Abstract
Collagen is an important biomarker of osteoporosis progression. Noninvasive, multispectral, photoacoustic (PA) techniques use pulsed laser light to induce PA signals to facilitate the visualization of chemical components that are strongly related to tissue health. In this study, the feasibility of multi-wavelength PA (MWPA) measurement of the collagen in bone, using the wavelength range of 1300–1800 nm, was investigated. First, the feasibility of this approach for detecting the collagen content of bone was demonstrated by means of numerical simulation. Then, ex vivo experiments were conducted on both animal and human bone specimens with different bone densities using the MWPA method. The relative collagen content was extracted and compared with the results of micro-computed tomography (micro-CT) and histology. The results showed that the “relative collagen content” parameter obtained using the MWPA approach correlated well with the bone volume ratio obtained from micro-CT images and histological analysis results. This study highlights the potential of the proposed PA technique for determining the collagen content of bones as a biomarker for bone health assessment.
Keywords: Collagen, Bone assessment, Multi-wavelength photoacoustic analysis, Bone metabolism information
1. Introduction
Osteoporosis is a systemic metabolic disease that affects the skeletal system and is characterized by decreased bone mineral density (BMD), destruction of bone microarchitecture (BMA), and changes in the total amount and types of proteins. Therefore, for screening and early diagnosis of osteoporosis, a diagnostic method that can comprehensively evaluate bone health and is easy to implement should be developed. Most of the recent clinically available diagnostic methods are based on the use of either X-rays or ultrasound [1,2]. The BMD information provided by dual-energy X-ray absorptiometry (DEXA) is currently recognized as the “gold standard” for osteoporosis diagnosis. However, the parameters provided by this method can only explain the changes in BMD and cannot measure BMA, bone elasticity, or other important factors that also determine the risk of fracture [3,4]. The quantitative ultrasound (QUS) bone assessment method primarily provides information associated with the speed of sound (SOS) and broadband ultrasound attenuation in bone tissue [5,6]. However, the specificity of QUS is limited for pathogenic bone diseases that are characterized by microstructural and chemical changes rather than changes in the bone mass [[1], [2], [3]].
Bone quantity and quality depend on the mass and structure of the non-organic mineral matrix, as well as the organic matrix, which is associated with bone blood flow and cellular metabolism. The main components of the organic matrix in bone are lipids, blood cells, collagen, and proteins. The mineral component of the non-organic matrix imparts the bones with hardness and rigidity, whereas the arrangement of the collagen fibers in the organic matrix provides strength. Previous studies based on clinical observation have shown that pathologies that affect the material properties of bone tissue by changing its mineralization, such as osteomalacia or osteopetrosis, increase the risk of fracture. However, diseases that primarily result from collagen defects and do not involve any inherent alteration of tissue mineralization can likewise increase the risk of fracture [1,2]. For example, collagen defects in a mouse model with osteogenesis imperfecta were found to reduce the post-yield deformation of the bone by 60 % and, consequently, reduce the work required to fracture [1]. Recently, magnetic resonance imaging (MRI) and magnetic resonance spectroscopy have shown potential as noninvasive imaging techniques for quantifying the chemical composition, including the lipid content and blood perfusion, of bone [6,7]. In addition, traditional optical spectroscopic techniques have been used to evaluate bone composition alterations related to aging or bone disease [8,9]. However, MRI cannot provide information about the collagen content, whereas traditional optical techniques suffer from limited spatial resolution and overwhelming optical scattering in bone tissue, reducing their efficacy for in vivo skeletal assessment. To overcome these issues, easy-to-apply biomarkers and new diagnostic approaches are required.
Among biomedical imaging modalities, the photoacoustic (PA) technique, which involves acoustic detection of signals triggered by light irradiation, has the unique ability to detect infrared light absorption contrast, which contains information regarding the molecular content deep within biological tissues [[10], [11], [12], [13], [14], [15], [16], [17]]. The past studies showed that, in bone health assessment, the PA technique has the potential to measure parameters related to BMD and BMA, but can also to measure the content and distribution of chemical constituents that are highly relevant to bone health, such as hemoglobin and lipids. Lashkari et al. observed changes in bone structure and density as well as collagen content using a dual backscattered ultrasound and PA radar system based on signals generated at two different wavelengths (805 and 1064 nm) [18,19]. Gu et al. performed photoacoustic Fourier-transform infrared spectroscopy to measure the mineral stoichiometry of cortical bone [20]. Wood et al. used an optimized PA imaging technique to assess the oxygen saturation in the bone marrow cavity during disease progression in a murine model of acute lymphoblastic leukemia, based on the wavelengths of 715, 730, 760, 800, 850, and 880 nm [21]. Further, Steinberg et al. used dual-modality multispectral PA system based on the wavelengths of 910 and 985 nm to quantify the blood/fat ratio in marrow, which has been correlated with molecular changes in long bones [22]. In addition, our group investigated the feasibility of using thermal PA measurements and PA spectral analysis to evaluate the BMD and BMA of trabecular bone in rat models [23,24]. Recently, our group obtained chemical information, such as that pertaining to the minerals, lipids, oxygenated hemoglobin, and deoxygenated hemoglobin, by performing multi-wavelength PA measurements in the traditional near-infrared (NIR) window (690–950 nm) on human calcaneus bone in vivo [25].
To date, although the use of wavelengths in the extended NIR (exNIR) region has been reported for collagen visualization [[26], [27], [28], [29]], to the best of our knowledge, PA measurement using these wavelengths has never been applied to the determination of collagen content for bone assessment. In this work, we studied the feasibility of a multi-wavelength PA (MWPA) analysis technique for assessing the collagen content of bone using numerical simulations and ex vivo experimental studies on animal models as well as human bone specimens. MWPA curves of bones with different collagen contents were obtained and decomposed. Subsequently, MWPA was used to determine the “relative collagen content” parameter, and this parameter for our samples was determined and compared with gold standard micro-computed tomography (micro-CT) images and histology results.
