regions that subserve cognitive control. The direction of effects between neurocognitive networks observed in the current study for the externalizing (i.e., hyperconnectivity) and internalizing (i.e., hypoconnectivity) dimensions are consistent with a previous study of youth from the Philadelphia Neurodevelopmental Cohort, aged 8 to 22 years (20). In contrast, that study also observed differentiated patterns of connectivity for the thought disorder dimension in the default mode and executive networks. Likewise, prior studies of patients with schizophrenia have identified altered connectivity within the default mode network (47–49). There is evidence to suggest that patterns of dysconnectivity linked to lower-order dimensions of psychopathology parallel clinical trajectories of these disorders. This may explain why associations for the thought disorder dimension did not pass FDR correction in the current study of preadolescents compared to prior studies in older populations (e.g., 20, 47, 48, 49). Externalizing aggression-focused syndromes, as captured in the present study, typically present earlier in childhood and have a more stable trajectory (50). Internalizing syndromes often manifest next, followed by thought disorder syndromes later in adolescence, often escalating in severity throughout adolescence and adulthood (51, 52). Likewise, preliminary evidence suggests that patterns of dysconnectivity associated with the externalizing dimension manifest earlier and have a more stable time-course, while patterns associated with the internalizing and thought disorder dimensions strengthen throughout adolescence and into young adulthood (20). Considering the current sample are preadolescents, it is expected that functional connectivity alterations associated with the internalizing and thought disorder dimensions will continue to diverge throughout adolescence and into young adulthood in parallel with symptom escalation.
Common and dissociable patterns of task-evoked activation across psychopathology dimensions
In addition to the robust finding that altered functional connectivity in neurocognitive networks (i.e., dorsal and ventral attention, retrosplenial-temporal, frontoparietal) is a transdiagnostic biomarker for psychopathology, the current study also identified that hypoactivation within the salience network (i.e., caudal middle frontal gyrus) during a working memory task is common across dimensions of psychopathology, in line with previous research (7, 21). This network is thought to be essential for adaptive switching between other neurocognitive networks (11). In contrast, externalizing pathology was uniquely associated with hyperactivation in the anterior cingulate cortex and subcortical regions. The anterior regions of the cingulate cortex are key nodes of the default mode network (53). Recent neuroanatomical modelling has also revealed that the thalamus and basal forebrain (including the nucleus accumbens, pallidum, putamen) are of central importance for the functioning of the default mode network (54). These findings dovetail the dissociable effects observed for functional connectivity, where the externalizing dimension was characterized by hyperconnectivity between the neurocognitive salience and ventral attention networks. As noted above, dissociable patterns of neural activation for the internalizing and thought disorder dimensions may become more pronounced with age.
Towards a neurocognitive network perspective of psychopathology
The current findings align with the triple network of psychopathology model (11) which posits that aberrant functional organization of the salience (including cingulo-opercular), frontoparietal (including dorsal and ventral attention), and default mode (including retrosplenial-temporal) networks and subnetworks, and their network interactions, underlie a wide range of psychopathologies, as does the amygdala which is crucial for the detection of biologically salient affective cues. While aberrant patterns were not observed throughout all neurocognitive networks, as noted above, it is anticipated that patterns will continue to diverge with age and severity of psychopathology symptoms. Longitudinal data spanning early development and adolescence are needed to establish the causal relationship between neurobiology and psychopathology. To date, evidence suggests that compromised brain health is an antecedent for psychopathology, whether that be through genetic susceptibilities (13, 55, 56), prenatal exposures (57), early life stressors (58), or some other mechanism. Interestingly, there is some evidence to suggest that psychopathology in children aged 8 has downstream effects on brain development at age 10 (59). A cascading interaction between psychopathology and the brain may exist during this critical developmental period, and future work utilizing this cohort when longitudinal data is available will help delineate these associations.
