Summary
Background
A single pre-operative antibiotic dose provides optimal prophylaxis against surgical site infection (SSI), but significant variability persists in adherence to prophylaxis guidelines. We describe a quality improvement project aiming to improve guideline-driven antibiotic prescribing within surgical teams at a tertiary hospital.
Methods
Face-to-face interviews with surgical teams and anonymous surveys of senior surgeons and anaesthetists were used to collect qualitative data on the perceptions and attitudes of prescribers. This informed intervention development, including a daily ward-round checklist using the acronymous ‘ABBDDOMM’, from A (antibiotics) to M (microbiology), combined with education and heightened guideline accessibility. A first audit cycle was performed for patients undergoing intra-abdominal surgery during a two-month period (cycle one). Post-implementation data were collected 12 months later (cycle two).
Findings
Interviews provided insight into common themes and barriers surrounding antibiotic prescribing, whilst surveys explored future solutions to these barriers. In cycle one, 100/205 (48.8%) patients received extended antibiotics beyond the single-dose prophylaxis. Following intervention, only 41/138 (29.7%) patients received extended antibiotic courses, demonstrating a 21.5% reduction in prolonged antibiotics (P<0.0005). In cycle one, 107/205 patients (52.2%) received antibiotics compliant with Trust Guidelines, compared to 80/138 (58.0%) in cycle two.
Conclusion
Our proposed checklist, alongside antimicrobial stewardship education, prompts daily review of important patient parameters and results to significantly reduce inappropriate post-operative antibiotic prescribing. Promoting the sustained use of similar checklists by junior doctors and focusing on measures to improve uptake of pre-operative induction antibiotic guidelines is required to achieve further benefits.
Keywords: Antibiotic decision-making, Antibiotic prophylaxis, Quality improvement, Checklist, Surgery
Introduction
Antibiotics have revolutionised the reach of surgical intervention, both by treating and preventing infection-related complications. However, inappropriate antibiotic use is increasingly leading to healthcare associated multi-drug resistant (MDR) infections. MDR infections cause 25,000 deaths per year in Europe and are associated with greater mortality and economic burden [1,2]. Inappropriate antibiotic prescribing is a key driver of antimicrobial resistance, which presents one of the most pressing global health challenges [1].
The World Health Organisation recommends a single dose of pre-operative antibiotic as optimal prophylaxis to reduce surgical site infection (SSI), whilst causing the least possible impact on patient microbiota and antimicrobial resistance [3]. Existing antibiotic prescribing guidelines aim to ensure a standardised approach to effective antibiotic choice according to local resistance patterns. However, significant variability in adherence to surgical prophylaxis guidelines exists globally [4], with concerns this poor compliance is mirrored locally. Surveillance in Northern Ireland suggests a large proportion of antibiotic prescribing in hospitals is inappropriate, highlighting prolonged surgical prophylaxis as a particular issue [5]. Possible reasons for poor compliance include poor awareness of guidelines and practising habitual prescribing based on previous clinical experiences [6].
Without consistent and sustained antimicrobial stewardship, we face losing the ability to practice safe surgery. Antimicrobial stewardship interventions, particularly those providing direct feedback and education for prescribers, have been shown to increase compliance with antibiotic guidelines and reduce hospital length of stay [6]. The success of ward-round checklists in quality improvement has been increasingly recognised, ensuring daily review of key post-operative measures [7] and reduced risk of iatrogenic infections following procedures [8].
We evaluated the baseline adherence to surgical antibiotic guidelines in a tertiary hospital and explored the attitudes of healthcare professionals underpinning poor prescribing practices. Based on findings, a three-pronged quality improvement intervention comprising 1) education, 2) ward-round checklist and 3) heightened guideline accessibility was implemented to successfully improve antimicrobial prescribing.
Methods
This mixed-methods study was conducted at a single National Health Service hospital between 2018-2020.
Qualitative data
Face-to-face interviews were conducted between 2018 -2019 by EC with fourteen members of the general surgery multi-disciplinary team [9,10]. Using open-ended questions, interviews explored participants' views and experiences of antibiotic prescribing in surgery and the key factors that influence decision-making. Interviews were recorded, anonymised and transcribed verbatim. A thematic analysis was conducted manually. Findings were organised into broad analytical, intermediate descriptive and detailed coded sub-themes of perceptions surrounding antibiotic use and the influence of team dynamics.
Pre-intervention anonymous online surveys assessed surgeons' prescribing practices and understanding of antibiotic guidelines. Questions were developed based on contextual information from face-to-face interviews and piloted with two junior doctors. Survey invitation was sent via email and responses were collected over six weeks. A similar anonymous survey for anaesthetists was developed based on responses from surgeons and piloted with two junior doctors. Survey participation occurred at an anaesthetic departmental meeting.
The intervention (Figure 1)
Figure 1.
Descriptive infographic of the three-stage intervention, detailing the daily ward-round checklist ‘ABBDDOMM’.
Education
Four educational sessions were delivered throughout the two-year period to three different staff groups. Each session was delivered as a PowerPoint presentation, using interactive questions to the audience, followed by discussion to gain feedback from general surgeons, anesthetists and junior doctors on perceived barriers to good antimicrobial prescribing and suggestions for improvement. All sessions included evidence on antimicrobial resistance and addressed knowledge gaps identified in the anonymous surveys, for example the impact of antibiotics on SSI risk. Sessions also covered the initial results of the QI project, an introduction to the ABBDDOMM checklist, and guidance on access to the Trust antibiotic guidelines via the mobile application.