2. Materials and methods
2.1. Theory
The amplitude of the PA signal generated by the bone, reaching the detector after ignoring the reflected acoustic waves from the boundary, can be expressed in a simplified form as follows [10]:
| (1) |
where Γ is the Grüneisen parameter, which can be expressed as ; β is the thermal coefficient of volume expansion; c is the SOS in the tissue; is the heat capacity at constant pressure; is the light fluence generated by the laser, and is the optical absorption coefficient. As the bone tissue samples used in this study were very thin (∼1 mm), light attenuation, , was neglected in this study. In this case, the amplitude of the PA signal received by the transducer can be simplified as
| (2) |
In this equation, can be obtained by calibration using the measured laser energies at different wavelengths, whereas and are independent of the wavelength. Therefore, and can be eliminated from the expression by dividing by at a reference wavelength of , i.e., :
| (3) |
From this equation, we can obtain the relative optical absorption coefficient using the relationship shown in Eq. (4).
| (4) |
Eq. (4) indicates that, by performing multi-wavelength PA measurements of bone samples, we can measure their relative optical absorption spectra . As was measured by using PA technique, we call it relative PA absorption spectra. However, owing to the strong acoustic attenuation in bone tissue, most of the PA signals received by the transducer are at frequencies lower than 5 MHz. Therefore, setting a high cut-off frequency f1 can help avoid high-frequency noise, whereas setting a low cut-off frequency f2 can help minimize system noise. Thus, the relative optical absorption coefficient can be obtained as follows:
| (5) |
| (6) |
Eqs. (5) and (6) indicate that by performing acoustic-frequency-resolved multi-wavelength PA measurements on bone samples, we can obtain the relative optical absorption spectra of the bone tissue with reduced system noise.
2.2. Signal processing
Multi-wavelength PA signals from each bone specimen were processed according to the steps listed below. In Step 1, the PA measurements at each wavelength for the bone samples were translated to the frequency domain. Frequency–wavelength PA spectra, which are also known as PA physio-chemical spectra (PAPCS) were obtained using Eq. (5) [28]. In Step 2, low-noise optical absorption spectra were obtained based on the PAPCS using Eq. (6). For this purpose, in Eq. (6) was set to 1300 nm in this study, and the relative optical absorption spectra for each bone specimen was obtained. Then, the relative PA absorption spectra for each bone specimen was normalized in the range of 0–1, and the normalized optical absorption curve was obtained. In Step 3, with the optical absorption spectrum of the bone sample measured and the optical absorption of the major chemical components of the bone known, a least-squares method was used to attempt to fit the measured PA optical absorption spectrum of the sample to a linear combination of the optical spectra of the components. In Step 4, the derived relative contribution of each chemical component to the measured PA optical absorption spectrum was assumed to correspond to the relative amount of each of the major chemical components in the bone.
2.3. Simulation study
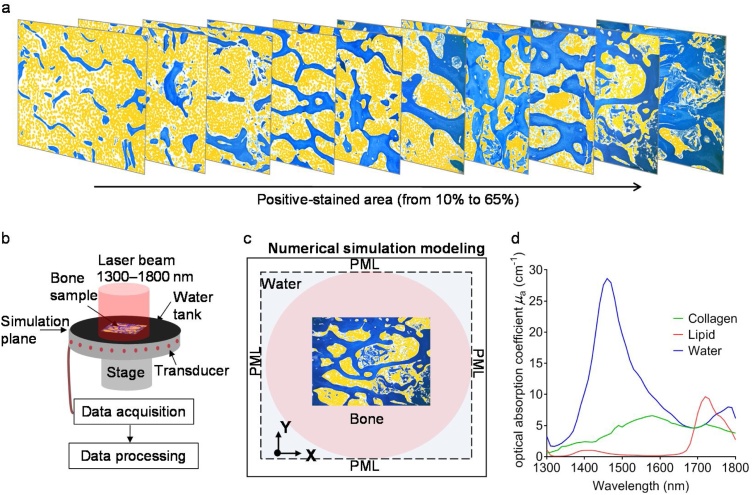
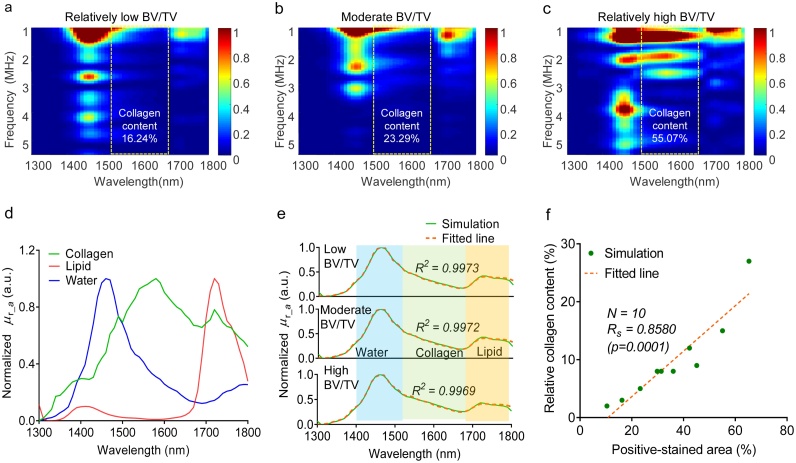
To evaluate the feasibility of the MWPA technique for quantifying the collagen content of bone, we first conducted PA simulations using bone specimens with different collagen contents. Ten collagen slices with a range of different collagen contents were selected from histologically stained images. For the bone simulation model, we filled the pores of the collagen network with lipids and water, hypothesizing that an appropriate lipid-to-water content ratio for the bone marrow was approximately 1:1. Fig. 1(a) depicts the 10 simulated bone samples, where the blue regions indicate collagen, yellow particles indicate lipids, and the remaining white areas indicate water.
Fig. 1.
Setup of the PA simulation. (a) Simulated bone samples after image processing based on the histological stained images. (b) Schematic diagram of the simulation setup. (c) Simulation area setting. (d) Optical absorption spectra of major chemical components of the bone—collagen, lipid, and water—with absorption bands at wavelengths in the range of 1300–1800 nm.
For the PA simulation, a 2D simulation of PA propagation in the bone was conducted based on the finite-difference time-domain (FDTD) method [30,31]. In total, 50 ultrasonic transducers were placed at different locations around the bone, as shown in Fig. 1(b), to receive the PA signal generated by the bone. The sound velocities of water, collagen, and lipid were set to 1414, 1540, and 1432 m/s, respectively, and their densities were set to 1000, 1050, and 975 kg/m3, respectively. The optical absorption coefficients of the dominant chemical components of bone in the wavelength range of 1300–1800 nm are shown in Fig. 1(d) [27,28,32]. For each bone sample, we simulated the PA signals received by the transducers at different locations that were evenly distributed around the sample for spanning the range of 1300–1800 nm with a constant interval of 10 nm (totally 51 wavelengths). The signal was propagated for 100 μs, which allowed it to travel through the entire bone section and beyond the specified domain. Using the non-normalized absorption spectra of the constituent chemicals of bone, i.e., collagen, lipids, and water, we obtained the PA signal in the wavelength range of 1300–1800 nm. These PA signals were processed using Step 1 and Step 2 of the data processing method described in Section 2.2. Then, the simulated relative PA optical absorption spectrum was obtained over the wavelength range of 1300–1800 nm for each transducer. In addition, the simulated PA absorption spectra obtained from 50 ultrasonic transducers were averaged to generate an averaged relative PA absorption spectrum for each model bone section. Finally, the PA absorption spectra were un-mixed, and the relative contents of the different chemical components were obtained for each model bone section using Step 3 and Step 4 of the method presented in Section 2.2.
