Summary
Ctenophores are a group of predatory macroinvertebrates whose controversial phylogenetic position has prompted several competing hypotheses regarding the evolution of animal organ systems. Although ctenophores date back at least to the Cambrian, they have a poor fossil record due to their gelatinous bodies. Here, we describe two ctenophore species from the Cambrian of Utah, which illuminate the early evolution of nervous and sensory features in the phylum. Thalassostaphylos elegans has 16 comb rows, an oral skirt, and an apical organ with polar fields. Ctenorhabdotus campanelliformis has 24 comb rows, an oral skirt, an apical organ enclosed by a capsule and neurological tissues preserved as carbonaceous films. These are concentrated around the apical organ and ciliated furrows, which connect to a circumoral nerve ring via longitudinal axons. C. campanelliformis deviates from the neuroanatomy of living ctenophores and demonstrates a substantial complexity in the nervous system of Cambrian ctenophores.
Subject areas: biological sciences, evolutionary biology, phylogenetics
Graphical abstract
Highlights
-
•
Two species of rare fossil ctenophores are described from the Cambrian of Utah
-
•
Fossil ctenophores preserve remains of nervous tissue and sensory structures
-
•
Neurological structures include an oral nerve ring and giant longitudinal axons
-
•
Cambrian ctenophores had a more complex neuroanatomy than living species
Biological sciences; Evolutionary biology; Phylogenetics
Introduction
Ctenophores are gelatinous, predatory animals that are key components of marine pelagic food webs (Purcell and Arai, 2001). Their evolutionary significance has recently been highlighted by their controversial phylogenetic position among metazoans inferred from phylogenomic data (Dunn et al., 2008; Pett et al., 2019; Ryan et al., 2010), which has cast great uncertainty on the relationships between the major animal clades. In analyses of molecular characters, ctenophores have been recovered as the sister group of all other metazoans (Dunn et al., 2008; Moroz et al., 2014), of a clade of cnidarians, placozoans, and bilaterians (Parahoxozoa) (Pisani et al., 2015; Ryan et al., 2010), or of Cnidaria alone (the clade Coelenterata) (Pett et al., 2019). Most of these competing phylogenies necessitate a complex pattern of convergent evolution of animal tissues and organs systems (Dohrmann and Wörheide, 2013; Dunn et al., 2015; Jékely et al., 2015). For example, the placement of ctenophores as the sister group of all other metazoans raises the possibility that the muscle and nervous systems of ctenophores and other complex animals have evolved independently (Jékely et al., 2015; Moroz et al., 2014).
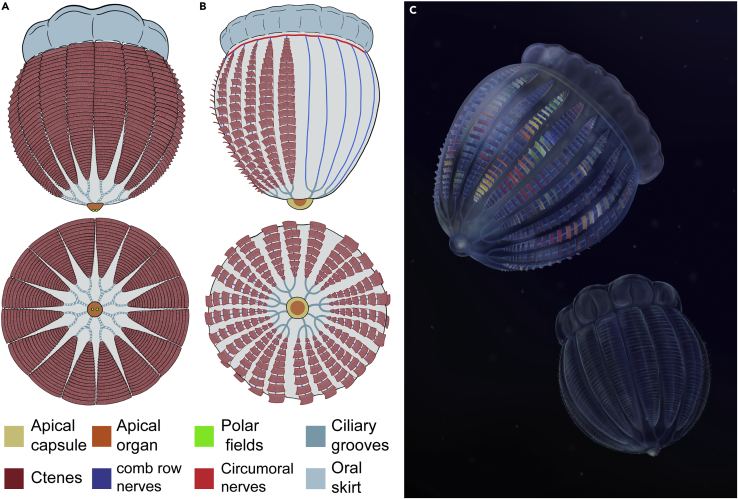
Living ctenophores possess a suite of unique morphological features that makes comparisons with extant members of other animal phyla difficult (Dunn et al., 2015); these include a biradial body symmetry, eight locomotive comb rows, a sensory apical organ, and a pair of tentacles with adhesive colloblasts. They possess a similar diploblastic body plan organization to cnidarians but also feature characters shared with bilaterians, including a functional through gut with two anal pores (Presnell et al., 2016) and mesoderm-like muscles (Martindale and Henry, 1999). Like cnidarians, extant ctenophores possess a relatively simple, diffuse nervous system. The nervous system of extant ctenophores consists primarily of mesogleal and ectodermal nerve nets (Jager et al., 2011; Moroz et al., 2014), and this pattern can be supplemented with functional specializations related to feeding, such as prominent nerves associated with the paired tentacles or sealing of the mouth in beroid ctenophores (Jager et al., 2011; Tamm and Tamm, 1995). The genus Euplokamis is unique among living representatives in the possession of “giant axons”, which underlie the comb rows and mediate a rapid escape swimming response (Mackie et al., 1992). Morphological phylogenetic analyses place ctenophores in various positions including as the sister group of cnidarians (Zhao et al., 2019), and within Bilateria (Nielsen et al., 1996), but never as the sister group of all other metazoans. Despite this uncertainty, morphological data do not necessarily require that the nervous system of ctenophores must have a common origin with that of other animals (Nielsen, 2019).
As they are soft-bodied, ctenophores are sparsely represented in the rock record. Fossil ctenophore species are mostly restricted to Cambrian Burgess Shale-type deposits (Conway Morris and Collins, 1996; Fu et al., 2019; Lerosey-Aubril et al., 2017; Ou et al., 2015), with a single record in the Ordovician (Young et al., 2012) and two species in the Devonian Hunsrück Slate (Stanley and Stürmer, 1987), although alternative interpretations of the Devonian specimens have been proposed (e.g. (Otto, 2000)). Cambrian ctenophores possess character combinations distinguishing them from any living species, including a greater number of comb rows (24 or as many as 80; Conway Morris and Collins, 1996), a sclerotized skeleton supporting the comb rows and/or the apical organ (well-developed in scleroctenophores; Ou et al., 2015), and a prominent circumoral structure known as the oral skirt (Conway Morris and Collins, 1996; Ou et al., 2015). Recently, a suite of Cambrian problematica with sessile polypoid lifestyles and radially arranged ciliated feeding organs have been interpreted as deep branches in the ctenophore stem group (Zhao et al., 2019), based primarily on the proposed homology of their feeding structures with the comb rows of ctenophores, and the presence of an internal organic skeleton comparable to that of scleroctenophores. Lastly, the Ediacaran fossil Eoandromeda has been interpreted as the earliest ctenophore, due to the presence of eight fold symmetry with spiraling arms and putative comb rows (Tang et al., 2011; although see Zhao et al., 2019 for an alternate view).
Here, we describe two ctenophore species from the Drumian (middle Cambrian) Marjum Formation of Utah. These specimens preserve exceptional details of the sensory and nervous systems and reveal the hitherto unrecognized complexity of the anatomical changes characterizing the early evolution of the ctenophore body plan.
Results
Systematic paleontology
Phylum: Ctenophora Eschscholtz 1829 Genus: Thalassostaphylos gen. nov.
Etymology: Genus name from Greek thalassa (sea) and staphylos (grape).
Type species:Thalassostaphylos elegans nov.
Diagnosis: Ctenophore with rounded body outline in lateral view and 16 broad comb rows that abut each other laterally. Comb rows terminate at the margin of an extensive oral skirt surrounding a large mouth opening. Oral skirt has a scalloped oral margin, with eight lobes present around its circumference. Apical sense organ with paired polar fields.
LSID: urn:lsid:zoobank.org:act:ACE08975-B2C8-4102-8599-0CB494E49E28.
Thalassostaphylos elegans sp. nov.
Etymology: From Latin elegans (elegant), in reference to the skirt and its scalloped margin.
Type material: UMNH.IP.6086 (holotype and only known specimen).
Locality and Horizon: House Range of western Utah, USA (see STAR Methods for locality details). Drumian strata (lower Ptychagnostus punctuosus Zone) of the middle part of the Marjum Formation, Miaolingian, Cambrian (see STAR Methods below).
Diagnosis: As for genus.
LSID: urn:lsid:zoobank.org:act:1B80E792-8624-47E8-A4AB-031D4A5023F0.