Strengths and Limitations
The current study has several strengths. The ABCD study is a multisite, demographically diverse, population-based study that is the largest of its kind to investigate child brain development. Here, lifetime mental disorders were highly prevalent, perhaps reflecting the use of parent- rather than clinician-report (60) and the relative sensitivity of the KSADS-5 to lower-level symptomatology (61). This sensitivity allowed for linkage of psychopathology with functional neurocircuitry in a manner not necessarily tied to strict clinician-rated disorder thresholds. The high incidence of psychiatric comorbidity was accounted for by utilizing factor analysis to examine general psychopathology and lower-order broad dimensional spectra representing latent liabilities towards externalizing, internalizing, and thought disorder pathology. Independent of the imaging analyses, this work demonstrates the coherence of symptoms across disorders in the preadolescent period, which are typically considered disparate. The large sample size allowed for inclusion of low base rate disorders that are rare in the preadolescent period. The narrow age range included in this study allowed for exploration of developmentally specific relationships between detailed neurobiological indices and psychopathology during a critical developmental period when the trajectories of the lower-order dimensions begin to shift, but prior to many mental disorders emerging.
These findings also need to be interpreted within the context of some limitations. ABCD data are cross-sectional at present and cannot determine causality between psychopathology and functional neurocircuitry aberrations. There were large amounts of excluded data for the functional MRI analyses (n = 2,647 to 5,575). Although the clinical characteristics and structure of psychopathology were similar for those included and excluded from the analyses, this excluded data may have affected the representativeness of the sample and thus the generalizability of the results. There was evidence of possible collinearity between the internalizing and thought disorder dimensions, and some caution should be taken when interpreting the dissociable effects for these dimensions. Furthermore, the current study analysed cortical parcellations and subcortical segmentations, however, future studies could explore voxelwise analytical approaches. Finally, replicating these findings in other large independent samples will be important to determine the robustness of the functional patterns underlying psychopathology found here, and at different stages throughout the lifespan.
Conclusions
The current study revealed that broad dimensions of psychopathology are characterized by common and dissociable patterns, particularly for externalizing pathology, of functional connectivity and task-evoked activation throughout neurocognitive networks in preadolescence, when adjusting for sex, race/ethnicity, cognitive function, and parental education. In the context of other studies, it appears that neural disruptions associated with psychopathology may become more pervasive across the life course, in parallel with clinical trajectories of each disorder. The broad dimensions examined in this study span multiple traditional disorder categories and provide further support for the consideration of mental diagnoses from a dimensional perspective, as suggested by RDoC and HiTOP. Future evaluations should examine the practical utility of identified functional biomarkers to predict clinical trajectories as well prevention and intervention responses.
Supplementary Material
Acknowledgements
Salary support was provided by the Australian National Health and Medical Research Council (GNT1169377 to BL; GNT1041756 and GNT1078407 to MT), National Institute on Drug Abuse (U01 DA041093 to LMS), National Institute of Mental Health (K23 MH104849 to LMM), Macquarie University (Research Fellowship to MKF), National Institute of Aging (R01AG053217 and U19AG051426 to RFK), and University of New South Wales (Research Fellowship to LM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Australian National Health and Medical Research Council or National Institute on Drug Abuse.
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children aged 9–10 and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners “under award numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, and U24DA041147”. A full list of supporters is available at https://abcdstudy.org/nih-collaborators. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/principal-investigators.html. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from the curated annual release 2.0.1.
Footnotes
Disclosures
The authors declare no biomedical financial interests or potential conflicts of interest.