2.4. Experimental setup
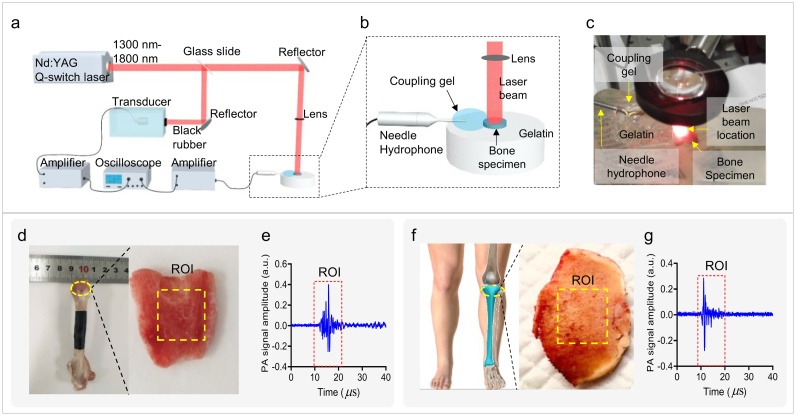
Each human sample was measured using the MWPA approach using multiple wavelengths in the range of 1300–1800 nm separated by 10 nm intervals (totally 51 wavelengths). Fig. 2(a) shows the experimental setup used in this study. The output of a Nd:YAG laser-pumped OPO (Vibrant B, OPOTEK, Carlsbad, CA, USA) was divided into two parts using a beam splitter. Ninety percent of the laser intensity was illuminated to the bone surface, as shown in Fig. 2(a, b). To decrease the laser beam size, the diameter of the laser beam illuminated to the bone surface was focused by a lens and kept at a value of about 8 mm. The total light fluence at the bone surface was controlled such that it remained less than 20 mJ/cm2, which is lower than the safety limit according to the American National Standards Institute. To minimize interference from water, commercial ultrasound coupling gel was used as the coupling medium. The coupling gel was placed in contact with the bone but did not cover it, as shown in Fig. 2(b–c). The PA signal generated by the bone was received by a needle hydrophone (HNC-1500, Onda Co., Sunnyvale, CA, USA) with a broad bandwidth ranging from 0 MHz to 10 MHz. Ten percent of the laser energy was guided to a black piece of rubber to determine the light energy. An ultrasonic transducer (fc = 1 MHz, V302, Olympus Corp., Tokyo, Japan) received the generated PA signal from the black rubber. Pre-amplifiers were connected to the outputs of the hydrophone and ultrasonic transducer to increase the signal-to-noise ratio (SNR), and the signals were then digitized and recorded using a digital oscilloscope (HDO6000, oscilloscope, Teledyne LeCroy, USA). The PA signal was averaged over 30 measured values to improve the SNR.
Fig. 2.
Experimental setup. (a) and (b) Schematics of the experiment. (c) Photograph of the experiment. To visualize the location of the laser beam, the laser wavelength was tuned to 700 nm for the acquisition of this photograph. The laser wavelength was tuned to various wavelengths in the range between 1300 and 1800 nm during the experiment. (d) Photograph of rabbit bone specimen. (e) Example PA signal generated by the rabbit bone specimen. (f) Photograph of human bone specimen. (g) Example PA signal generated by the human bone specimen.
By adjusting the laser wavelength, we obtained a PA signal in the wavelength range of 1300–1800 nm as well as the PA absorption curve for each bone specimen. To enhance stability and reduce measurement error, the PA signals from each bone specimen were detected from two different locations. The PA spectra from the two different locations were averaged for subsequent analyses. Finally, spectral unmixing based on the least-squares regression method was conducted, and the average PA absorption spectrum of each bone group was decomposed to obtain the proportions of its constituent chemicals.
2.5. Animal and human ex vivo bone specimens
In this study, we used two types of bone specimens to investigate the feasibility of collagen detection using the PA technique. In the first instance, well-established rabbit models of bone loss and preservation were employed. Female New Zealand rabbits (3–4 months old) were subjected to sham surgery (Sham, N = 6) and ovariectomy (OVX, N = 6). The OVX rabbit model was validated based on early bone turnover that produces bone loss due to estrogen withdrawal. Twenty weeks after surgery, the rabbits were euthanized, and the femora were dissected and subjected to PA assessment as shown in Fig. 2(d). Using the setup depicted in Fig. 2, the PA measurements were performed ex vivo on bone tissue at the distal end of the femur. In the second instance, human bone specimens were used, as shown in Fig. 2(f). In total, 17 fresh human trabecular bone specimens were extracted from patients undergoing joint replacement surgery. The use of these specimens was approved by the patients and by the Institutional Review Board of the Institute of Drum Tower Hospital, School of Medicine, Nanjing University (No. 2009022). The bone specimens were initially stored at −80 °C. During the subsequent procedures, the samples were maintained at room temperature (20 °C).
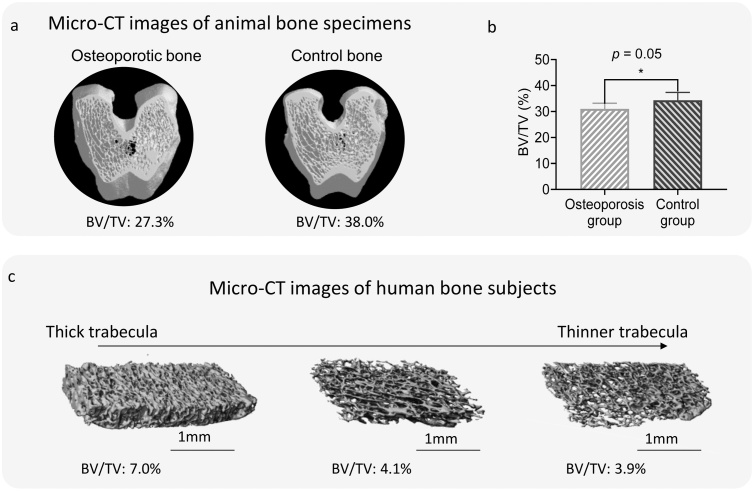
To validate the PA measurement results obtained using the two types of bone specimens, all the bone specimens were scanned using a micro-CT system (SCANCO, vivaCT 80). To validate the PA measurements acquired using the human bone specimens, bone volume/total volume (BV/TV) data were obtained from the micro-CT images and used for further analyses, as shown in Fig. 3. The micro-CT images of the rabbit bone specimens, as shown in Fig. 3(a,b), verify that the mean BV/TV value of the OVX group was significantly decreased, with an 3.3 % reduction with respect to that of the control group. Fig. 3(c) shows the micro-CT results for three human bone specimens with different BV/TV values. This ratio was obtained for each human bone specimen, and the values ranged from 1.1% to 12.1%. This analysis based on micro-CT imaging confirmed the variation among the specimens, for both the animal and human bone specimens used in this study.