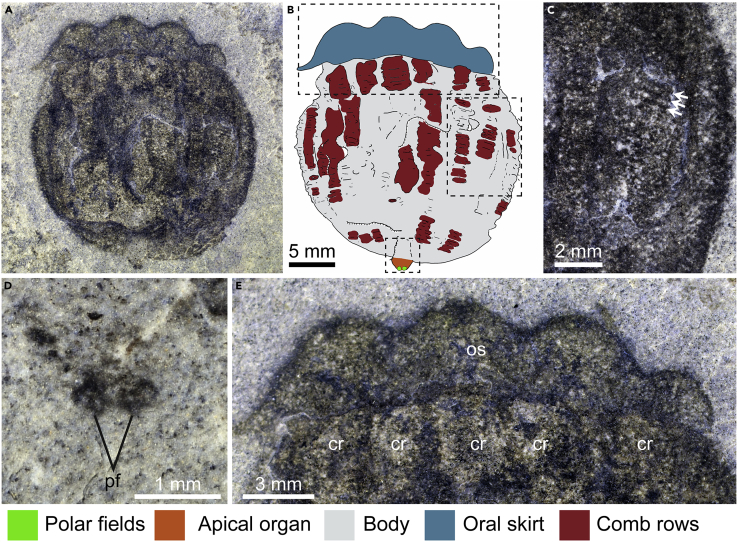
Description: The specimen of Thalassostaphylos elegans gen. et sp. nov (Figure 1) is 27 mm long along the oral-aboral axis and is preserved in a lateral view. The body is complete, with a maximum width of 24 mm, and preserves the comb rows (Figure 1C), the apical organ (Figure 1D), and a well-developed oral skirt (Figure 1E). Exclusive of the oral skirt and apical organ, the main body is sub-circular in outline but for a straighter margin along the oral skirt. The comb rows are expressed as longitudinally aligned patches on the main body and extend to the junction with the oral skirt. These comb rows are broad (∼3 mm wide at body center) and narrowly spaced (∼500 μm), and they exhibit transverse features interpreted as cushion plates. At least eight, probably nine, comb rows can be observed on the exposed surface of the body. However, the left margin of the specimen appears at least slightly disturbed (Figure 1A), and it is probable that the comb row visible in this region is from the other side of the body. Therefore, we reconstruct this taxon as possessing 16 comb rows in total, based on this observation, as well as the four-fold repetition of the scalloping of the oral skirt described below. The oral skirt is wide (2.8–3.7 mm) and its outer margin is scalloped, defining four lobes aligned with two comb rows each (Figure 1E). The apical organ projects ∼1.3 mm from the aboral surface and preserves two discrete dark dots, 450 μm wide and 420 μm long each. A potential interpretation of these features is that they represent statoliths; however they differ in size by an order of magnitude of statoliths of similarly sized living ctenophores (e.g. in Mnemiopsis; Tamm, 2014), as well as their position on the surface of, rather than within the apical organ. The number, position, and size of these dark dots are consistent with their interpretation as polar fields (Figure 1D). Based on these observations, we reconstruct Thalassostaphylos elegans as having a biradial symmetry, resulting from the superimposition of a bilateral symmetry (e.g. pair of polar fields) and an octaradial symmetry (e.g. 16 comb rows, eight skirt lobes).
Figure 1.
Holotype specimen of Thalassostaphylos elegans gen. et sp. nov. from the Drumian Marjum Formation in Utah
(A) Whole specimen UMNH.IP.6086 photographed underwater using cross polarized light.
(B) Interpretative drawing of whole specimen showing main body regions and anatomy.
(C) Details of comb row, white arrows indicate position of transverse bars interpreted as the cushion plates that underlie the comb rows.
(D) Detail of apical organ polar fields.
(E) Close up of the oral skirt and the comb rows closest to the oral region.
Abbreviations: cr – comb row, os – oral skirt, pf – polar fields.
Genus: Ctenorhabdotus Conway Morris and Collins, 1996.
Ctenorhabdotus campanelliformis sp. nov.
Etymology: From Latin campanella (small bell) and formis (shape), in reference to the bell-shaped outline of the body.
Type species:Ctenorhabdotus capulus (Conway Morris and Collins, 1996).
Type material: UMNH.IP.6125 (holotype and only known specimen).
Locality and Horizon: House Range of western Utah, USA (see STAR Methods for locality details). Most likely recovered from the Drumian strata (lower Ptychagnostus punctuosus Zone) of the middle part of the Marjum Formation, Miaolingian, Cambrian (see STAR Methods below).
Diagnosis: Ctenophore with bell-shaped body outline in lateral view and 24 comb rows. Comb rows terminate at the margin of an extensive oral skirt surrounding a large mouth opening. Aborally, comb rows merge with trifurcating ciliary grooves arising from the apical organ. Apical sense organ enclosed in a capsule.
LSID: urn:lsid:zoobank.org:act:BA8A97CD-E84C-4E08-82CA-6641DBE6DEC1.
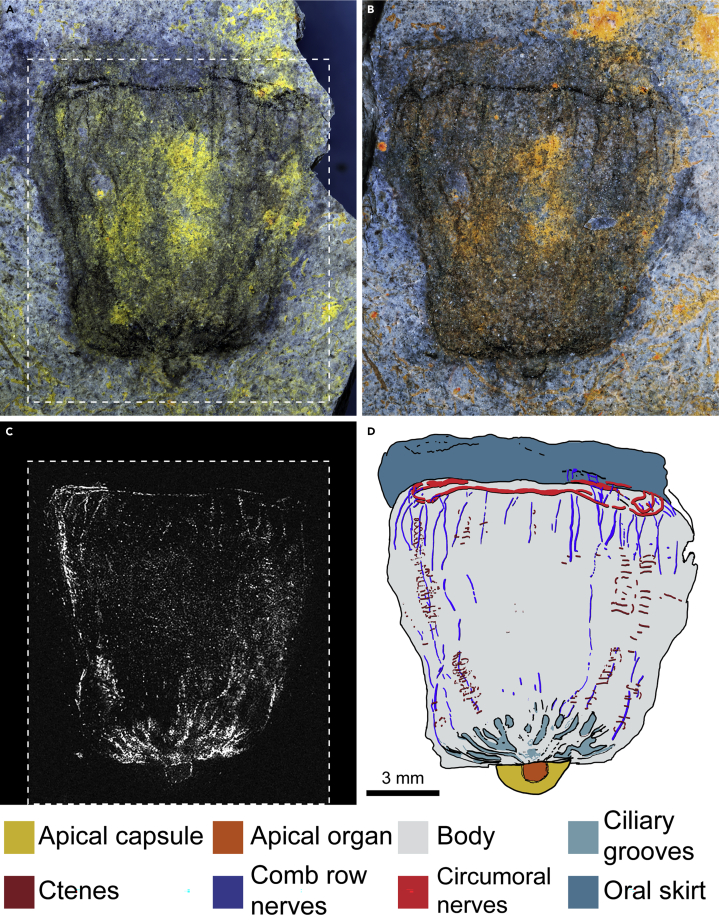
Description:Ctenorhabdotus campanelliformis sp. nov. is known from a single complete specimen (UMNH.IP.6125) preserved as a slightly oblique lateral compression (Figure 2). The body outline is subtrapezoidal (Figures 2A–2D), with a narrower aboral end (∼8.5 mm in width) and a maximum width of 12.6 mm reached at 8.7 mm from the apical organ. The total length of the specimen is 14.5 mm, the main body measuring 11.2 mm. The apical organ (Figure 3D) protrudes 0.8 mm from the aboral surface and is enclosed within a semicircular capsule 1.4 mm long and 3 mm wide. Unlike the body, the outline of the apical capsule is smooth and regular, suggesting that it was more rigid in life, indicative of light sclerotization. At the oral end, the body constricts prior to a well-developed oral skirt (Figure 2), which is 1.9 mm long and 11.5 mm wide.
Figure 2.
Holotype specimen of Ctenorhabdotus campanelliformis sp. nov. from the Drumian Marjum Formation in Utah
(A and B) Counterpart and part (mirrored) of UMNH.IP.6125, photographed immersed in water under cross polarized light; dotted line in panel (A) indicates area examined for elemental analysis.
(C) Energy-dispersive spectroscopy (EDS) elemental map of carbon in the counterpart.
(D) Interpretative drawing of whole specimen, colors as in Figure 1 and as shown.