Sessions one and two were delivered after cycle one. Session one was presented at a general surgery morbidity and mortality meeting to around 50 members of the general surgery department including consultants, registrars, junior doctors and nurse practitioners. Session two was presented at a departmental anaesthetist meeting, to around 30 anaesthetic consultants and registrars. Anaesthetist survey responses were collected during this session. Session three was delivered prior to checklist implementation at an induction session for new junior doctors in general surgery (seven doctors), focusing on the introduction and use of the checklist in ward round documentation. Session four occurred following cycle two at another general surgery morbidity and mortality meeting, with an audience of around 50 general surgeons and junior doctors.
The ‘ABBDDOMM’ checklist
Knowledge from interview s provided contextual information and formed a thematic basis for the fundamentals of a simple, user-friendly intervention. Daily ward rounds were observed to assess the frequency of review of patient parameters. The acronymous ward round checklist ‘ABBDDOMM’ was developed to include parameters most commonly assessed by surgeons, alongside microbiological parameters: A (Antibiotics), B (Blood results), B (Bowels), D (Drain output), D (Diet), O (Observations), M (Mobility) and M (Microbiology results). The checklist is displayed in Appendix 1. Various iterations were piloted by one junior doctor (GP) during ward rounds, with input from senior surgeons. Checklist usability was piloted with four junior doctors for two weeks before implementation.
Promotion of trust antimicrobial guidelines
General surgeons, anaesthetists and junior doctors received an education session on how to access the Trust antimicrobial guidelines. Based on anaesthetist feedback, QR codes (easily scanned by mobile phone devices with cameras) for point-of-care guideline reference will be implemented in cycle three of this project.
Quantitative data
Cycle one reviewed surgeries undertaken between 1st January and 28th February 2019. Cycle two reviewed surgeries undertaken between 1st January and 28th February 2020, following intervention. All patients over 18-years-old who had intra-abdominal surgery during this period were included. The setting was a specialist tertiary centre for bariatric surgery with independent guidelines, therefore bariatric surgeries were excluded due to comparison limitations with the local Trust and national surgical standards. There is no existing guideline for antibiotic prophylaxis in diagnostic laparoscopy, therefore diagnostic laparoscopies were also excluded from analysis. In cycle one, 205 surgeries were included for analysis and 138 in cycle two.
Data collection
Patients were identified using theatre lists. Retrospective data was collected using online patient notes and prescription charts. Information on the choice and duration of antibiotics prescribed at anaesthetic induction and the surgery characteristics were collected. Data on SSI was collected for 30 days post-operatively. SSIs were graded according to the ASEPSIS score as mild, moderate or severe [11]. Microbiology, culture and sensitivity (MCS) results were correlated with any antibiotic used for treatment. Identical data was collected 12 months apart, with additional data on the utilisation of the checklist in ward round notes during cycle two.
Data analysis
Antibiotic choice and duration used at induction was compared to the Trust antibiotic guidelines to assess compliance. Continued prescriptions of antibiotics on the ward, and any documented indications, were analysed.
Data was stored and analysed using Microsoft Excel. P-values and confidence intervals were calculated using the Chi-squared test, using Medcalc Statistical Software Version 19.8.
Results
Surgery characteristics
Surgery characteristics are detailed in Table I.
Table I.
Characteristics of surgical procedures performed in Cycles One and Two
| Procedure Type | Cycle 1 |
Cycle 2 |
||||
|---|---|---|---|---|---|---|
| Elective (n=116) | Emergency (n=89) | Total (n=205) | Elective (n=91) | Emergency (n=47) | Total (n=138) | |
| Appendicectomy | 5 | 46 | 51 | 1 | 23 | 24 |
| Cholecystectomy | 21 | 4 | 25 | 12 | 4 | 16 |
| Trauma exploration | 0 | 10 | 10 | 0 | 5 | 5 |
| Hernia repair | 62 | 8 | 70 | 35 | 2 | 37 |
| Gastroduodenal surgery | 5 | 11 | 16 | 6 | 4 | 10 |
| Colorectal surgery | 24 | 9 | 33 | 37 | 9 | 46 |
Antibiotic choice: prescription at anaesthetic induction
The choice of antibiotic regimen used for all surgeries, in comparison to the Trust guideline, are listed in Table II.
Table II.