Fig. 3.
Micro-CT images of the rabbit and human bone specimens. (a) Example micro-CT images of bones from the osteoporosis and control groups. (b) Statistical analysis of bone volume/total volume (BV/TV) for the rabbit bone specimens from the osteoporosis and control groups. (c) Example micro-CT images of human bone specimens with different BV/TVs.
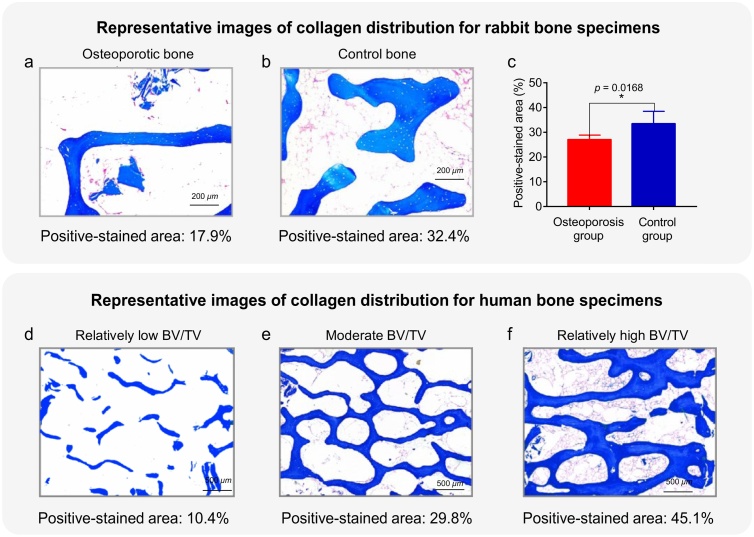
In this study, the collagen in the rabbit and human bone specimens was examined and quantified via histological analysis. After harvesting, all the bone specimens were fixed in 4% paraformaldehyde (E672002, Sangon Biotech), decalcified with ethylene diamine tetraacetic acid (EDTA) solution (E671001, Sangon Biotech) for 2 months and embedded in paraffin (8002-74-2, Sigma-Aldrich). Serial longitudinal sections (5 μm thick) were cut, and the sections were stained with Masson’s trichrome (HT15, Sigma-Aldrich) according to standard laboratory protocols. The morphological changes and collagen content of the human and animal samples were observed using microscopy, as shown in Fig. 4. The positive-stained area, calculated using MATLAB software for each sample slice, was averaged, and this mean value was employed as the gold standard for the amount of collagen in the bone specimens. These positive-stained areas were compared with the relative collagen parameter values quantified from the MWPA results.
Fig. 4.
Histological results with collagen quantification obtained using Masson’s trichrome (TriC) staining for rabbit and human bone specimens. (a, b) Representative images of rabbit bone specimens from the osteoporosis and control groups, respectively. (c) Statistical analysis results for the positive-stained areas of rabbit bone specimens from the osteoporosis and control groups. (d–f) Representative images of human bone specimens with different collagen contents.
3. Results
3.1. Simulation results
Fresh bone mainly contains minerals (hydroxyapatite), water, collagen, lipids, and hemoglobin [33]. In the spectral range of 1300–1800 nm, the main optically absorbing components in bone are collagen, water, and lipids, whereas a small amount of light is absorbed by other materials in this range [6,32,[34], [35], [36], [37]]. According to the literature, collagen has a strong absorption peak at 1530 nm, whereas water and lipids have absorption peaks near 1450 and 1730 nm, respectively, as shown in Fig. 1(d) [32].
Fig. 5 presents the MWPA simulation results for bone samples with different collagen contents. Fig. 5(a–c) depict the PAPCS in the frequency–wavelength domain to elucidate both the physical and chemical information in the PA signal acquired from the bone tissue. As shown in Fig. 5(a–c), differences are evident among the PAPCS of the bone samples with different collagen contents. Compared with the bone samples with low collagen content, the PAPCS of the bone with high levels of collagen exhibits a broader and more intense power distribution in the range of 1300–1800 nm, which is attributed to the optical absorption of both collagen and water. To present the PA absorption curves in the wavelength range of 1300–1800 nm, we summed the energy of the PA signal in a specified frequency range using Eq. (6) for each wavelength. In this study, for consistency with the experimental work, the specified frequency range in the PAPCS of each bone sample was set as 1–5 MHz. As an example, Fig. 5(e) depicts the simulated PA absorption curve.
Fig. 5.
Results of simulation of collagen detection using multi-wavelength photoacoustic (MWPA) analysis. (a–c) PA physio-chemical spectra (PAPCS) of bone specimens with different BV/TVs. (d) Normalized optical absorption coefficient μa versus wavelength for collagen, lipid, and water. (e) Comparison of simulated PA spectra with fitted spectra obtained using the least-squares method for the model bone specimens with different BV/TVs. (f) Analysis of correlation between the relative collagen content as obtained via MWPA and the positive-stained area metric for the collagen content extracted from the histological images.
To obtain the relative contribution of each chemical component, spectral decomposition was performed for each PA absorption curve via the least-squares method. Using the normalized optical absorption coefficient spectrum of the main optically absorbing components in bone, shown in Fig. 5(d), the simulated PA absorption curve of the bone sample was fitted as illustrated in Fig. 5(e) (R2 ≈ 0.997). The relative collagen content of each bone sample can be obtained by unmixing. To study the relationship between the relative collagen content parameter extracted from the experimental MWPA data and the ground truth, the correlation between the MWPA-derived relative collagen content and the positive-stained area in the model bone specimens was analyzed using Spearman’s rank correlation coefficient, as shown in Fig. 5(f). The results demonstrated that the relative collagen content, as obtained via MWPA, is closely correlated to the positive-stained area value from the histological results (p-value ≈ 0.0001, RS ≈ 0.86).