Factor structure, factor loadings and item thresholds were constrained to be equal across groups.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Caspi A, Houts RM, Ambler A, Danese A, Elliott ML, Hariri A, et al. Longitudinal Assessment of Mental Health Disorders and Comorbidities Across 4 Decades Among Participants in the Dunedin Birth Cohort Study. JAMA Network Open. 2020;3(4):e203221-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustün TB. Age of onset of mental disorders: a review of recent literature. Curr Opin Psychiatry. 2007;20(4):359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21(3):169–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conway CC, Forbes MK, Forbush KT, Fried EI, Hallquist MN, Kotov R, et al. A Hierarchical Taxonomy of Psychopathology Can Transform Mental Health Research. Perspect Psychol Sci. 2019;14(3):419–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caspi A, Moffitt TE. All for One and One for All: Mental Disorders in One Dimension. American Journal of Psychiatry. 2018;175(9):831–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sha Z, Wager TD, Mechelli A, He Y. Common Dysfunction of Large-Scale Neurocognitive Networks Across Psychiatric Disorders. Biological psychiatry. 2019;85(5):379–88. [DOI] [PubMed] [Google Scholar]
- 7.McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A. Identification of Common Neural Circuit Disruptions in Cognitive Control Across Psychiatric Disorders. The American journal of psychiatry. 2017;174(7):676–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McTeague LM, Rosenberg BM, Lopez JW, Carreon DM, Huemer J, Jiang Y, et al. Identification of Common Neural Circuit Disruptions in Emotional Processing Across Psychiatric Disorders. American Journal of Psychiatry. 2020;177(5):411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sprooten E, Rasgon A, Goodman M, Carlin A, Leibu E, Lee WH, et al. Addressing reverse inference in psychiatric neuroimaging: Meta-analyses of task-related brain activation in common mental disorders. Human brain mapping. 2017;38(4):1846–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15(10):483–506. [DOI] [PubMed] [Google Scholar]
- 12.Gatt JM, Burton KLO, Williams LM, Schofield PR. Specific and common genes implicated across major mental disorders: A review of meta-analysis studies. Journal of Psychiatric Research. 2015;60:1–13. [DOI] [PubMed] [Google Scholar]
- 13.Cross-Disorder Group of the Psychiatric Genomics Consortium. Genomic Relationships, Novel Loci, and Pleiotropic Mechanisms across Eight Psychiatric Disorders. Cell. 2019;179(7):1469–82.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Insel T, Cuthbert B, Garvey M, Heinssen R, Kozak MJ, Pine DS, et al. Research Domain Criteria (RDoC): Developing a valid diagnostic framework for research on mental disorders. American Journal of Psychiatry. 2010;167(7):748–51. [DOI] [PubMed] [Google Scholar]
- 15.Kotov R, Krueger RF, Watson D, Achenbach TM, Althoff RR, Bagby RM, et al. The Hierarchical Taxonomy of Psychopathology (HiTOP): A dimensional alternative to traditional nosologies. J Abnorm Psychol. 2017;126(4):454–77. [DOI] [PubMed] [Google Scholar]
- 16.Latzman RD, DeYoung CG, Afzali MH, Allen TA, Althoff RR, DeYoung CG, et al. Using empirically-derived dimensional phenotypes to accelerate clinical neuroscience: the Hierarchical Taxonomy of Psychopathology (HiTOP) framework. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2020;45(7):1083–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lahey BB, Krueger RF, Rathouz PJ, Waldman ID, Zald DH. A hierarchical causal taxonomy of psychopathology across the life span. Psychological bulletin. 2017;143(2):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, et al. The p factor: one general psychopathology factor in the structure of psychiatric disorders? Clinical Psychological Science. 2014;2(2):119–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lahey BB, Applegate B, Hakes JK, Zald DH, Hariri AR, Rathouz PJ. Is there a general factor of prevalent psychopathology during adulthood? Journal of abnormal psychology. 2012;121(4):971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia CH, Ma Z, Ciric R, Gu S, Betzel RF, Kaczkurkin AN, et al. Linked dimensions of psychopathology and connectivity in functional brain networks. Nat Commun. 2018;9(1):3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shanmugan S, Wolf DH, Calkins ME, Moore TM, Ruparel K, Hopson RD, et al. Common and Dissociable Mechanisms of Executive System Dysfunction Across Psychiatric Disorders in Youth. The American journal of psychiatry. 2016;173(5):517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lees B. Functional brain correlates of dimensional psychopathology: ABCD secondary data analysis. Retrieved from osfio/234ny. 2020, May25. [Google Scholar]