The choice of antibiotic regimens used for different categories of surgery during Cycle One and Cycle Two. ∗For patients with penicillin-allergy, gentamicin is substituted for cefuroxime in all guidelines recommending cefuroxime
| Type of Surgery | Guideline | Antibiotics administered | Number |
|
|---|---|---|---|---|
| Cycle 1 | Cycle 2 | |||
| Appendicectomy | Cefuroxime∗ and Metronidazole | Cefuroxime and Metronidazole | 41 | 18 |
| Cefuroxime | 0 | 2 | ||
| Co-amoxiclav | 7 | 3 | ||
| Co-amoxiclav and Metronidazole | 0 | 1 | ||
| Ceftriaxone and Metronidazole | 1 | 0 | ||
| Clindamycin | 2 | 0 | ||
| Laparoscopic cholecystectomy | Cefuroxime and Metronidazole | Cefuroxime and Metronidazole | 6 | 6 |
| Co-amoxiclav | 7 | 4 | ||
| Cefuroxime | 7 | 4 | ||
| Clindamycin | 3 | 0 | ||
| Ciprofloxacin | 2 | 0 | ||
| Amoxicillin and Clarithromycin | 0 | 1 | ||
| None | 0 | 1 | ||
| Abdominal trauma | Cefuroxime and Metronidazole | Cefuroxime and Metronidazole | 3 | 4 |
| Co-amoxiclav | 5 | 0 | ||
| Gentamicin | 0 | 1 | ||
| Vancomycin | 1 | 0 | ||
| Hernia repair with mesh | Cefuroxime | Cefuroxime | 14 | 8 |
| Cefuroxime and Metronidazole | 20 | 6 | ||
| Co-amoxiclav | 17 | 10 | ||
| Clindamycin | 4 | 1 | ||
| Ciprofloxacin | 1 | 0 | ||
| Vancomycin | 0 | 2 | ||
| Ceftriaxone and Metronidazole | 1 | 0 | ||
| None | 4 | 1 | ||
| Hernia repair without mesh | No prophylaxis | None | 2 | 1 |
| Co-amoxiclav | 3 | 2 | ||
| Cefuroxime | 2 | 6 | ||
| Ceftriaxone and Metronidazole | 1 | 0 | ||
| Gastroduodenal surgery | Cefuroxime and Metronidazole | Cefuroxime and Metronidazole | 9 | 8 |
| Ciprofloxacin | 1 | 0 | ||
| Metronidazole | 1 | 0 | ||
| Clindamycin | 1 | 0 | ||
| Cefuroxime, Metronidazole and Gentamicin | 0 | 2 | ||
| Gentamicin and Metronidazole | 1 | 0 | ||
| Cefuroxime, Metronidazole and Fluconazole | 2 | 0 | ||
| Colorectal surgery | Cefuroxime and Metronidazole | Cefuroxime and Metronidazole | 25 | 31 |
| Co-amoxiclav | 1 | 1 | ||
| Cefuroxime | 0 | 1 | ||
| Co-amoxiclav and Metronidazole | 2 | 0 | ||
| Co-amoxiclav and Gentamicin | 0 | 1 | ||
| Clindamycin and Metronidazole | 1 | 1 | ||
| Gentamicin and Metronidazole | 0 | 7 | ||
| Gentamicin, Metronidazole and Ciprofloxacin | 1 | 0 | ||
| Gentamicin | 0 | 1 | ||
| Vancomycin | 0 | 1 | ||
| Metronidazole | 1 | 0 | ||
| Piperacillin-tazobactam | 0 | 2 | ||
| Meropenem | 1 | 0 | ||
| Linezolid | 1 | 0 | ||
Bold text identifies the number of procedures performed using Trust recommended antibiotics.
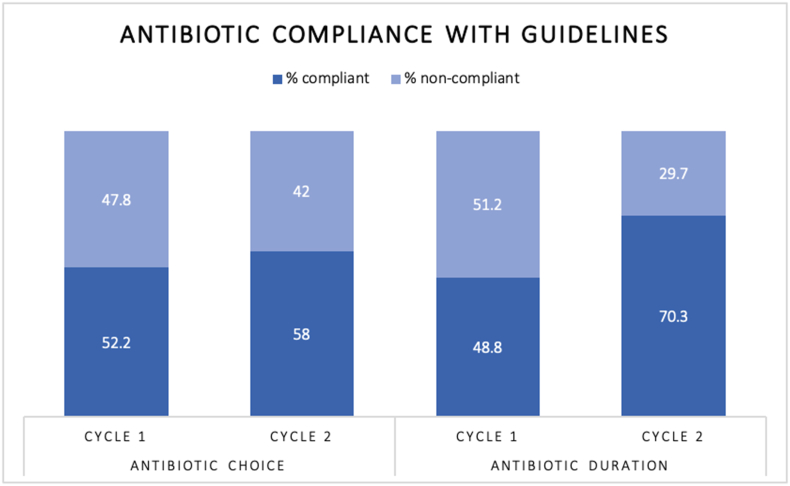
Cycle one: In 107/205 operations (52.2%), the correct choice of antibiotics according to Trust guidelines was used. In two of these operations, a non-adherent antibiotic had been advised by a microbiologist, therefore were considered compliant. In 98/205 procedures (47.8%), the antibiotics used were non-compliant with Trust guidelines.
Cycle two: In 80/138 operations (58%), the correct choice of antibiotics according to Trust guidelines was used. In 58/138 procedures (42%), the antibiotics used were non-compliant with Trust guidelines.
This demonstrates an increase in uptake of Trust guidelines by 5.8% (CI -4.9%–16.2%, p-value 0.29), see Figure 2.
Figure 2.
Comparison of compliance to Trust antibiotic guidelines for antibiotic choice and duration between Cycles One and Two.
Antibiotic duration: post-operative prescriptions
Cycle one: 100/205 patients (48.8%) received an extended course of antibiotics (>one dose at induction or >two doses for operations four hours or longer). In 60 of these patients (60%) there was a clear indication for prolonged antibiotic use. Forty (66.7%) of given indications were for intra-operative findings including purulent fluid, abscess or perforated viscus. Eight (13.3%) were prophylaxis for contaminated wounds or foreign bodies. Twelve (20%) were for intercurrent active infection: eight patients had intra-abdominal sepsis on admission requiring emergency surgery, two patients had elective surgeries but became febrile and septic post-operatively and two elective patients were being treated for intercurrent infection (cellulitis and urinary tract infection). In 40 (40%) of the 100 patients receiving prolonged antibiotics there was no apparent or documented indication. In seven of these cases (17.5%), antibiotics were not discontinued despite explicit instruction to in the operation note.
Cycle two: 41/138 patients (29.7%) received extended antibiotics. In 16 of these patients (39%) there was a clear indication documented for a prolonged duration of antibiotics; ten (62.5%) for intra-operative findings, four (25%) for prophylaxis of contaminated wounds and two (12.5%) for intercurrent infection (emergency procedures for patients with intra-abdominal sepsis). In cycle two, 70.3% of patients received the correct duration of antibiotics compared to 48.8% in cycle one, demonstrating an increase in compliance by 21.5% (CI 10.9%–31.2%, p-value 0.0001).