3.2. Experimental results obtained from rabbit bone models
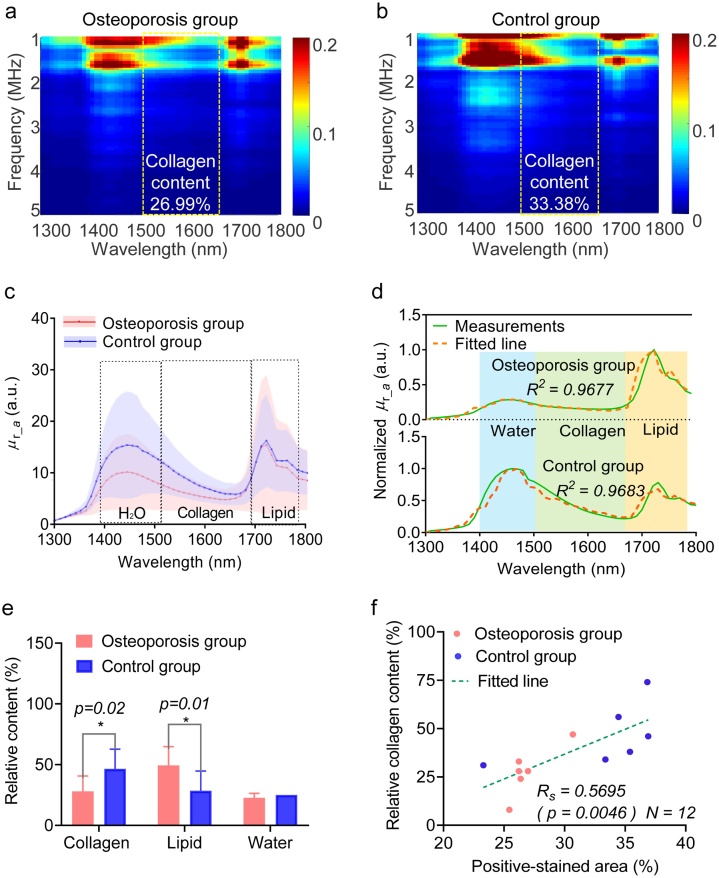
Fig. 6 shows the experimental MWPA measurements obtained from the rabbit bone models. Fig. 6(a–b) presents the average (mean) PAPCS for the osteoporosis and control groups. To avoid low- and high-frequency system noise, we set the low and high cut-off frequencies to 1 MHz and 5 MHz, respectively. Differences are evident between the PAPCS of the osteoporosis and control groups in Fig. 6 (a,b). Compared with the PAPCS of the osteoporosis group in Fig. 6(a), the PAPCS of the control group in Fig. 6(b) has a broader and more intense absorption feature in the range of 1500–1650 nm, which is attributed to both collagen and water, whereas it shows narrow and weak absorption in the range of 1650–1750 nm for the control group, which is assigned to the absorption of lipids. These experimental findings are consistent with the simulation results.
Fig. 6.
Multi-wavelength photoacoustic analysis (MWPA) results for the osteoporosis and control rabbit model groups. (a) Photoacoustic physio-chemical spectra (PAPCS) of a rabbit bone specimen from the osteoporosis group. (b) PAPCS of a rabbit bone specimen from the control group. (c) MWPA curves of rabbit bone specimens from the osteoporosis and control groups. (d) Example MWPA curve fits for the osteoporosis and control groups. (e) Statistical analysis results for the relative collagen, lipid, and water contents of the osteoporosis and control groups. (f) Analysis of correlation between the relative collagen content as obtained via MWPA and the positive-stained area for the collagen content extracted from the histological images.
To analyze the spectra more carefully, we calculated the wavelength dependence of the relative optical absorption coefficient using Eq. (6) for each bone specimen in the osteoporosis and control groups. The results are presented in Fig. 6(c). The lines indicate the average PA spectra, whereas the shaded areas overlapping the lines indicate the standard deviations. The overall shape of the PA spectrum is in good agreement with results reported in the literature [38]. Compared with the PA absorption spectrum from the control group, we observe relatively weak absorption in the region of 1500–1650 nm and strong absorption at 1650–1750 nm in osteoporosis group. These differences between the PAPCS and multi-wavelength PA curves for the two experimental groups arise because the osteoporotic bone contains less collagen but more lipids with respect to the control bone, which is consistent with the results of previous studies [39,40].
As the absorption peaks of water and collagen are close to one another, the relative collagen content is difficult to determine from the absolute value of the measured PA optical spectrum. Using the known optical absorption spectra of all the major chemical components of bone, the relative contribution of each chemical component to the PA spectrum can be derived by performing spectral unmixing. A least-squares method was used to fit the PA spectrum via a linear combination of the optical spectra of all the chemical components. Fig. 6(d) compares example PA spectra from the control and osteoporosis groups with the spectral fits based on the least-squares method. An R2 value of 0.97 was achieved, demonstrating successful fitting with high accuracy through spectral unmixing.
The relative collagen content can be obtained after spectral unmixing, as shown in Fig. 6(e,f). As the optical absorption data used were not based on the molar concentration of the chemical components, the relative content derived via spectral unmixing reflects the mass, rather than the molar amount, of each absorber in the bone. The results in Fig. 6(e,f) show that the osteoporosis group has lower collagen content than the control group. To examine whether the changes in the collagen content in the bone were significant, unpaired t-tests were performed to compare the results in Fig. 6(e). The collagen and lipid contents of the osteoporosis and control groups were statistically significant different (p-value: 0.02 (collagen) and 0.01 (lipid)). The differences in the water content between the two groups were not significant. Furthermore, the correlation between the relative collagen content parameter obtained from MWPA and the positive-stained area obtained via histology was analyzed using Spearman’s rank correlation method, as shown in Fig. 6(f). The results demonstrate that the relative collagen content parameter correlates well with the positive-stained area metric (p-value ≈ 0.0046, RS ≈ 0.57).
3.3. Experimental results obtained using human bone specimens
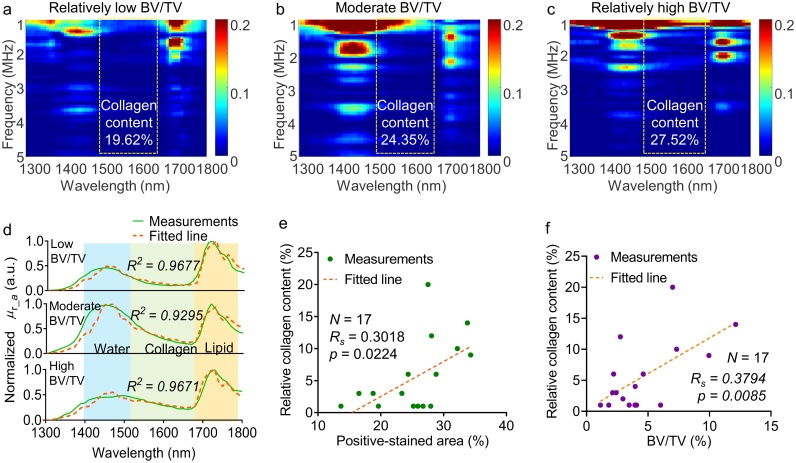
Fig. 7 shows the MWPA results for human bone specimens with different BV/TVs. Fig. 7(a–c) provide example PAPCS for human bone specimens with BV/TVs of 1.1 %, 2.3 %, and 7.0 %, respectively. With increasing BV/TV, the PA spectrum in the range of 1500–1650 nm becomes broader and more intense, consistent with the simulation and experimental results obtained using the animal model.