- 23.Hagler DJ, Hatton SN, Makowski C, Cornejo MD, Fair DA, Dick AS, et al. Imaging processing and analysis methods for the Adolescent Brain Cognitive Development Study. bioRxiv 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobak KA, Kratochvil C, Stanger C, Kaufman J. Computerized screening of comorbidity in adolescents with substance or psychiatric disorders. Anxiety Disorders and Depression(La Jolaa, CA). 2013. [Google Scholar]
- 25.Townsend L, Kobak K, Kearney C, Milham M, Andreotti C, Escalera J, et al. Development of three web-based computerized versions of the Kiddie Schedule for Affective Disorders and Schizophrenia child psychiatric diagnostic interview: preliminary validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 2020;59(2):309–25. [DOI] [PubMed] [Google Scholar]
- 26.Merikangas KR, He J-P, Burstein M, Swanson SA, Avenevoli S, Cui L, . . ., et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication –Adolescent Supplement (NCSA). Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(10):980–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, et al. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev Cogn Neurosci. 2018;32:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fair DA, Miranda-Dominguez O, Snyder AZ, Perrone A, Earl EA, Van AN, et al. Correction of respiratory artifacts in MRI head motion estimates. NeuroImage. 2020;208:116400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE. Generation and Evaluation of a Cortical Area Parcellation from Resting-State Correlations. Cerebral cortex (New York, NY : 1991). 2016;26(1):288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miranda-Dominguez O, Feczko E, Grayson DS, Walum H, Nigg JT, Fair DA. Heritability of the human connectome: A connectotyping study. Network Neuroscience. 2017;2(2):175–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grayson DS, Fair DA. Development of large-scale functional networks from birth to adulthood: A guide to the neuroimaging literature. NeuroImage. 2017;160:15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–73. [DOI] [PubMed] [Google Scholar]
- 33.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–80. [DOI] [PubMed] [Google Scholar]
- 34.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–55. [DOI] [PubMed] [Google Scholar]
- 35.Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, et al. The p Factor: One General Psychopathology Factor in the Structure of Psychiatric Disorders? Clin Psychol Sci. 2014;2(2):119–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romer AL, Elliott ML, Knodt AR, Sison ML, Ireland D, Houts R, et al. Pervasively Thinner Neocortex as a Transdiagnostic Feature of General Psychopathology. American Journal of Psychiatry. 2020:appi.ajp.2020.19090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romer AL, Knodt AR, Sison ML, Ireland D, Houts R, Ramrakha S, et al. Replicability of structural brain alterations associated with general psychopathology: evidence from a population-representative birth cohort. Molecular psychiatry. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carragher N, Teesson M, Sunderland M, Newton NC, Krueger RF, Conrod PJ, et al. The structure of adolescent psychopathology: a symptom-level analysis. Psychological medicine. 2016;46(5):981–94. [DOI] [PubMed] [Google Scholar]
- 39.Sunderland M, Forbes MK, Mewton L, Baillie A, Carragher N, Lynch SJ, et al. The structure of psychopathology and association with poor sleep, self-harm, suicidality, risky sexual behavior, and low self-esteem in a population sample of adolescents. Development and Psychopathology. 2020:1–12. [DOI] [PubMed] [Google Scholar]
- 40.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:14065823. 2014. [Google Scholar]
- 41.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological). 1995;57(1):289–300. [Google Scholar]
- 42.Kaczkurkin AN, Park SS, Sotiras A, Moore TM, Calkins ME, Cieslak M, et al. Evidence for Dissociable Linkage of Dimensions of Psychopathology to Brain Structure in Youths. The American journal of psychiatry. 2019;176(12):1000–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American journal of psychiatry. 2007;164(10):1476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sabatinelli D, Fortune EE, Li Q, Siddiqui A, Krafft C, Oliver WT, et al. Emotional perception: meta-analyses of face and natural scene processing. Neuroimage. 2011;54(3):2524–33. [DOI] [PubMed] [Google Scholar]