Surgical site infections
Cycle one: 18/205 operations (8.8%) were complicated by SSI, of which seven (38.9%) were elective and 11 (61.1%) were emergency procedures. Twelve (66.7%) occurred following open surgery and six (33.3%) occurred at sites of laparoscopic surgery. Two patients developed a collection in addition to wound infection. Eleven infections were classified as mild, five as moderate and two as severe.
Eleven patients (61.1%) were started on antibiotics. Nine of those started on antibiotics (81.8%) had swabs taken for MCS. Antibiotics were appropriate following MCS results in three patients (33.3%): in one patient antibiotics were changed based on resistance, and in two patients there was no need to change antibiotics. In six patients (66.6%), antibiotics were not appropriately changed following MCS results that demonstrated resistance to the prescribed antibiotic.
Cycle two: eight of 138 operations (5.8%) were complicated by SSI, of which four (50%) were elective and four (50%) were emergency procedures. Six (75%) occurred following open surgery and two (25%) occurred at sites of laparoscopic surgery. Three patients developed wound infections, four developed collections and one patient developed both. Of the four wound infections, one was classified as a “disturbance of wound healing”, one as a minor SSI and two as moderate. Following intervention there was a reduction in SSI rate of 3.0% (CI -3.1%–8.5%), p-value 0.30.
One patient remained in intensive care post-operatively, under the care of multiple teams. Given inability to access intensive care online patient notes, conclusions could not be accurately drawn on the management of the SSI, therefore this patient was excluded from further analysis of SSI data. All seven other patients had swabs or cultures taken for MCS. Six patients (85.7%) were treated with antibiotics for SSI. Antibiotics were appropriate following MCS results in six patients (85.7%); in five patients antibiotics were changed based on resistance, and in one patient there was no need to change antibiotics. In one patient, antibiotics were not thought clinically indicated but the subsequent wound swab grew klebsiella with resistance; there is no documented evidence of this result being reviewed by the surgical team. Table III summarises the positive microbiology, culture and sensitivity results for patients with SSI.
Table III.
Summary of positive microbiology, culture and sensitivity results for patients with surgical site infection (SSI)
| Patient | Antibiotics used at induction | Antibiotics prolonged Y/N | Antibiotics used to treat SSI | Culture source | Organism grown | Sensitivity | Resistance | |
|---|---|---|---|---|---|---|---|---|
| sCycle 1 | A | Cefuroxime, Metronidazole | Y | Cefuroxime, metronidazole | Wound exudate | Escherichia coli | Amoxicillin Co-amoxiclav Metronidazole |
None reported |
| Wound swab | Pseudomonas aeruginosa | None reported | None reported | |||||
| B | Cefuroxime, Metronidazole | Y | Co-amoxiclav, switched to ciprofloxacin | Wound swab 1 | Pseudomonas aeruginosa | Ceftazidime Ciprofloxacin Gentamicin |
None reported | |
| Escherichia coli | Amoxicillin Co-amoxiclav Gentamicin |
None reported | ||||||
| Wound swab 2 | Pseudomonas aeruginosa | Amikacin Ceftazidime, Ciprofloxacin Gentamicin Meropenem |
None reported | |||||
| Escherichia coli | Amoxicillin Ciprofloxacin Gentamicin Co-amoxiclav Co-trimoxazole Ertapenem |
None reported | ||||||
| Wound swab 3 | Pseudomonas aeruginosa | Amikacin Ciprofloxacin Gentamicin |
None reported | |||||
| C | Cefuroxime, Metronidazole | Y | Co-amoxiclav | Wound swab | Staphylococcus aureus | Erythromycin Flucloxacillin Tetracycline |
Penicillin | |
| Pseudomonas aeruginosa | Ciprofloxacin | None reported | ||||||
| D | Cefuroxime, Metronidazole | Y | Co-amoxiclav | Wound swab 1 | Pseudomonas aeruginosa | Ceftazidime Ciprofloxacin Gentamicin Meropenem |
None reported | |
| Wound swab 2 | Pseudomonas aeruginosa | Ceftazidime Ciprofloxacin Gentamicin Meropenem |
None reported | |||||
| E | Cefuroxime, Metronidazole | Y | Co-amoxiclav | Wound swab | Methicillin resistant staphylococcus aureus (MRSA) | Clindamycin Tetracycline Mupirocin Trimethoprim |
Flucloxacillin Penicillin |
|
| F | Cefuroxime, Metronidazole | Y | Cefuroxime, Metronidazole, switched to Co-amoxiclav | Collection fluid | Escherichia coli | Ciprofloxacin Co-trimoxazole |
Amoxicillin Co-amoxiclav Piperacillin-tazobactam |
|
| Rectal wound swab | Escherichia coli | Amikacin Ciprofloxacin Co-trimoxazole Ertapenem Gentamicin Tigecycline |
Amoxicillin Piperacillin-tazobactam Co-amoxiclav Temocillin |
|||||
| G | Clindamycin | N | Not clinically indicated | Wound swab | Pseudomonas aeruginosa | None reported | None reported | |
| Cycle 2 | H | Gentamicin, Metronidazole | Y | Meropenem | Collection fluid | Escherichia coli | Ciprofloxacin Gentamicin Meropenem |
Amoxicillin Ciprofloxacin |
| I | Cefuroxime, Metronidazole | N | Not clinically indicated | Wound swab | Klebsiella pneumoniae | Ciprofloxacin Gentamicin Meropenem |
Ceftazidime Ceftriaxone Co-amoxiclav Co-trimoxazole |
|
| J | Vancomycin | Y | Co-amoxiclav “to cover chest” | Wound swab 1 | Escherichia coli, ESBL | Amikacin Co-trimoxazole Temocillin |
None reported | |
| Sputum | Pseudomonas aeruginosa | Ciprofloxacin Piperacillin-tazobactam |
None reported | |||||
| Wound swab 2 | Escherichia coli | None reported | None reported | |||||
| K | Cefuroxime, Metronidazole | Y | Piperacillin-tazobactam | Collection fluid | Pseudomonas aeruginosa | Amikacin Ceftazidime Gentamicin Piperacillin-tazobactam |
Ciprofloxacin | |
| L | Gentamicin, Metronidazole | N | Meropenem, Vancomycin | Abdominal drain fluid | Enterococcus species | Linezolid Tigecycline Vancomycin |
Amoxicillin Gentamicin |
|
| Blood culture | Coagulase negative staphylococcus | None reported | None reported |
Use of the ABBDDOMM checklist
During cycle two, 103 patients were admitted to the surgical ward post-operatively. 76 of these patients (74%) had the ABBDDOMM checklist used in the ward round documentation at least once during their inpatient stay, and 38 patients (37%) had the checklist used in the ward round documentation every day. Antibiotics were reviewed daily 100% of the time when ABBDDOMM was used, and 30% of the time when not used.