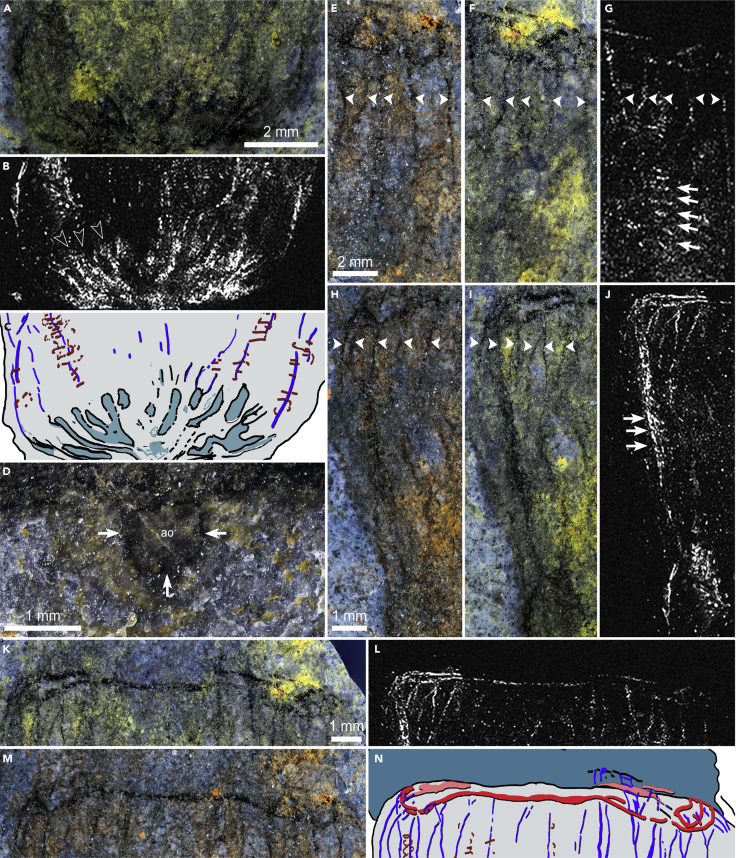
Figure 3.
Anatomical details of Ctenorhabdotus campanelliformis sp. nov
(A and B) Close up of the aboral region and ciliary grooves shown in UMNH.IP.6125b under cross polarized light (A) and in EDS carbon map (B).
(C) Interpretative drawing of region shown in (A and B) based on both the part and counterpart.
(D) Close-up of the apical organ and the capsule surrounding it in UMNH.IP.6125a, white arrows indicate the carbon film lining the apical organ.
(E and F) Details of the comb rows and nerves in the oral region of UMNH.IP.6125a (E) and UMNH.IP.6125b (F), arrowheads indicate the position of giant axons.
(G) EDS carbon map in the region shown in (F), and white arrows show the transverse ctenes.
(H and I) Details of the comb rows and nerves from the oral to aboral regions of part of UMNH.IP.6125a (H) and UMNH.IP.6125b (I).
(J) EDS carbon map for the region shown in I, white arrows show the transverse ctenes.
(K–N) Details of the oral nerve ring of UMNH.IP.6125b (M) and UMNH.IP.6125a (K). (L) EDS carbon map in the region shown in (K). (N) Interpretative drawing of region shown in (K and L) based on features preserved in both the part and counterpart. The images of UMNH.IP.6125b have been mirrored to facilitate easier comparison between the part and counterpart.
UMNH.IP.6125 preserves internal anatomical structures as dark compressions (Figure 3), which represent organic carbon films detectable with energy dispersive spectroscopy (EDS)-based elemental mapping (Figures 3B, 3G, 3J, and 3L). Both part and counterpart contain complementary carbon films, which allowed us to reconstruct their overall organization (Figure 2D). Carbonaceous structures are concentrated at the apical pole and feature trifurcating ciliary grooves arising from the apical organ (Figures 3A–3C), which are also evident in Ctenorhabdotus capulus (Conway Morris and Collins, 1996). The outer limit of the apical organ within the capsule is marked by a narrow band of dark material that lines the interior of the apical capsule (Figure 3D). This likely represents the remains of the ciliated lining observed in extant taxa. Narrow carbonaceous strands originate from the ciliary grooves in UMNH.IP.6125 (Figure 3B). Based on their morphology and preservation, we interpret these narrow strands as fossilized longitudinal nerves, comparable to the giant axons of extant Euplokamis (see discussion for taphonomy and possible alternative interpretations). The axons are occasionally overlain by transverse bars (Figures 3G and 3J) that represent remains of the comb rows (and/or their supporting polster cells) similar to those observed in fossil ctenophores from other lower and mid-Cambrian localities (Conway Morris and Collins, 1996; Ou et al., 2015). Unlike Ctenorhabdotus capulus (Conway Morris and Collins, 1996), however, UMNH.IP.6125 shows no visible signs of morphological differentiation between comb rows (Figure 3). The longitudinal nerves are approximately 19–40-μm-thick measured on the oral region of the counterpart (Figures 3E–3J). The axons appear to bifurcate at random locations on both the part (Figure 3G) and counterpart (Figure 3J). This lack of alignment of the bifurcation points most likely represents a preservation artifact resulting from the superimposition of features from both sides of the body, after burial at a slightly oblique angle and subsequent deformation of the gelatinous body. The longitudinal axons traverse the body parallel to the oral-aboral axis, meeting a thicker (∼150-μm-wide) transverse dark strand at the margin between the body and the oral skirt (Figures 2K–2N). The perpendicular carbon strand surrounds the base of the oral skirt in a circumoral configuration, forming a discrete oral nerve ring (Figure 3N). Besides the highly concentrated carbon strands that conform the longitudinal axons and the circumoral nerve ring, UMNH.IP.6125 shows a diffuse carbon signature throughout the body. However, elemental mapping did not reveal additional concentrations of carbon in the oral skirt nor is there any dark carbonaceous material visible in this region under polarized light. It is noteworthy that the margins of the fossil are not associated with a dark line, or any carbon enrichment detectable with EDS, which gives support to our interpretation of the carbon strands within it representing internal features, rather than compaction artifacts.
Phylogenetic position
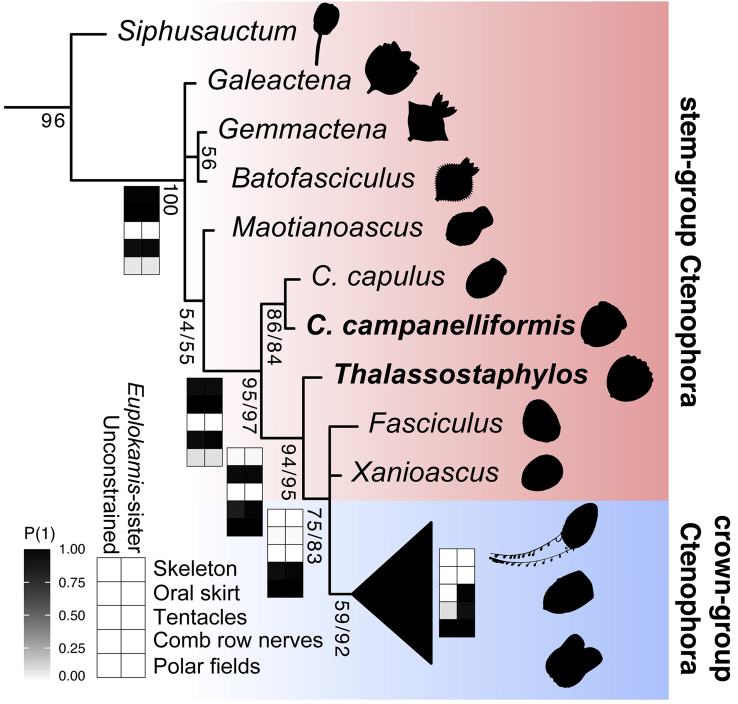
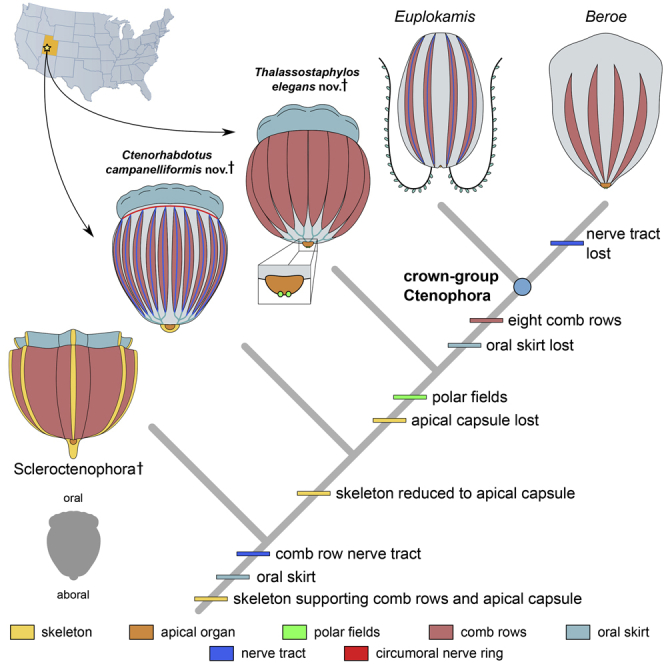
Our Bayesian phylogenetic analysis under the mki model consistently recovers Ctenorhabdotus campanelliformis as the sister taxon of Ctenorhabdotus capulus (Conway Morris and Collins, 1996) within the ctenophore stem-group (Figures 5, S1, and S2), supported by the shared presence of an apical capsule and trifurcating ciliary grooves. Thalassostaphylos elegans is recovered in a phylogenetic position that is more crownward, as the sister taxon of a clade composed of Xanioascus and the ctenophore crown group. We performed constrained analyses aiming to explore how the position of ctenophores among animals and the in-group relationships of living ctenophore lineages impact tree topologies. Topologies with (Figure S3A) and without (Figures S3B, S4A, and S4B) molecular constraints on relationships within living ctenophores (Whelan et al., 2017), yield similar results within total-group Ctenophora to previous analyses using this data set (Zhao et al., 2019), albeit with improved resolution for taxa proximal to the crown group. The positions of Ctenorhabdotus campanelliformis and Thalassostaphylos elegans are also stable in the analyses that constrain non-bilaterian relationships (i.e. ctenophores as the sister-group of other animals (Dunn et al., 2008), or the monophyly of the Parahoxozoa (Ryan et al., 2010)) (Figure S4). These treatments also changed the placement of Cambrian dinomischiids from stem-group ctenophores as supported by our unconstrained morphological analyses (Figure 5 and S3A) and one previous study (Zhao et al., 2019), to placement as stem group cnidarians, as in another previous study (Ou et al., 2017) (Figure S4). However, these alternative topologies are not as well supported based on Bayes factor analysis (see Tables S1 and S2).