- 45.Snyder HR, Miyake A, Hankin BL. Advancing understanding of executive function impairments and psychopathology: bridging the gap between clinical and cognitive approaches. Frontiers in psychology. 2015;6:328-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gualtieri CT, Morgan DW. The frequency of cognitive impairment in patients with anxiety, depression, and bipolar disorder: an unaccounted source of variance in clinical trials. J Clin Psychiatry. 2008;69(7):1122–30. [DOI] [PubMed] [Google Scholar]
- 47.Whitfield-Gabrieli S, Ford JM. Default Mode Network Activity and Connectivity in Psychopathology. Annual review of clinical psychology. 2012;8(1):49–76. [DOI] [PubMed] [Google Scholar]
- 48.Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, et al. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97(1–3):194–205. [DOI] [PubMed] [Google Scholar]
- 49.Pankow A, Deserno L, Walter M, Fydrich T, Bermpohl F, Schlagenhauf F, et al. Reduced default mode network connectivity in schizophrenia patients. Schizophr Res. 2015;165(1):90–3. [DOI] [PubMed] [Google Scholar]
- 50.Bongers IL, Koot HM, van der Ende J, Verhulst FC. Developmental trajectories of externalizing behaviors in childhood and adolescence. Child Dev. 2004;75(5):1523–37. [DOI] [PubMed] [Google Scholar]
- 51.Petersen IT, Lindhiem O, LeBeau B, Bates JE, Pettit GS, Lansford JE, et al. Development of internalizing problems from adolescence to emerging adulthood: Accounting for heterotypic continuity with vertical scaling. Dev Psychol. 2018;54(3):586–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGrath JJ, Saha S, Al-Hamzawi AO, Alonso J, Andrade L, Borges G, et al. Age of Onset and Lifetime Projected Risk of Psychotic Experiences: Cross-National Data From the World Mental Health Survey. Schizophr Bull. 2016;42(4):933–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the Default Mode Network: Distinct Contributions of the Ventral and Dorsal Posterior Cingulate Cortex to Cognitive Control. The Journal of Neuroscience. 2011;31(9):3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alves PN, Foulon C, Karolis V, Bzdok D, Margulies DS, Volle E, et al. An improved neuroanatomical model of the default-mode network reconciles previous neuroimaging and neuropathological findings. Communications Biology. 2019;2(1):370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smoller JW, Andreassen OA, Edenberg HJ, Faraone SV, Glatt SJ, Kendler KS. Psychiatric genetics and the structure of psychopathology. Molecular psychiatry. 2019;24(3):409–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, Duncan L, et al. Analysis of shared heritability in common disorders of the brain. Science. 2018;360(6395). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lees B, Mewton L, Jacobus J, Valadez E, Stapinski LA, Teesson M, et al. Association of Prenatal Alcohol Exposure With Psychological, Behavioral, and Neurodevelopmental Outcomes in Children From the Adolescent Brain Cognitive Development Study. The American journal of psychiatry. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zou R, Tiemeier H, van der Ende J, Verhulst FC, Muetzel RL, White T, et al. Exposure to Maternal Depressive Symptoms in Fetal Life or Childhood and Offspring Brain Development: A Population-Based Imaging Study. The American journal of psychiatry. 2019;176(9):702–10. [DOI] [PubMed] [Google Scholar]
- 59.Muetzel RL, Blanken LME, van der Ende J, El Marroun H, Shaw P, Sudre G, et al. Tracking Brain Development and Dimensional Psychiatric Symptoms in Children: A Longitudinal Population-Based Neuroimaging Study. The American journal of psychiatry. 2018;175(1):54–62. [DOI] [PubMed] [Google Scholar]
- 60.Townsend L, Kobak K, Kearney C, Milham M, Andreotti C, Escalera J, et al. Development of Three Web-Based Computerized Versions of the Kiddie Schedule for Affective Disorders and Schizophrenia Child Psychiatric Diagnostic Interview: Preliminary Validity Data. J Am Acad Child Adolesc Psychiatry. 2020;59(2):309–25. [DOI] [PubMed] [Google Scholar]
- 61.Barch DM, Albaugh MD, Avenevoli S, Chang L, Clark DB, Glantz MD, et al. Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: Rationale and description. Dev Cogn Neurosci. 2018;32:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.