Surgical team perceptions of antibiotic use
Face-to-face interviews
Fourteen face-to-face interviews were conducted with multidisciplinary healthcare professionals within the general surgery team (two consultant surgeons, one consultant physician, six specialty registrars, two foundation doctors, one pharmacist and two advanced nurse practitioners). For complete thematic analysis results, with analytical, descriptive and sub-themes, see Table IV.
Table IV.
Overarching analytical themes, descriptive themes and detailed sub-themes describing current barriers to surgical team antibiotic prescribing
| Analytical Themes | Descriptive Themes | Sub-themes |
|---|---|---|
| Uncertainty about which teams take overall responsibility for surgical antibiotic prescribing | Reliance on senior surgeons on the advice given by microbiology teams | “Surgeons have quite significantly advocated responsibility to the microbiologists…they are the experts…I just let them make the call” [consultant surgeon] “Personally, I usually ask the juniors to contact microbiology” [consultant surgeon] “In order to go with the guidelines because I don't know, the guidelines for antibiotics change every day sometimes, so I know that I am not updated all the time, from time to time I check them, but especially when we have somebody who we think that the antibiotics are not working properly I will ask microbiology opinion before we change antibiotics and give them. And I think it works to be honest because I have seen improvement of patients after changing the antibiotics according to microbiology.” [consultant surgeon] |
| Ambiguity between anaesthetists and surgeons about who has overall responsibility for the choice of antibiotic pre-operatively | “For elective cases usually I ask for a standard antibiotic or sometimes if the anaesthetist is more updated than me on the guidelines of the hospital they say, oh, shall we give this one instead of the other? And I will say, yes, if it's, if that's the guidelines currently I don't mind. Now if we have a sick patient and I need some extra antibiotics personally I will ask them and say, give please a dose of gentamicin because we have pus in the abdomen from a perforated appendix or whatever.” [consultant surgeon] “I know we'll have a discussion, the anaesthetist will ask me, and they will say do you have a strong opinion? And if I do not give an opinion, they will say, OK, well I'm going to give this. Right, so the anaesthetist will sometimes make a choice, but generally they will say, do you want antibiotics, yes or no, and I will say yes, or I will say no, and they won't give them unless I say yes or say no. And then once I've said yes, then we might have a, we might have a more flexible discussion about the sort that might give.” [consultant surgeon] “It's definitely the surgeon's because they will always ask us, do you want antibiotics? So it's always up to you.” [surgical registrar] |
|
| Apparent inconsistencies in the antibiotic practices of senior surgeons from the perspective of junior doctors | “I don't know how the surgeons decide how long to give antibiotics for because for some patients it's two doses post-op and I'm like, huh? Because the last patient with a similar condition had five days' worth. It's not clear how they make these decisions about prophylactic antibiotics” [junior doctor] “So those are the standard things [antibiotics] we go for, so we assume that the juniors are able to manage that…And then I think because the [antibiotic] guidelines are so good we rely on the juniors being able to follow them.” [surgical registrar] |
|
| Surgical cultural barriers to good antibiotic prescribing | The historic beliefs and previous experiences of senior surgeons driving decision-making rather than contemporaneously adapted guidelines | “I have my personal favourites and I give them simply because I trust them and I've used them [regardless of what the policy is].” [consultant surgeon] “I honestly don't refer to it [guidelines] because I know the standard treatments.” [surgical registrar] “I think that most of the Surgeons don't use antibiotic guidelines a lot. They are based more on practice and experience.” [consultant surgeon] “And actually that's compounded by the fact that most surgeons don't have expert knowledge of microbiology. Most surgeons basically are fairly dogmatic in their prescribing practices. They prescribe the handful of antibiotics that they know, and they don't really understand the fundamental clinical science in what they're doing.” [consultant surgeon] |
| Surgical culture driving prioritising short-term outcomes over long-term effects of antibiotics | “Surgery is an incredibly defensive branch of medicine” [consultant surgeon] “I know that my patient gets a wound infection for example, my case will be discussed at a Morbidity and Mortality meeting. I've got one tomorrow and my patients are on it, and infection is an outcome that's discussed, it affects my data … and my outcome data will be on a website, so yeah I'm going to practise pretty defensive medicine, absolutely.” [consultant surgeon] “I think because we're so careful and cautious about complications, we're very pro-covering for post-op complications” [surgical registrar] “They don't really care what the evidence is, and they don't really care what the problems with antibiotic resistance are, because, to be honest it's their patient, if they're going to get an infection they'd rather prescribe the antibiotic.” [consultant surgeon] “To be honest I don't think about antibiotic resistance.” [consultant surgeon] “Often antibiotics aren't prioritised” and “it's not that Surgeons don't think antibiotics are important, it's just not high on their priorities” [consultant surgeon] “I think people know globally that we shouldn't be over using them because of that issue, but I think the standard thing is if you have raised inflammatory markers, or a temperature, or both, or with appendicitis that isn't going to get to theatre, you start them on antibiotics”. [surgical registrar] “They're not seeing the knock-on effect of what happens to them [the patients] after a blast of Meropenem.” [surgical pharmacist] “Yeah, yeah, we couldn't do it. So but that whole culture is totally embedded in our practice, and to change that is extremely difficult.” [consultant surgeon] “It's not that surgeons don't think antibiotics are important, it's just not high on their priorities” [consultant surgeon] |