Figure 5.
Phylogeny and ancestral state reconstruction incorporating Ctenorhabdotus campanelliformis and Thalassostaphylos elegans
Phylogenetic inferred from a character matrix of animals consisting of 285 characters. Note that the tree has been truncated at the node subtending Siphusauctum (see Figures S3 and S4 for full results). Numbers at nodes are posterior probabilities for the unconstrained analysis (top) and an analysis where Euplokamis is constrained as the sister group of all extant ctenophores (bottom). Tile plots illustrate posterior probabilities of ancestral states at selected nodes from Bayesian analysis of both the constrained and unconstrained analysis.
Ancestral state reconstruction supports a loss of discrete longitudinal nerves underlying the comb rows within either the ctenophore stem-group or the crown-group, depending on if Euplokamis is recovered as the sister-group of all other extant ctenophores or not (Figure 5). Our ancestral state reconstruction reveals that the oral skirt and skeleton were lost prior to the origin of the ctenophore crown group (Figure 5) and that nerves that lie beneath the comb rows have a deep origin in the ctenophore total group. Such nerves may have been lost and then evolved independently in Euplokamis, again depending on the position of this taxon relative to all other extant ctenophores (Figure 5). Polar fields evolved prior to the loss of the oral skirt that is characteristic of most Cambrian ctenophore species, including Thalassostaphylos and Ctenorhabdotus. The reconstruction of the ctenophore crown ancestor is dependent on the phylogenetic position of Euplokamis and the Beroida. If the former is the sister group of all other modern ctenophores, the ancestral ctenophore possessed giant axons and paired tentacles bearing colloblasts (Whelan et al., 2017).
Discussion
Taphonomy
The discovery of Thalassostaphylos elegans and Ctenorhabdotus campanelliformis contributes toward the sparse fossil record of ctenophores and reveals important details of their external and internal anatomy. In Ctenorhabdotus campanelliformis, we identify carbonaceous remains encircling the mouth, which are linked with strands that run beneath comb rows and connect to the aboral sense organ. The ctenophore body plan features a number of different radially arranged internal features that follow the oral-aboral axis including parts of the gastrovascular system, ciliated features, and the nervous system. Based on morphological and taphonomic evidence, we argue that these features represent both the ciliated grooves and nervous system, which complement the record of exceptionally preserved nervous systems of bilaterian animals in the fossil record (Ortega-Hernández et al., 2019).
Carbonaceous films representing the remains of nervous tissue have been described from a number of Cambrian localities in a range of phyla (Ma et al., 2015; Ortega-Hernández et al., 2019; Parry and Caron, 2019; Parry et al., 2018), although predominantly from panarthropods (see Ortega-Hernández et al., 2019 for a recent summary). Although these fossilized tissues have not been without controversy (e.g. Sansom, 2016), a growing body of evidence shows that these features display a consistent pattern of carbonaceous preservation through time and space in many Cambrian BSTs deposits (Ma et al., 2015; Ortega-Hernández et al., 2019; Park et al., 2018; Parry and Caron, 2019; Parry et al., 2018). It has been suggested that some of these fossilized brains and nerve cords could instead represent biofilms formed during early decay, after the rupture of the gut wall has allowed intestinal microbes to proliferate within body cavities (Liu et al., 2018). Although the recognition of Cambrian neurological structures requires caution, the critical examination of key aspects of these remains (carbonaceous composition, bilateral symmetry, morphological complexity, congruence between specimens and localities; Ortega-Hernández et al., 2019) contributes to their reliable identification as fossilized neuroanatomical features.
The morphology and topological relationships of the internal structures observed in Ctenorhabdotus campanelliformis are incompatible with an alternative interpretation as remains of the gut. The gastrovascular system of living ctenophores includes a pharynx, a gastric cavity, and the infundibulum, a complex network of canals arising from the gastric cavity in the mid body region and running underneath the comb rows (Mayor, 1912). Ctenorhabdotus campanelliformis exhibits traces of the cellular gelatinous body outline but no features comparable to any of the most prominent components of the ctenophore gastrovascular system. In fact, digestive structures are rarely preserved in stem-group ctenophores other than dinomischiids (Zhao et al., 2019); none are known in the Burgess Shale taxa (Conway Morris and Collins, 1996), and only one specimen of Galeactena from Chengjiang preserves evidence of an aboral canal (Ou et al., 2015). The carbon films in Ctenorhabdotus campanelliformis can also be traced to the apical organ (Figures 3A–3C), a structure that is not connected to the gastrovascular system in modern representatives. Moreover, the structures that we interpret as giant axons are much thinner (∼30 μm in width for a ∼12 mm long body) than typical meridional canals or the comb rows (∼500 μm), both of which are typically of similar widths in living species. They are however, consistent with organically preserved features previously identified as remnants of nervous tissues in ctenophores and related fossil groups, such as the “giant axons” of Fasciculus vesanus (Conway Morris and Collins, 1996) and the nerves that subtend paired comb rows and ciliary organs in scleroctenophores and dinomischiids (Zhao et al., 2019). These longitudinal features in Fasciculus and Ctenorhabdotus campanelliformis most closely resemble the giant axons in Euplokamis (Mackie et al., 1992), but are distinguished by their larger diameter (∼30 μm vs 8.5–12 μm). This may reflect the presence of cell bodies in the comb row nerves rather than only axons.
Another alternative is that the longitudinal features that we interpret as axons represent remains of the interplate ciliary grooves. The latter structures are extensions of the ciliary grooves toward the oral end of the body, which underlie the comb rows and contribute to the transmission of ciliary beating (Tamm, 1973). These features differ from the giant axons in that they are interrupted at the junctions between comb plates (Tamm, 1973), a detail that would be difficult to observe in compressed Cambrian fossils. We consider that this interpretation is unlikely, as interplate ciliary grooves are restricted to phylogenetically derived lobate ctenophores, a clade (that now includes Cestida) that is deeply nested within the ctenophore crown group (Whelan et al., 2017; Zhao et al., 2019). Lastly, the delicate morphological complexity captured in the longitudinal axons and the transverse oral ring argue against the possibility of fossilized biofilms (see Liu et al., 2018), whereas biofilms developed from the ruptured gut and indiscriminately invade any internal body cavities, the carbonaceous films in Ctenorhabdotus campanelliformis are clearly organized in discrete strands, supporting their interpretation as legitimate anatomical details rather than decay artifacts.
Fossil ctenophores are frequently identified by the serially repeated comb plates and/or their underlying polster cells (Conway Morris and Collins, 1996; Ou et al., 2015; Zhao et al., 2019). In Ctenorhabdotus campanelliformis and Thalassostaphylos elegans, the comb rows are not conspicuously preserved but expressed as transverse features, especially on elemental maps (Figure 3G). In contrast, ciliary grooves (Figure 3B) and polar fields (Figure 1D) are well preserved, highlighting tissue-specific preservation variability between Burgess Shale-type deposits (Parry et al., 2018).
We reconstruct Ctenorhabdotus campanelliformis as a nektonic stem-group ctenophore with a complex nervous system, composed of an apical organ that gives rise to longitudinal axons running under the comb rows and connected to a circumoral nerve ring positioned aborally of the oral skirt (Figure 4). It is likely that Ctenorhabdotus campanelliformis also possessed a diffuse reticulate nerve net, given that this feature is present in living ctenophores and other early branching animals, such as cnidarians and acoel flatworms (Martinez and Sprecher, 2020). These reticulate nerve nets, however, may be too small and/or delicate for preservation in carbonaceous compression fossils.