Fig. 7.
Multi-wavelength photoacoustic analysis (MWPA) results for the human bone specimens. (a–c) PA Physio-chemical spectra (PAPCS) of the bone specimens with different BV/TV values. (d) Example MWPA curve fitting for human bone specimens with different BV/TVs. (e) Analysis of correlation between the relative collagen content as obtained via MWPA and the positive-stained area metric for the collagen content extracted from the histological images. (f) Analysis of the correlation between BV/TV and the positive-stained areas in the histological images.
To analyze the spectrum more carefully, we conducted MWPA analysis on the human bone specimens and obtained the MWPA curve and relative collagen content via decomposition. To examine whether the relative collagen content as obtained via MWPA was correlated with the gold-standard results, analyses of the correlation between the MWPA-derived relative collagen content and the positive-stained area identified in the histological analysis and between the MWPA-derived relative collagen content and the BV/TV value obtained from the micro-CT results were carried out. The relative collagen content was found to correlate well with the positive-stained area (p-value ≈ 0.0224, RS ≈ 0.30) as well as the BV/TV results (p-value ≈ 0.0085, RS ≈ 0.38).
4. Discussion and conclusion
This study demonstrated the potential role of the MWPA method as a contrast-agent-free technique for the detection of collagen as a biomarker for bone health assessment. Using numerical simulations and experiments performed using a clinically relevant rabbit bone model and human bone specimens, we demonstrated that the MWPA method can provide information about the collagen content, which is closely related to bone mineral mass and bone metabolism. In our previous work, we demonstrated the capability of MWPA analysis in the short-wavelength NIR range for bone assessment by detecting differences in blood content, oxygen saturation, and lipid content in vivo [24]. To date, although the use of exNIR wavelengths has been reported, to the best of our knowledge, these wavelengths less been applied to bone collagen content detection. Our findings in this work suggest that the assessment of the collagen content of the bone using MWPA analysis is suitable for distinguishing osteoporotic and healthy bones as well as monitoring the degeneration of collagen in human bone.
The present study had some limitations. First, light attenuation in the bone samples was not considered. The bone specimens used were very thin (∼1 mm) in this study, therefore, the results were negligibly affected by light attenuation in the bone. However, in vivo clinical studies have shown that a large bone size leads to significant ultrasound and light attenuation in the bone. Therefore, compensation for these ultrasound and light losses should be considered in future clinical studies. Furthermore, in clinical studies, because of spectral coloring in soft tissue and bone, linear unmixing of photoacoustic spectra can rarely be used to acquire accurate chemical composition information from uncorrected photoacoustic spectra of tissue. In our previous clinical study on the human calcaneus bone, wavelength-dependent light attenuation was partially addressed based on the bone model [25]. Previous studies from other groups have also indicated that spectral coloring can be eliminated using model-based or machine-learning-based methods [[41], [42], [43], [44], [45], [46], [47]]. Therefore, in future clinical studies, PA absorption curves affected by spectral coloring should be further studied and corrected.
Second, we used a needle hydrophone to receive the PA signal in this study, which may be unsuitable for clinical research. Compared with the majority of other types of commercial ultrasonic transducers, needle hydrophones have broad frequency responses in the range of 0–10 MHz. As the PA signal generated by tissue is rich in information in this frequency range, the use of the needle hydrophone facilitated the acquisition of this information-rich PA signal in our ex vivo study. However, in clinical investigations, the frequency ranges of the PA signals are expected to be much lower than those of the PA signals acquired in ex vivo studies because of the strong ultrasound attenuation; thus, ultrasound transducers with lower center frequencies should be considered for clinical studies.
Third, in this work, some discrepancies between the measured and fitted lines were observed, especially in the human study. In this case, the bone specimens were extracted from patients undergoing joint replacement surgery. Therefore, parts of the bone specimens were stained by blood, which is rich in water and strongly absorbs in the wavelength range of 1300–1800 nm. This affected the results of the human study (Fig. 7(d–f)). For the animal study, the bone model is well established and controlled. In this case, the specimens were extracted after the rabbit had been euthanized, leading to less blood staining. This is one of the major reasons that the experimental results from the rabbit model were more consistent with the fitting results (Fig. 6 (d,f)). In the future work, well-controlled experiments on human bone specimens should be carried out using a large number of human bone specimens.
Despite these limitations, this study successfully proved the feasibility of using PA techniques to access the collagen content of bone. Compared with the established gold-standard DEXA imaging modality, the presented PA bone assessment method is target-specific, non-ionizing, low-cost, and patient-friendly. Furthermore, developing PA techniques can help accurately quantify organ-level chemical and molecular changes, such as lipid content, blood content (perfusion), and hydroxyapatite content, and can provide physical information about bone that is closely related to BMD and BMA. Considering that the PA technique can provide information about both the organic and non-organic tissues in the bone non-invasively, it has excellent potential for the early identification of changes in bone metabolism, bone disease assessment, and treatment monitoring.
Funding
This work was supported by the National Natural Science Foundation of China [grant numbers 12034015, 11827808, 11704188, 11674249]; the National Key Research and Development Project [grant numbers 2017YFC0111400 and 2016YFA0100800]; and the Postdoctoral Science Foundation of China [grant number 2019M651564].
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Biographies

Ting Feng is a lecturer at the School of Electronic and Optical Engineering in Nanjing University of Science and Technology. Concurrently, she is a postdoctoral research fellow at the School of Physics Science and Engineering in Tongji University. In addition, she was the visiting scholar at the University of Michigan in 2018 and 2019. Before working as an independent investigator, Ting Feng received her Graduate degree, Master's degree and Ph. D. degree from Nanjing University in 2010, 2012 and 2016, respectively. She was the joint-PhD student at the University of Michigan in 2013−2015. Her current research interest includes photoacoustic imaging and measurements. A major part of her research is focused on clinical application of photoacoustic techniques for bone assessment.

Yuxiang Ge is a Ph.D. candidate student from Fudan University. He received Bachelor degree from Nanjing Medical University and Master degree from Nanjing University. Now he is in his second year of Ph.D. and his current research focuses on the pathogenesis and treatment of osteoarthritis and intervertebral disc degeneration.

Yejing Xie is a master student from Nanjing University of Science and Technology. She received Bachelor degree from Jiangsu Normal University. She is now in the second year of her master's degree and her current research focuses on Multi-wavelength photoacoustic spectroscopy.