|
| Surgical knowledge barriers to good antibiotic prescribing | Limitations of knowledge of antibiotic theory | “They prescribe the handful of antibiotics that they know, and they don't really understand the fundamental clinical science in what they're doing. So asking a surgeon to go onto an antibiotic ward round, it's a bit like, you might as well be asking them to go onto, I don't know, a cardiology ward round. They just don't have any working knowledge of it. They can probably tell you what a gram positive or a gram-negative bacteria is. They can probably tell you headline functions of the major antibiotics, but beyond that very little. So I think there's a lack of working knowledge and a lack of interest which is the major barriers.” [consultant surgeon] |
| Difficulties in remembering to review antibiotics | “Probably 60–80% of the time they have prescribed anti-infectives that are appropriate. Now, what they are not so good at is the review and follow-up of antibiotics that are started. [surgical pharmacist] “[Surgeons] often forget to review the antibiotics if infection was not the primary reason for admission.” [consultant surgeon] “We as consultants sometimes forget they're on antibiotics and forget to review whether they should still be on them or not.” [consultant surgeon] “[Surgeons] don't actually very regularly ask how many days' worth they've had, which is strange” [junior doctor] |
|
| Physical barriers to good antibiotic prescribing | Lack of point-of-care access or awareness of guidelines | “The guidelines for antibiotics change every day, from time to time I check them.” [consultant surgeon] “Sometimes we don't have time to look at the guidelines”. [consultant surgeon] “Yeah, this [the Antibiotic Guideline Smartphone App] was really helpful for me, when I was a registrar it was very, very helpful.” [consultant surgeon] |
| Discrepancy in awareness between senior and junior surgical colleagues of the guidelines | “I'm not convinced that all of them know that there is a treatment infection guideline.” [surgical pharmacist] “Really useful. I always look at the policy before phoning microbiology.” [junior doctor] |
|
| Physical layout of Trust antibiotic charts | “When you used to have the physical drug card there you would look at it and say, this patient has been on 20 days of Tazocin, what are we doing?” [surgical registrar] |
Anonymous survey findings
Thirteen responses were collected from the surgical team (seven consultants, four registrars, and two junior doctors). Cited reasons for not using the guidelines were they are “not easily accessible” and “vague and non-specific”. One surgeon was not aware of the Trust guidelines. Eleven surgeons stated the correct number of doses of induction antibiotic prophylaxis was one. Seven out of 13 surgeons either did not agree that prolonged antibiotic use does not reduce the risk of SSI, or did not know: one did not agree, and six did not know, whilst six surgeons did understand that prolonged antibiotic use does not reduce SSI risk. When asked to cite indications for prolonged antibiotics post-operatively, surgeons gave examples of intra-operative findings such as perforation, collection, contaminated wounds, concurrent sepsis and patient factors including diabetes and smoking status.
Twenty-five anaesthetic responses were collected (thirteen consultants, eight specialty registrars, four junior doctors). Twenty-one reported anaesthetists prescribed the prophylactic antibiotics at induction, whilst four reported the surgeons did. Twenty-three were unsure who should actually be responsible for prescribing the prophylactic antibiotics. All anaesthetists were aware of Trust guidelines for antibiotic prescribing in surgery prophylaxis. None of the anaesthetists had used the Trust antibiotic prescribing app that is available on their phones. Eighteen anaesthetists reported one antibiotic dose should be given at surgery induction, depending on the surgery duration, whilst seven thought multiple doses should be given. Suggestions for interventions to improve adherence included posters of the guidelines displayed in induction rooms and availability of a point-of-care guideline reference on mobile phones.
Discussion and recommendations
This study highlights two crucial time-points where antibiotic prescribing practices can be targeted in general surgery: 1) at pre-operative induction and 2) post-operative prescribing of antibiotics on the ward. A small improvement in the compliance to guidelines for choice of antibiotics at induction was observed between cycles one and two, and a significant improvement was seen in prolonged post-operative prescribing of antibiotics. There was no significant change in SSI rates post-intervention, as expected in line with extensive evidence in this area, [3,12] and incidence remained around the national average for small bowel and colorectal surgery of 6.6% and 8.3% respectively [12]. Finally, this project furthers understanding of cultural and physical barriers to appropriate antibiotic prescribing at a tertiary NHS hospital, and investigates end-user intervention suggestions to promote future antimicrobial stewardship.
Despite improvement in guideline compliant pre-operative antibiotic choice, the overall compliance rate in cycle two remained poor. A similar audit, communicated locally, of antibiotic prescribing by counterpart medical teams demonstrated 100% of prescribed antibiotics were adherent to Trust guidelines, exposing disparity between medical and surgical practices. In contrast, inappropriate post-operative antibiotic prescribing by surgeons is mirrored in international studies; for example, Vessal found only 8 of 106 patients at an Iranian hospital received the recommended pre-operative prophylactic antibiotic regimen, and antibiotic treatment was extended in 83% of cases despite only being indicated in 37% [13,14].