Figure 4.
Reconstruction of Thalassostaphylos elegans and Ctenorhabdotus campanelliformis
(A and B) Technical drawings showing major anatomical features of T. elegans (A) and C. campanelliformis (B).
(C) Life reconstructions of T. elegans (bottom) and C. campanelliformis (top).
Artwork in all panels by Holly Sullivan.
Ecology and mode of life
The plesiomorphic feeding mode of crown-group ctenophores consists of prey capture using colloblast-bearing tentacles (Whelan et al., 2017) innervated by prominent tentacular nerve (Jager et al., 2011). Cambrian ctenophores (with one possible exception in Fu et al., 2019) lack paired tentacles, and these are likewise absent in Ctenorhabdotus campanelliformis and Thalassostaphylos elegans. Most Cambrian ctenophores also feature a constriction between the oral region, represented by a well-developed oral skirt or lappets (Ou et al., 2015), and the rest of the body. The oral skirt however has no equivalent among living species, and its precise function in feeding in extinct taxa remains speculative. Yet, given the presence of this large circumoral structure in taxa seemingly lacking tentacles, we believe that it might have facilitated engulfment of large food items. A comparable feeding mode is well-documented in a clade of living ctenophores, the deeply nested beroids, which suggests multiple independent adaptations for this feeding strategy in total-group Ctenophora (Whelan et al., 2017). Interestingly, beroids possess a large mouth associated with a specialized giant nerve net (but no discrete nerve ring) involved in closing the mouth when not feeding (Tamm and Tamm, 1995). The circumoral nerve ring of Ctenorhabdotus campanelliformis might have played an analogous function in controlling the oral skirt during engulfment-type feeding. If such a functional relationship truly existed, a circumoral nerve might have been a common, but rarely preserved feature in Cambrian ctenophores.
Evolutionary implications
Thalassostaphylos elegans and Ctenorhabdotus campanelliformis illustrate the progressive modification of the ctenophore body plan from skeletonized forms, with oral skirts presumably used in prey capture and robust encapsulated apical organs, to unsclerotised crown group representatives in which prey capture is typically performed using paired tentacles, and the apical organ is equipped with sensory polar fields (Figure 4). In Ctenorhabdotus campanelliformis, and Ctenorhabdotus capulus, the apical organ is surrounded by a capsule. This appears to have been rigid in life, maintaining a smooth, circular (aboral view) or semicircular (lateral view) outline even in specimens that were substantially deformed (e.g. figure 19A in Conway Morris and Collins, 1996).
The rigid apical capsule in Ctenorhabdotus campanelliformis and Ctenorhabdotus capulus (Conway Morris and Collins, 1996) represents a vestigial skeletonized region, suggesting that the skeletal organization of scleroctenophores (Ou et al., 2015) was lost earlier in the comb-bearing region than in apical region. The enclosing of the apical organ within a sclerotized capsule suggests it might have functioned differently in these Cambrian representatives compared with living species. A rigid skeleton is absent in most middle Cambrian forms, having been lost at the node that subtends Thalassostaphylos. In extant ctenophores, the apical organ is equipped with ciliated polar fields, which are absent in all Cambrian taxa, except for the Burgess Shale Xanioascus (Conway Morris and Collins, 1996) and Thalassostaphylos (Figure 1). In Xanioascus, the oral skirt that is characteristic of most of the Cambrian taxa is absent, but present in Thalassostaphylos, indicating loss of the oral skirt after the acquisition of the polar fields, a scenario that is supported by our ancestral state reconstruction (Figure 5).
The complex neuroanatomy of Ctenorhabdotus campanelliformis contrasts with that of extant ctenophores, which typically lack discrete nerves underlying the comb rows or a circumoral nerve center. Euplokamis is unique among living species in this regard, as the comb rows are equipped with giant axons that regulate a rapid escape swimming response (Mackie et al., 1992; Norekian and Moroz, 2020). These axons in Euplokamis are ∼12 μm wide and therefore slightly narrower than the features we describe in Ctenorhabdotus campanelliformis (∼19–40 μm wide). This observation may result from a concentration of neurons in the nerve tracts beneath the comb rows in Ctenorhabdotus campanelliformis, contrasting with the presence of only giant axons in Euplokamis. Similar longitudinal “axons” are found in the Burgess Shale ctenophore Fasciculus and species from Chengjiang (Conway Morris and Collins, 1996). Narrow dark strands that may correspond to the same structures also occur between paired comb rows in scleroctenophores, such as Galeactena (Ou et al., 2015; Zhao et al., 2019), and along the circumoral ciliated feeding tentacles in dinomischiids (Zhao et al., 2019). Ctenorhabdotus campanelliformis adds further evidence that nerve tracts beneath the comb rows were widespread in early members of total-group Ctenophora, rather than representing an autapomorphy of Euplokamis (Mackie et al., 1992), as is traditionally considered (Conway Morris and Collins, 1996). Euplokamis has recently been resolved as the sister-group of all other living ctenophores (Whelan et al., 2017) and so this phylogenetic placement, combined with the widespread occurrence of longitudinal comb-row nerves in Cambrian taxa, suggests that the presence of giant axons is plesiomorphic for crown-group ctenophores, as also indicated by our ancestral state reconstruction (Figure 5).
The extent to which the ctenophore nervous system has been modified over time has been difficult to evaluate due to limited variability among living species, and the elusive identity of the ctenophore sister-group (Dunn et al., 2008; Pett et al., 2019; Ryan et al., 2010; Zhao et al., 2019). Unlike many cnidarians, living ctenophores lack an oral nerve center (e.g. a circumoral nerve ring) (Mackie et al., 1992), which raises the question of whether this results from secondary loss or a primitive absence in the phylum (Arendt et al., 2016). The circumoral nerve ring of Ctenorhabdotus campanelliformis reveals the presence of a cnidarian-like (Zhao et al., 2019), bipolar nervous system in a Cambrian stem-group ctenophore (Figure 5), an organization that may have been secondarily lost in extant representatives. The absence of circumoral nerve rings, but the presence of diffuse nerve nets in living ctenophores may be an adaptation for their holopelagic lifestyle, as nerve nets are well suited for sensing in an isotropic environment where stimuli can arise from all around the organism (Martinez and Sprecher, 2020). A placement of ctenophores as the sister-group of all other animals, as supported by some phylogenomic analyses (Dunn et al., 2008; Moroz et al., 2014), suggests that the ctenophore nervous system is not homologous with that of cnidarians and bilaterians (Moroz et al., 2014). In this scenario, the complex neuroanatomy of C. campanelliformis would indicate that the parallel acquisition of the nervous system must have taken place at an early stage of ctenophore evolution (at least 518 million years ago) relative to the much younger origin of the crown-group ∼350 ± 88 MYA (Whelan et al., 2017).
The presence of a circumoral concentration of neurons (the blastoporal nervous system) in non-bilaterians has been regarded as a key precursor in the evolution of bilaterian nervous systems (Arendt et al., 2016). In cnidarians, circumoral nerve rings are present in both medusozoan medusae and anthozoan polyps. In the cnidarian model organism Nematostella, there is a circumoral concentration of neurons that may form a nerve ring (Marlow et al., 2009) (although see Kelava et al., 2015, where its presence was not confirmed), as well as discrete longitudinal tracts that follow the parietal muscles either side of the mesenteries (Kelava et al., 2015); this organization resembles the oral and aboral nerve centers linked by giant axons in Ctenorhabdotus campanelliformis (Figure 3). However, it is difficult to confidently infer the homology of the nerve ring in cnidarians and Ctenorhabdotus campanelliformis, given the uncertain homology of these structures within the Cnidaria (Arendt et al., 2016), and the potential large scale anatomical modifications at the origin of the ctenophore body plan. For instance, the position of the mouth may not be topologically equivalent among these taxa (Zhao et al., 2019). The presence of such an oral nerve ring could also represent an autapomorphy of Ctenorhabdotus campanelliformis, since its presence has not been documented in other fossil ctenophores. The description of this feature in a Cambrian representative demonstrates that early ctenophores were neuroanatomically complex, regardless of whether the nerve ring is a plesiomorphy that has been lost in crown-group ctenophores.