Weiya Xie is a Ph.D. candidate student from Institute of Acoustics, School of Physics Science and Engineering of Tongji University. She received Bachelor degree from Ocean University of China. Her current research focuses on photoacoustic measurement of bone health and photoacoustic imaging.

Chengcheng Liu is currently a young Professor at the Academy for Engineering and Technology, Fudan University, Shanghai, China. He received the B.S. and Ph.D. degrees from the Department of Electronic Engineering, Fudan University, Shanghai, China, in 2009 and 2014, respectively. He was a Post-Doctor in Fudan University and an Assistant Professor in Tongji University, Shanghai. His research interests include biomedical ultrasound, ultrasonic signal processing, and ultrasonic imaging.

Nan Li is a research assistant and post-doctor at Nanjing Drum Tower Hospital, Medical School of Nanjing University, Nanjing, China. He received the Ph.D. degree from the School of Mechanical Engineering, Southeast University, Nanjing, China, in 2020. His research interests include 3D printing, biomaterials, and bone/cartilage regeneration.

Dean Ta (Member, IEEE) received M.S. and Ph.D degrees from the Institute of Acoustics in Shaanxi Normal University and Tongji University, China in 1999 and 2002, respectively. He is currently vice head of Electronic Engineering Department of Fudan University, Vice President of the Acoustical Society of China (ASC) and Chairman of Biomedical Ultrasound Engineering Speciality of ASC. He was the Principal Investigators of more than 20 projects. In the last decade, he has contributed more than 200 papers, coauthored 5 books and 17 patents. His research interests include bone ultrasound, biomedical ultrasound and diagnosis system, and medical signal processing. The generation, propagation and applications of ultrasonic guided waves in bone and NDT & E. The applications of numerical techniques in ultrasound.

Qing Jiang had been engaged in orthopedic and sports medicine clinical and basic research since 1989. He is not only the first sports medicine clinical doctor cultivated by China, and also is the only winner of National Outstanding Youth Fund of the sports medicine profession. Prof. Jiang Mainly engaged in the basic and clinical research of sports system diseases, and published 239 Chinese core articles, 165 SCI articles, included Nature Medicine, Nature Genetic, ACS NANO, Advanced Functional Materials, Angew. Chem. Int. Ed. Engl., Nano Research, FASEB J., Annals of the Rheumatic Diseases and so on. The total number of citations is 2198, H index is 27. He is the first domestic scholars who hold the post of committee member of the OARSI, and he is the vice chair of China branch of basic research branch of SICOT, the vice chair of China branch of ICRS, the vice chairman of sports medicine branch of Chinese medical association, the chairman of sports medicine branch of Jiangsu province, vice chairman of orthopedics branch, vice chairman of trauma branch, the head of Nanjing Drum Tower hospital orthopedics laboratory of ICMRS, etc.

Qian Cheng is the group leader for photoacoustic imaging and quantitative analysis at the Tongji University. Her main research interest is in the clinical translation of photoacoustic imaging and quantitative analysis, and in particular for tumor diagnosis and evaluation. She studied acoustics at the Tongji University and received her master degree in physics. After that, she studied at Laboratory of Physical Mechanics at First University of Bordeaux in France as an exchange student and received her PhD in 2006 from Tongji University. She has over 10 years’ experience developing ultrasound and photoacoustic imaging and quantitative analysis methods for clinical studies.
Contributor Information
Qing Jiang, Email: qingj@nju.edu.cn.
Qian Cheng, Email: q.cheng@tongji.edu.cn.
Appendix A
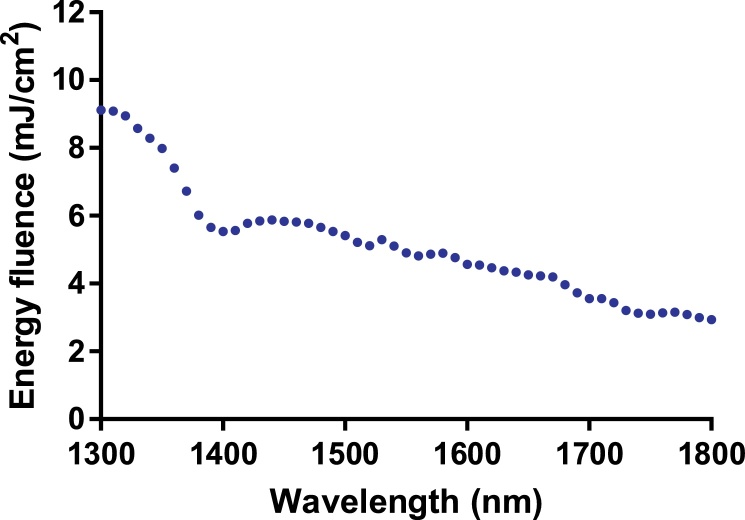
The energy fluence of the laser source across the wavelength range of interest (1300–1800 nm) is shown in Fig. A1.
Fig. A1.
Energy fluence of the laser source across the wavelength range of interest (1300–1800 nm).
Appendix B
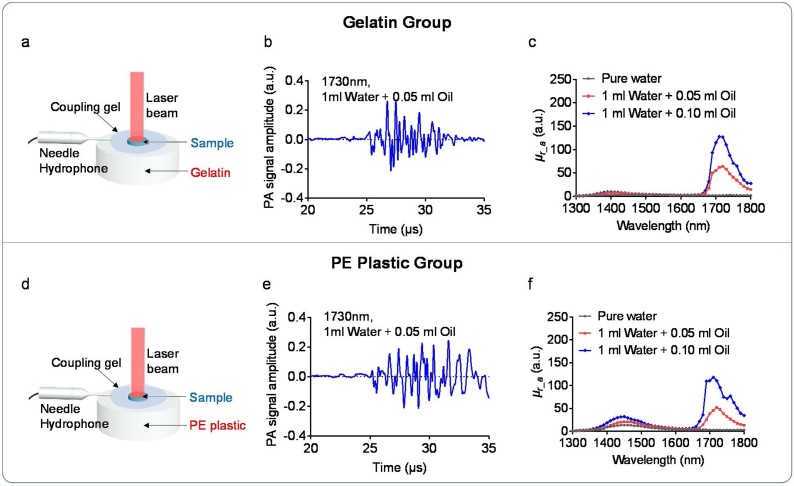
To test the concept of our research, we have conducted phantom studies with water and oil inclusions by using the experimental setup using two different types of background materials (i.e., gelatin and PE plastic). These experimental setups that are shown in Fig. B1 (a) and (d) were similar to that used in animal and human study shown in Fig. 2 (a–b). The samples used in this phantom study were water and oil inclusions having constituencies as follows: 1) sample #1: 1 ml water (water only), 2) sample #2: 1 ml water +0.05 ml oil, and 3) sample #3: 1 ml water +0.10 ml oil. The results for the gelatin and PE plastic groups are shown in Figs B (b–c) and (e–f), respectively. Fig. B1 (b) and (e) show the PA signal acquired at 1730 nm by using the gelatin and PE plastic background, respectively. Compared to the study that used gelatin background, the PA signal in the PE plastic background contains more reverberation, as shown in Fig. B1 (e). This might be due to the different mechanical properties of gelatin and PE plastic. Fig. B1 (c) and (f) show the relative PA absorption curves acquired from the three samples by using the experimental setup with different background materials (i.e., gelatin and PE plastic). This shows that all the relative PA absorption curves have an absorption peak at 1730 nm (the optical absorption peak of lipid) as we expected. Furthermore, the relative PA absorption at 1730 nm of the sample #3 (1 m water +0.10 ml oil) is approximately twice higher than that of sample #2 (1 ml water +0.05 ml oil) in the results of both gelatin group and PE plastic group. In conclusion, despite the differences shown in Fig. B1 (b) and (e), our results shown in Fig. B1 (c) and (f) indicate that the method used in this study can sense the chemical changes in different samples with less system noises.