Most surgeons acknowledged the need for rapidly changing antibiotic guidelines according to resistance patterns, yet poor surgical adherence to guidelines implies decision-making is influenced by other factors. An interesting observation during interviews was the ambiguity between surgeons and anaesthetists regarding who takes responsibility for pre-operative antibiotic prescribing decisions at induction, which may stem from observed reluctance to challenge inappropriate behaviour within the hierarchical dynamics of an operating team [15]. Moreover, some surgeons admitted prescribing was driven by consideration for short-term individual patient outcomes rather than the negative consequences of extended antibiotic use. The McDonnel group theorise two explanations for this attitude; 1) the action and consequence are so widely separated in time that the relationship goes unrecognised, and 2) surgeons acting in “rational self-interest” feel their individual contribution to antimicrobial resistance is insignificant [16]. Troughton et al. identified that surgeons' fear of adverse patient outcomes, including SSI, negatively impacting on their professional reputation is a salient influence as surgeons feel “personal accountability” for SSI rates, despite a consensus that SSI prevention is a team responsibility [15]. Similarly, Charani describes a “defensive antibiotic decision-making” pattern seen in surgical antibiotic prescribing, which is primarily driven by surgical outcome data and the short-term procedure success [10]. Understanding the cultures underpinning poor prescribing is essential to facilitating sustainable change; our exploration of local attitudes and perceptions to target intervention may in part explain the project's success. Based on conflicting perceptions of prescribing responsibility for infection-related complications, we recommend clinical accountability for pre-operative prescribing should be defined and agreed between general surgeons and anaesthetists.
Consistent uptake of the ‘ABBDDOMM’ checklist suggests it plays an important role in reducing inappropriate post-operative antibiotic prescribing, particularly given the much greater improvement observed in duration of antibiotics compared to pre-operative choice of antibiotic. Microbiology results were reviewed and appropriately acted upon more frequently in cycle two. Implementation of the ABBDDOMM checklist may have contributed to this improvement, but it is difficult to determine causation in this small cohort, particularly as several patients with complex SSIs in cycle two had extensive microbiology team involvement in their care. Wider literature demonstrates the potential for point-of-care reference applications to improve guideline-driven prescribing, with Yoon et al. finding an 8% increase in adherence to guidelines for treating urinary tract infections following introduction of a smartphone application [17]. Whilst a smartphone application exists within this Trust, our interviews revealed limited usage, suggesting poor awareness or barriers to application accessibility. Influenced by feedback from anaesthetists, cycle three of this project will introduce QR codes into anaesthetic induction rooms to provide instant access to the Trust guidelines, potentially overcoming any accessibility problems and focusing on increasing uptake of pre-operative antibiotic guidelines.
Study limitations
This is a single-centre study focusing on one surgical department, therefore data may not be representative of other surgical departments within the Trust or nationally.
Given that there were multiple interventions implemented at the same time, it is difficult to ascertain the relative impact of any one of these on the observed outcomes. Since the checklist targeted antibiotic prescribing on wards post-operatively, whereas education should additionally target prescribing at induction, it can be inferred that the checklist led to the greatest benefit seen in prolonged antibiotic prescribing. Alternative factors contributing to the observed improvements in compliance rates and post-operative antibiotic prescribing should also be considered, such as increased media coverage of antibiotic resistance, and the Trust switching to an entirely computerised prescribing system.
SSIs were only included if they aligned with the WHO definition [18], and if patients remained an inpatient or re-presented to hospital with SSI symptomology within 30-days post-operatively. The true SSI rate in our population may therefore be under-estimated, as we were unable to capture patients presenting to Primary Care. Online notes were reviewed retrospectively, and so interpretation of SSI severity and management is limited by the quality of the documentation. Furthermore, larger sample sizes are needed to assess the impact of this intervention on SSI rate.
Conclusions
Improved adherence to surgical prophylaxis antibiotic guidelines and reduced post-operative antibiotic use was observed following introduction of the ‘ABBDDOMM’ checklist, alongside education. This checklist could be easily replicated as a simple and cost-effective antimicrobial stewardship intervention, specifically focusing on the decision-making dynamics of surgical teams. Further work is needed to establish interventions that target pre-operative antibiotic choice, which was less impacted by our intervention; specifically the benefit of QR codes as a tool for point-of-care access to guidelines to improve guideline-driven antibiotic prescribing for surgical prophylaxis.
Acknowledgements
We would like to thank the Trust general surgery and anaesthetic departments for their open and willing contribution to this study through participation in interviews and surveys.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Funding sources
Charani and Holmes acknowledge funding from the National Institute for Health Research, UK Department of Health [HPRU-2012-10047] in partnership with Public Health England.
Appendix 1.
The checklist “ABBDDOMM”, as presented to junior doctors for use in daily ward-round documentation:
A– Antibiotics
-
-
Review the type, route, dose, indication and intended duration for any prescribed antibiotics
B–Blood results
-
-
Review most recent full blood count (FBC), C-reactive protein (CRP), renal function and liver function. Review dose levels for monitored antibiotics.
B – Bowels
-
-
Review patient bowel frequency and form; assess for signs of post-operative ileus
D–Drains
-
-
Clinically review any post-operative drains or indwelling urinary catheters, and review output over 24 hours.
D – Diet
-
-
Review permitted diet and oral intake
O – Observations
-
-
Review trends in vital signs over the past 24 hours
M –Mobility
-
-
Review post-operative mobility and recovery
M – Microbiology Results
-
-
Review any microbiology, culture and sensitivity (MCS) results for blood cultures, wound cultures and intra-operative samples. This section also provided an opportunity to flag up any ‘sent’ samples that require chasing.