Ctenorhabdotus campanelliformis documents the broader distribution of circumoral nerve rings and linked oral and aboral nerve centers among non-bilaterian metazoans during the Cambrian compared to the Recent, implying a more intricate history of these features among extant phyla than previously recognized. Depending on the precise phylogenetic position of ctenophores, this organization could represent a synapomorphy of eumetazoans that was modified to form the complex bilaterian nervous system (Arendt et al., 2016), a synapomorphy uniting ctenophores and cnidarians as Coelenterata (Zhao et al., 2019), or a case of astonishing convergence during the earliest stages of animal evolution.
Along with other Cambrian taxa, the two ctenophores from the Marjum Formation expand the known morphological diversity of total-group Ctenophora. Numerous comb rows, sclerotized body parts (including an encapsulated apical organ), an oral skirt, or a circumoral nerve ring are all major departures from the morphologies displayed by crown-group representatives. These morphological features have been pruned from the body plan of living ctenophores by extinction over the last 500 million years of their evolutionary history. Critically, Ctenorhabdotus campanelliformis demonstrates that at least some of the diversity of ctenophores in the Cambrian were more neuroanatomically complex than most living species.
Limitations of the study
The current controversy surrounding the deep phylogenetic relationships of five main groups of animals (i.e. Porifera, Cnidaria, Ctenophora, Placozoa, Bilateria) results in the lack of a clear framework for understanding the early evolution of key metazoan innovations, including nervous systems (Jékely et al., 2015; Li et al., 2021; Moroz et al., 2014; Pett et al., 2019; Pisani et al., 2015; Ryan et al., 2013; Whelan et al., 2017; Zhao et al., 2019). Although fossil data combined with the morphology of living species strongly support the traditional monophyly of Coelenterata over other alternatives (Ou et al., 2017; Zhao et al., 2019) (see also Tables S1 and S2), simulated morphological datasets suggest that such difficult phylogenetic questions (e.g. ancient and rapid radiations and/or very short internodes) may typically be out of the reach of the topological improvements provided by fossils (Mongiardino Koch et al., 2021). In addition, the phylogenetic placement of fossil taxa (e.g. dinomischiids) is labile when different alternatives of the sister group of all animals are imposed (Figures S3 and 4). However, this would require convergence among certain features shared by dinomischiids and ctenophores (particularly scleroctenophores), notably the presence of ciliary pumping organs and a skeleton that supports both the aboral region (calyx/apical organ) and the ciliated tentacles/ctenes. Consequently, we are unable to determine the broader significance of some of the morphological features of the fossil ctenophores that we describe, such as if the oral nerve ring of Ctenorhabdotus campanelliformis is homologous to cnidarian nerve rings or evolved independently. This is compounded by the paucity of neuroanatomical data available for Cambrian ctenophores, particularly the absence of oral nerve centers. The presence of a circumoral nerve ring has been suggested for (but not observed in) the dinomischiid Daihua (Zhao et al., 2019), which would likely not be topologically equivalent to the circumoral nerve ring in C. campanelliformis if the evolutionary transitions from a polypoid organism to nektonic ctenophore proposed in Zhao et al., 2019 are correct. Consequently, we make no attempt to reconstruct the early evolutionary history of this trait in our ancestral state reconstructions but description of this feature nonetheless expands the known morphological diversity of ctenophores.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Ctenorhabdotus campanelliformis fossil specimen | Natural History Museum of Utah, Salt Lake City | UMNH.IP.6125 |
| Thalassostaphylos elegans fossil specimen | Natural History Museum of Utah, Salt Lake City | UMNH.IP.6086 |
| Deposited data | ||
| Morphological character matrix in NEXUS format | This study | https://doi.org/10.5061/dryad.wh70rxwnr |
| Software and algorithms | ||
| MrBayes 3.2.7 | Huelsenbeck and Ronquist (2001) | https://nbisweden.github.io/MrBayes/download.html |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Javier Ortega-Hernández (jortehernandez@fas.harvard.edu)
Materials availability
Specimen UMNH.IP.6125 and UMNH.IP.6086 are both deposited in the Natural History Museum of Utah in Salt Lake City. The phylogenetic dataset used in phylogenetic analyses is available from Dryad (https://doi.org/10.5061/dryad.wh70rxwnr).
Experimental model and subject details
Specimen UMNH.IP.6125 (part and counterpart) belongs to a collection of Cambrian fossils from the House Range of Utah (Wheeler, Marjum, and Weeks Formations) deposited in the Natural History Museum of Utah in Salt Lake City. The accompanying label does not provide stratigraphical or geographical data, but characteristics of the fossil and the matrix surrounded it strongly suggest that it was recovered from the gray layers of the Marjum Formation, as is the case of many fossils from the same collection. Only the Drumian strata (lower Ptychagnostus punctuosus agnostoid Zone) of the middle part of the Marjum Formation have yielded exceptionally preserved fossils (Pates et al., 2021). An origin from the underlying Wheeler Formation is unlikely, but it cannot be entirely ruled out. In such a case, UMNH.IP.6125 would still be Drumian in age, although slightly older (Ptychagnostus atavus agnostoid Zone). Specimen UMNH.IP.6086 belongs to the same collection of fossils housed at the Natural History Museum of Utah. Associated label clearly stipulates that it was found in the Marjum Formation, which is confirmed by the lithological characteristics of the surrounding matrix. However, the precise location of the collection site within the House Range of Utah is unknown.
Method details
Fossil imaging
Fossil specimens were photographed either dry, underwater, and using cross-polarized light in order to maximize the contrast of different anatomical features. Large-area multi-detector energy dispersive spectroscopy (EDS) was performed using a TESCAN VEGA GMU variable pressure scanning electron microscope equipped with two Bruker XFlash 5030 X-ray detectors. Mapping data were acquired at a 15 mm analytical working distance and an accelerating voltage of 20 keV. All maps were acquired from the native fossil (with no mounting or surface coatings) at a chamber pressure of 20 Pa to minimize potential sample damage.
Quantification and statistical analysis
Phylogenetic analysis
The character matrix used in the phylogenetic analyses was derived from Zhao et al. (2019) which in turn was derived from previous analyses focusing on fossil cnidarians and ctenophores (Ou et al., 2015, 2017). Alterations of this matrix included correction of coding errors and addition of new characters, and changes made are described and justified as part of the dataset available on Dryad (https://doi.org/10.5061/dryad.wh70rxwnr). The character matrix contains some taxonomic redundancy inherited from previous matrices upon which it is built (Duan et al., 2017) and poorly known genera with high levels of missing data (e.g. some scleroctenophores). Redundant taxa were identified and pruned using safe taxonomic reduction in Claddis (Lloyd, 2016). The taxon ‘Cydippida’ has been replaced by the genus Euplokamis as ‘Cydippida’ is not monophyletic (Whelan et al., 2017). Lobate ctenophores are now represented by the exemplar genus Mnemiopsis. Character scores for these genera are based on published descriptions (Mackie et al., 1992; Mayor, 1912; Mills, 1987) and the presence of tentacles and colloblasts was scored using the table in Babonis et al. (2018).
Although the phylogenetic position of ctenophores based on phylogenomic data within animals is contested, their internal relationships are more stable with strong support for paraphyly of ‘Tentaculata’ with respect to beroids and Euplokamis representing the earliest diverging lineage in crown Ctenophora (Whelan et al., 2017). Consequently, we applied a backbone constraint in MrBayes 3.2.7 (Huelsenbeck and Ronquist, 2001) using the “constraint partial” command to enforce constraints based on recent phylogenomic analyses for the ingroup relationships of ctenophores. In addition, to assess if the phylogenetic position of Ctenorhabdotus campanelliformis is sensitive to the position of ctenophores among animals, we performed analyses constraining ctenophores as the sister-group of all other animals, as well as the monophyly of Planulozoa/Parahoxozoa (i.e. the monophyly of a clade of all animals except sponges and ctenophores). In unconstrained phylogenetic analyses, all fossil taxa were left unconstrained and therefore could occupy any branch in the tree. Constrained analyses included ctenophores as the sister group of all other animals (Dunn et al., 2008), the monophyly of a clade of all animals except sponges and ctenophores, i.e. Parahoxozoa/Planulozoa (Ryan et al., 2010) and a constraint for the ingroup relationships of ctenophores where Tentaculata is paraphyletic (Whelan et al., 2017).
Phylogenetic analyses were conducted in MrBayes 3.2.7 under the mki model (Lewis correction for scoring only parsimony informative characters (Lewis, 2001)) with rate variation among characters modeled using four discrete gamma categories. 40,000,000 generations were requested, with analyses terminated once the average deviation of split frequencies dropped below 0.01, with convergence checked for all parameters (ESS >200, PSRF ∼1.0) using the output of the sump command in MrBayes. Convergence was achieved after <10,000,000 generations in all analyses.
In order to assess support for the competing topologies from the constrained and unconstrained phylogenetic analyses we carried out stepping stone sampling in MrBayes 3.2.7(Ronquist et al., 2012). For steppingstone analyses, 60 million generations were requested, approximately ten times more than the number needed to reach convergence in the MCMC analyses. Steppingstone sampling analyses used 50 steps. We compared four different topologies in these analyses, namely H0 = monophyly of Coelenterata (equivalent to the unconstrained analysis), H1 = monophyly of Coelenterata with in group ctenophore relationships as in Whelan et al. (2017), H2 = monophyly of Parahoxozoa, and H3 = ctenophores as the sister group of all other animals. We compared topologies employing similar levels of constraints, as otherwise Bayes factors can be biased in favor of constrained topologiesm (Bergsten et al., 2013). Constraints for Parahoxozoa and Ctenosister are the same as above, but the constraints for H0 and H1 included a single constraint on the monophyly of Coelenterata (i.e. a clade of cnidarians and ctenophores), which like all other constraints did not included constraints on the phylogenetic position of any fossil taxa. Marginal likelihoods of the different topologies are shown in Table S1.
Separate phylogenetic analyses were performed that monitored ancestral states of nodes of interest. Monitoring of ancestral states in this fashion requires the use of hard constraints, necessitating separate analyses from those concerned with just tree topology. Ancestral state reconstruction was performed in MrBayes using the approach described in ref (Huelsenbeck and Bollback, 2001), such that the analyses integrate over the uncertainty in all model parameters, including the tree topology. Ancestral states were estimated on topologies where relationships within the ctenophore crown were left unconstrained and where Euplokamis is recovered as the sister group of all other ctenophores. Ancestral states were estimated for the presence of the scleroctenophore type skeleton, oral skirt, paired tentacles, nerve tracts associated with the comb rows and polar fields. The results are summarized as tileplots in Figure 5.
Acknowledgments
Carolyn Levitt-Bussian and Randal B. Irmis facilitated the study of the specimens and kindly assisted R.L.-A and J.O.H. during their visits at the Natural History Museum of Utah. LAP was supported by a Donnelley Postdoctoral Fellowship from Yale University and an early career fellowship awarded by St. Edmund Hall, University of Oxford. The Bureau of Land Management, particularly Scott E. Foss and Greg McDonald, deposited these specimens in the Natural History Museum of Utah and provided curation assistance. Holly Sullivan (www.sulscientific.com) created the illustrations in Figure 4.
Author contributions
R.L.-A., J.O.-H., and L.A.P. conceived and designed the study and photographed material. J.C.W. produced the elemental maps. L.A.P. assembled morphological data set and ran phylogenetic analyses. L.A.P. wrote first draft of manuscript and drafted figures with input from R.L.-A. and J.O.-H. All authors discussed results, read, and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Published: September 24, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102943.
Contributor Information
Luke A. Parry, Email: luke.parry@seh.ox.ac.uk.
Rudy Lerosey-Aubril, Email: rudy_lerosey@fas.harvard.edu.
Javier Ortega-Hernández, Email: jortegahernandez@fas.harvard.edu.
Supplemental information
Data and code availability
The phylogenetic data set used in the phylogenetic analyses, character list, details of changes to character scores, and commands to implement topological constraints are available from Dryad (doi:10.5061/dryad.wh70rxwnr). The nomenclatural acts contained in this work have been registered at ZooBank. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Arendt D., Tosches M.A., Marlow H. From nerve net to nerve ring, nerve cord and brain—evolution of the nervous system. Nat. Rev. Neurosci. 2016;17:61. doi: 10.1038/nrn.2015.15. [DOI] [PubMed] [Google Scholar]
- Babonis L.S., DeBiasse M.B., Francis W.R., Christianson L.M., Moss A.G., Haddock S.H., Martindale M.Q., Ryan J.F. Integrating embryonic development and evolutionary history to characterize tentacle-specific cell types in a Ctenophore. Mol. Biol. Evol. 2018;35:2940–2956. doi: 10.1093/molbev/msy171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsten J., Nilsson A.N., Ronquist F. Bayesian tests of topology hypotheses with an example from diving beetles. Syst. Biol. 2013;62:660–673. doi: 10.1093/sysbio/syt029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway Morris S., Collins D. Middle Cambrian ctenophores from the Stephen Formation, British Columbia, Canada. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996;351:279–308. [Google Scholar]
- Dohrmann M., Wörheide G. Novel scenarios of early animal evolution—is it time to rewrite textbooks? Integr. Comp. Biol. 2013;53:503–511. doi: 10.1093/icb/ict008. [DOI] [PubMed] [Google Scholar]
- Duan B., Dong X.-P., Porras L., Vargas K., Cunningham J.A., Donoghue P.C. The early Cambrian fossil embryo Pseudooides is a direct-developing cnidarian, not an early ecdysozoan. Proc. R. Soc. B. 2017;284:20172188. doi: 10.1098/rspb.2017.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn C.W., Hejnol A., Matus D.Q., Pang K., Browne W.E., Smith S.A., Seaver E., Rouse G.W., Obst M., Edgecombe G.D. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature. 2008;452:745–749. doi: 10.1038/nature06614. [DOI] [PubMed] [Google Scholar]
- Dunn C.W., Leys S.P., Haddock S.H. The hidden biology of sponges and ctenophores. Trends Ecol. Evol. 2015;30:282–291. doi: 10.1016/j.tree.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Fu D., Tong G., Dai T., Liu W., Yang Y., Zhang Y., Cui L., Li L., Yun H., Wu Y. The Qingjiang biota—a Burgess Shale–type fossil Lagerstätte from the early Cambrian of South China. Science. 2019;363:1338–1342. doi: 10.1126/science.aau8800. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck J.P., Bollback J.P. Empirical and hierarchical Bayesian estimation of ancestral states. Syst. Biol. 2001;50:351–366. [PubMed] [Google Scholar]
- Huelsenbeck J.P., Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Jager M., Chiori R., Alié A., Dayraud C., Quéinnec E., Manuel M. New insights on ctenophore neural anatomy: immunofluorescence study in Pleurobrachia pileus (Müller, 1776) J. Exp. Zool. B Mol. Dev. Evol. 2011;316:171–187. doi: 10.1002/jez.b.21386. [DOI] [PubMed] [Google Scholar]
- Jékely G., Paps J., Nielsen C. The phylogenetic position of ctenophores and the origin (s) of nervous systems. Evodevo. 2015;6:1. doi: 10.1186/2041-9139-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelava I., Rentzsch F., Technau U. Evolution of eumetazoan nervous systems: insights from cnidarians. Philos. Trans. R. Soc. B Biol. Sci. 2015;370:20150065. doi: 10.1098/rstb.2015.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerosey-Aubril R., Paterson J.R., Gibb S., Chatterton B.D. Exceptionally-preserved late Cambrian fossils from the McKay Group (British Columbia, Canada) and the evolution of tagmosis in aglaspidid arthropods. Gondwana Res. 2017;42:264–279. [Google Scholar]
- Lewis P.O. A likelihood approach to estimating phylogeny from discrete morphological character data. Syst. Biol. 2001;50:913–925. doi: 10.1080/106351501753462876. [DOI] [PubMed] [Google Scholar]
- Li Y., Shen X.-X., Evans B., Dunn C.W., Rokas A. Rooting the animal tree of life. Mol. Biol. Evol. 2021 doi: 10.1093/molbev/msab170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Steiner M., Dunlop J.A., Shu D. Microbial decay analysis challenges interpretation of putative organ systems in Cambrian fuxianhuiids. Proc. R. Soc. B. 2018;285:20180051. doi: 10.1098/rspb.2018.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd G.T. Estimating morphological diversity and tempo with discrete character-taxon matrices: implementation, challenges, progress, and future directions. Biol. J. Linn. Soc. 2016;118:131–151. [Google Scholar]
- Ma X., Edgecombe G.D., Hou X., Goral T., Strausfeld N.J. Preservational pathways of corresponding brains of a Cambrian euarthropod. Curr. Biol. 2015;25:2969–2975. doi: 10.1016/j.cub.2015.09.063. [DOI] [PubMed] [Google Scholar]