References
- 1.Laxminarayan R., Duse A., Wattal C., Zaidi A.K.M., Wertheim H.F.L., Sumpradit N. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13(12):1057–1098. doi: 10.1016/S1473-3099(13)70318-9. 2014. [published correction appears in Lancet Infect Dis. 2014 Jan;14(1):11] [DOI] [PubMed] [Google Scholar]
- 2.de Kraker M.E.A., Davey P.G., Grundmann H., on behalf of the BURDEN study group Mortality and Hospital Stay Associated with Resistant Staphylococcus aureus and Escherichia coli Bacteremia: Estimating the Burden of Antibiotic Resistance in Europe. PLoS Med. 2011;8(10) doi: 10.1371/journal.pmed.1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Guidelines for Safe Surgery (2009): safe surgery saves lives. Available at: http://whqlibdoc.who.int/publications/2009/9789241598552 eng.pdf [accessed on 14.10.2014]. [PubMed]
- 4.Gouvêa M., Novaes Cde O., Pereira D.M., Iglesias A.C. Adherence to guidelines for surgical antibiotic prophylaxis: a review. Braz J Infect Dis. 2015 Sep-Oct;19(5):517–524. doi: 10.1016/j.bjid.2015.06.004. Epub 2015 Aug 5. PMID: 26254691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aldeyab M.A., Kearney M.P., McElnay J.C., Magee F.A., Conlon G., MacIntyre J., ESAC Hospital Care Subproject Group A point prevalence survey of antibiotic use in four acute-care teaching hospitals utilizing the European Surveillance of Antimicrobial Consumption (ESAC) audit tool. Epidemiol Infect. 2012 Sep;140(9):1714–1720. doi: 10.1017/S095026881100241X. 2011. Epub 2011 Nov 24. PMID: 22115422. [DOI] [PubMed] [Google Scholar]
- 6.Davey P., Marwick C.A., Scott C.L., Charani E., McNeil K., Brown E. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2017;2(2) doi: 10.1002/14651858.CD003543.pub4. Published 2017 Feb 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishnamohan N., Maitra I., Shetty V.D. The surgical ward round checklist: improving patient safety and clinical documentation. J Multidiscip Healthc. 2019;12:789–794. doi: 10.2147/JMDH.S178896. Published 2019 Sep 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor J.E., McDonald S.J., Earnest A., Buttery J., Fusinato B., Hovenden S. A quality improvement initiative to reduce central line infection in neonates using checklists. Eur J Pediatr. 2017 May;176(5):639–646. doi: 10.1007/s00431-017-2888-x. Epub 2017 Mar 10. PMID: 28283785. [DOI] [PubMed] [Google Scholar]
- 9.Charani E., Tarrant C., Moorthy K., Sevdalis N., Brennan L., Holmes A.H. Understanding antibiotic decision making in surgery-a qualitative analysis. Clin Microbiol Infect. 2017 Oct;23(10):752–760. doi: 10.1016/j.cmi.2017.03.013. Epub 2017 Mar 21. PMID: 28341492. [DOI] [PubMed] [Google Scholar]
- 10.Charani E., de Barra E., Rawson T.M., Gill D., Gilchrist M., Naylor N.R. Antibiotic prescribing in general medical and surgical specialties: a prospective cohort study. Antimicrob Resist Infect Control. 2019;8:151. doi: 10.1186/s13756-019-0603-6. Published 2019 Sep 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson A.P., Treasure T., Sturridge M.F., Grüneberg R.N. A scoring method (ASEPSIS) for postoperative wound infections for use in clinical trials of antibiotic prophylaxis. Lancet. 1986 Feb 8;1(8476):311–313. doi: 10.1016/s0140-6736(86)90838-x. [DOI] [PubMed] [Google Scholar]
- 12.Public Health England . 2020. Surveillance of surgical site infections in NHS hospitals in England 2019 to 2020.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/945712/SSI_Annual_Report_2019_20.pdf Available at: [Google Scholar]
- 13.Vessal G., Namazi S., Davarpanah M.A., Foroughinia F. Evaluation of prophylactic antibiotic administration at the surgical ward of a major referral hospital, Islamic Republic of Iran. East Mediterr Health J. 2011 Aug;17(8):663–668. PMID: 21977569. [PubMed] [Google Scholar]
- 14.Machowska A., Sparrentoft J., Dhakaita S.K., StålsbyLundborg C., Sharma M. Perioperative antibiotic prescribing in surgery departments of two private sector hospitals in Madhya Pradesh, India. Perioper Med (Lond) 2019;8:10. doi: 10.1186/s13741-019-0121-3. [published correction appears in Perioper Med (Lond). 2019 Oct 28;8:12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Troughton R., Mariano V., Campbell A., Hettiaratchy S., Holmes A., Birgand G. Understanding determinants of infection control practices in surgery: the role of shared ownership and team hierarchy. Antimicrob Resist Infect Control. 2019 Jul 15;8:116. doi: 10.1186/s13756-019-0565-8. PMID: 31341614; PMCID: PMC6631607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonnell Norms Group Antibiotic overuse: the influence of social norms. J Am Coll Surg. 2008 Aug;207(2):265–275. doi: 10.1016/j.jamcollsurg.2008.02.035. Epub 2008 May 12. PMID: 18656057. [DOI] [PubMed] [Google Scholar]
- 17.Yoon C.H., Ritchie S.R., Duffy E.J., Thomas M.G., McBride S., Read K. Impact of a smartphone app on prescriber adherence to antibiotic guidelines in adult patients with community acquired pneumonia or urinary tract infections. PLoS One. 2019;14(1) doi: 10.1371/journal.pone.0211157. Published 2019 Jan 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO SSI surveillance protocol. 2018. https://www.who.int/infection-prevention/tools/surgical/SSI-surveillance-protocol.pdf [Google Scholar]