- Mackie G., Mills C., Singla C. Giant axons and escape swimming in Euplokamis dunlapae (Ctenophora: cydippida) Biol. Bull. 1992;182:248–256. doi: 10.2307/1542118. [DOI] [PubMed] [Google Scholar]
- Marlow H.Q., Srivastava M., Matus D.Q., Rokhsar D., Martindale M.Q. Anatomy and development of the nervous system of Nematostella vectensis, an anthozoan cnidarian. Dev. Neurobiol. 2009;69:235–254. doi: 10.1002/dneu.20698. [DOI] [PubMed] [Google Scholar]
- Martindale M.Q., Henry J.Q. Intracellular fate mapping in a basal metazoan, the ctenophore Mnemiopsis leidyi, reveals the origins of mesoderm and the existence of indeterminate cell lineages. Dev. Biol. 1999;214:243–257. doi: 10.1006/dbio.1999.9427. [DOI] [PubMed] [Google Scholar]
- Martinez P., Sprecher S.G. Of circuits and brains: the origin and diversification of neural architectures. Front. Ecol. Evol. 2020;8:82. [Google Scholar]
- Mayor A.G. Carnegie institution of Washington; 1912. Ctenophores of the Atlantic Coast of North America. [Google Scholar]
- Mills C.E. Revised classification of the genus Euplokamis Chun, 1880 (Ctenophora: cydippida: Euplokamidae n. fam.) with a description of the new species Euplokamis dunlapae. Can. J. Zool. 1987;65:2661–2668. [Google Scholar]
- Mongiardino Koch N., Garwood R.J., Parry L.A. Fossils improve phylogenetic analyses of morphological characters. Proc. R. Soc. B. 2021;288:20210044. doi: 10.1098/rspb.2021.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz L.L., Kocot K.M., Citarella M.R., Dosung S., Norekian T.P., Povolotskaya I.S., Grigorenko A.P., Dailey C., Berezikov E., Buckley K.M. The ctenophore genome and the evolutionary origins of neural systems. Nature. 2014;510:109–114. doi: 10.1038/nature13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen C. Early animal evolution: a morphologist's view. R. Soc. Open Sci. 2019;6:190638. doi: 10.1098/rsos.190638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen C., Scharff N., Eibye-Jacobsen D. Cladistic analyses of the animal kingdom. Biol. J. Linn. Soc. 1996;57:385–410. [Google Scholar]
- Norekian T.P., Moroz L.L. Comparative neuroanatomy of ctenophores: neural and muscular systems in Euplokamis dunlapae and related species. J. Comp. Neurol. 2020;528:481–501. doi: 10.1002/cne.24770. [DOI] [PubMed] [Google Scholar]
- Ortega-Hernández J., Lerosey-Aubril R., Pates S. Proclivity of nervous system preservation in Cambrian Burgess Shale-type deposits. Proc. R. Soc. B. 2019;286:20192370. doi: 10.1098/rspb.2019.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M. Supposed soft tissue preservation in the Hunsrückschiefer (Lower Devonian, rheinisches schiefergebirge): the example of brachiopods. Paläontol. Z. 2000;74:79–89. [Google Scholar]
- Ou Q., Han J., Zhang Z., Shu D., Sun G., Mayer G. Three Cambrian fossils assembled into an extinct body plan of cnidarian affinity. Proc. Natl. Acad. Sci. U S A. 2017:8835–8840. doi: 10.1073/pnas.1701650114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Q., Xiao S., Han J., Sun G., Zhang F., Zhang Z., Shu D. A vanished history of skeletonization in Cambrian comb jellies. Sci. Adv. 2015;1:e1500092. doi: 10.1126/sciadv.1500092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park T.-Y.S., Kihm J.-H., Woo J., Park C., Lee W.Y., Smith M.P., Harper D.A., Young F., Nielsen A.T., Vinther J. Brain and eyes of Kerygmachela reveal protocerebral ancestry of the panarthropod head. Nat. Commun. 2018;9:1019. doi: 10.1038/s41467-018-03464-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry L., Caron J.-B. Canadia spinosa and the early evolution of the annelid nervous system. Sci. Adv. 2019;5:eaax5858. doi: 10.1126/sciadv.aax5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry L.A., Smithwick F., Nordén K.K., Saitta E.T., Lozano-Fernandez J., Tanner A.R., Caron J.B., Edgecombe G.D., Briggs D.E., Vinther J. Soft-bodied fossils are not simply rotten carcasses–toward a holistic understanding of exceptional fossil preservation. BioEssays. 2018;40(1):1700167. doi: 10.1002/bies.201700167. [DOI] [PubMed] [Google Scholar]
- Pates S., Lerosey-Aubril R., Daley A.C., Kier C., Bonino E., Ortega-Hernández J. The diverse radiodont fauna from the Marjum Formation of Utah, USA (Cambrian: Drumian) PeerJ. 2021;9:e10509. doi: 10.7717/peerj.10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pett W., Adamski M., Adamska M., Francis W.R., Eitel M., Pisani D., Wörheide G. The role of homology and orthology in the phylogenomic analysis of metazoan gene content. Mol. Biol. Evol. 2019;36:643–649. doi: 10.1093/molbev/msz013. [DOI] [PubMed] [Google Scholar]
- Pisani D., Pett W., Dohrmann M., Feuda R., Rota-Stabelli O., Philippe H., Lartillot N., Wörheide G. Genomic data do not support comb jellies as the sister group to all other animals. Proc. Natl. Acad. Sci. U S A. 2015;112:15402–15407. doi: 10.1073/pnas.1518127112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presnell J.S., Vandepas L.E., Warren K.J., Swalla B.J., Amemiya C.T., Browne W.E. The presence of a functionally tripartite through-gut in Ctenophora has implications for metazoan character trait evolution. Curr. Biol. 2016;26:2814–2820. doi: 10.1016/j.cub.2016.08.019. [DOI] [PubMed] [Google Scholar]
- Purcell J.E., Arai M.N. Interactions of pelagic cnidarians and ctenophores with fish: a review. Hydrobiologia. 2001;451:27–44. [Google Scholar]
- Ronquist F., Teslenko M., van der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J.F., Pang K., Mullikin J.C., Martindale M.Q., Baxevanis A.D. The homeodomain complement of the ctenophore Mnemiopsis leidyi suggests that Ctenophora and Porifera diverged prior to the ParaHoxozoa. EvoDevo. 2010;1:9. doi: 10.1186/2041-9139-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J.F., Pang K., Schnitzler C.E., Nguyen A.-D., Moreland R.T., Simmons D.K., Koch B.J., Francis W.R., Havlak P., Smith S.A. The genome of the ctenophore Mnemiopsis leidyi and its implications for cell type evolution. Science. 2013;342:1242592. doi: 10.1126/science.1242592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom R.S. Preservation and phylogeny of Cambrian ecdysozoans tested by experimental decay of Priapulus. Sci. Rep. 2016;6:32817. doi: 10.1038/srep32817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley G.D., Stürmer W. A new fossil ctenophore discovered by X-rays. Nature. 1987;328:61–63. [Google Scholar]
- Tamm S., Tamm S.L. A giant nerve net with multi-effector synapses underlying epithelial adhesive strips in the mouth of Beroe (Ctenophora) J. Neurocytol. 1995;24:711–723. doi: 10.1007/BF01179820. [DOI] [PubMed] [Google Scholar]
- Tamm S.L. Mechanisms of ciliary co-ordination in ctenophores. J. Exp. Biol. 1973;59:231–245. [Google Scholar]
- Tamm S.L. Formation of the statolith in the ctenophore Mnemiopsis leidyi. Biol. Bull. 2014;227:7–18. doi: 10.1086/BBLv227n1p7. [DOI] [PubMed] [Google Scholar]
- Tang F., Bengtson S., Wang Y., Wang X.l., Yin C.y. Eoandromeda and the origin of Ctenophora. Evol. Dev. 2011;13:408–414. doi: 10.1111/j.1525-142X.2011.00499.x. [DOI] [PubMed] [Google Scholar]
- Whelan N.V., Kocot K.M., Moroz T.P., Mukherjee K., Williams P., Paulay G., Moroz L.L., Halanych K.M. Ctenophore relationships and their placement as the sister group to all other animals. Nat. Ecol. Evol. 2017;1:1737. doi: 10.1038/s41559-017-0331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young G., Rudkin D., Dobrzanski E., Robson S., Cuggy M., Demski M., Thompson D. Great Canadian Lagerstätten 3. Late Ordovician Konservat-Lagerstätten in Manitoba. Geosci. Can. 2012;39:201–213. [Google Scholar]
- Zhao Y., Vinther J., Parry L.A., Wei F., Green E., Pisani D., Hou X., Edgecombe G.D., Cong P. Cambrian sessile, suspension feeding stem-group ctenophores and evolution of the comb jelly body plan. Curr. Biol. 2019;29:1112–1125.e1112. doi: 10.1016/j.cub.2019.02.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The phylogenetic data set used in the phylogenetic analyses, character list, details of changes to character scores, and commands to implement topological constraints are available from Dryad (doi:10.5061/dryad.wh70rxwnr). The nomenclatural acts contained in this work have been registered at ZooBank. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.