Fig. B1.
Phantom study of measuring the PA absorption curves by using different samples and different background materials (i.e., gelatin and PE plastic). (a) Schematic of the experimental setup with gelatin as the background material. (b) PA signal acquired by the experimental setup with gelatin as the background. (c) Relative PA absorption curves of three different samples (i.e., pure water, 1 ml water +0.05 ml oil, 1 ml water +0.1 ml oil) for the gelatin group. (d) Schematic of the experimental setup with PE plastic as the background. (f) PA signal acquired by the experimental setup with PE plastic as the background material. (f) Relative PA absorption curves of three different samples (i.e., pure water, 1 ml water +0.05 ml oil, 1 ml water +0.1 ml oil) for the PE plastic group.
References
- 1.Töyräs J., Nieminen M., Kröger H., Jurvelin J. Bone mineral density, ultrasound velocity, and broadband attenuation predict mechanical properties of trabecular bone differently. Bone. 2002;31:503–507. doi: 10.1016/s8756-3282(02)00843-8. [DOI] [PubMed] [Google Scholar]
- 2.Liu C. The relationship between ultrasonic backscatter and trabecular anisotropic microstructure in cancellous bone. J. Appl. Phys. 2014;115 [Google Scholar]
- 3.Njeh C., Boivin C., Langton C. The role of ultrasound in the assessment of osteoporosis: a review. Osteoporos. Int. 1997;7:7–22. doi: 10.1007/BF01623454. [DOI] [PubMed] [Google Scholar]
- 4.Burr D.B. The contribution of the organic matrix to bone’s material properties. Bone. 2002;31:8–11. doi: 10.1016/s8756-3282(02)00815-3. [DOI] [PubMed] [Google Scholar]
- 5.Fratzl P., Gupta H.S., Paschalis E.P., Roschger P. Structure and mechanical quality of the collagen–mineral nano-composite in bone. J. Mater. Chem. 2004;14:2115–2123. [Google Scholar]
- 6.Griffith J.F., Yeung D.K., Antonio G.E. Vertebral marrow fat content and diffusion and perfusion indexes in women with varying bone density: MR evaluation. Radiology. 2006;241:831–838. doi: 10.1148/radiol.2413051858. [DOI] [PubMed] [Google Scholar]
- 7.Patsch J.M., Li X., Baum T., Yap S.P. Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J. Bone Miner. Res. 2013;28:1721–1728. doi: 10.1002/jbmr.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pifferi A. Optical biopsy of bone tissue: a step toward the diagnosis of bone pathologies. J. Biomed. Opt. 2004;9:474–481. doi: 10.1117/1.1691029. [DOI] [PubMed] [Google Scholar]
- 9.Morris M.D., Mandair G.S. Raman assessment of bone quality. Clin. Orthop. Relat. Res. 2011;469:2160–2169. doi: 10.1007/s11999-010-1692-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu M., Wang L.V. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006;77 [Google Scholar]
- 11.Cox B., Laufer J.G., Arridge S.R., Beard P.C. Quantitative spectroscopic photoacoustic imaging: a review. J. Biomed. Opt. 2012;17 doi: 10.1117/1.JBO.17.6.061202. [DOI] [PubMed] [Google Scholar]
- 12.Upputuri P.K., Pramanik M. Recent advances toward preclinical and clinical translation of photoacoustic tomography: a review. J. Biomed. Opt. 2016;22 doi: 10.1117/1.JBO.22.4.041006. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Y. Towards clinical translation of LED-based photoacoustic imaging: a review. Sensors. 2020;20:2484. doi: 10.3390/s20092484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian C., Zhang W., Mordovanakis A., Wang X., Paulus Y.M. Noninvasive chorioretinal imaging in living rabbits using integrated photoacoustic microscopy and optical coherence tomography. Opt. Exp. 2017;25:15947–15955. doi: 10.1364/OE.25.015947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinberg I., Huland D.M., Vermesh O., Frostig H.E., Gambhir S.S. Photoacoustic clinical imaging. Photoacoustics. 2019;14:77–98. doi: 10.1016/j.pacs.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schellenberg M.W., Hunt H.K. Hand-held optoacoustic imaging: a review. Photoacoustics. 2018;11:14–27. doi: 10.1016/j.pacs.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang W. Dual-modality X-ray-induced radiation acoustic and ultrasound imaging for real-time monitoring of radiotherapy. BME Frontiers. 2020;2020 doi: 10.34133/2020/9853609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lashkari B., Yang L., Mandelis A. The application of backscattered ultrasound and photoacoustic signals for assessment of bone collagen and mineral contents. Quant. Imaging Med. Surg. 2015;5:46–56. doi: 10.3978/j.issn.2223-4292.2014.11.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lashkari B., Mandelis A. Coregistered photoacoustic and ultrasonic signatures of early bone density variations. J. Biomed. Opt. 2014;19(19) doi: 10.1117/1.JBO.19.3.036015. [DOI] [PubMed] [Google Scholar]
- 20.Gu C., Katti D.R., Katti K.S. Photoacoustic FTIR spectroscopic study of undisturbed human cortical bone. Spectrochim. Acta A. 2013;103:25–37. doi: 10.1016/j.saa.2012.10.062. [DOI] [PubMed] [Google Scholar]
- 21.Wood C., Harutyunyan K., Sampaio D.R., Konopleva M., Bouchard R. Photoacoustic-based oxygen saturation assessment of murine femoral bone marrow in a preclinical model of leukemia. Photoacoustics. 2019;14:31–36. doi: 10.1016/j.pacs.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinberg I., Shiloh L., Gannot I. First-in-human study of bone pathologies using low-cost and compact dual-wavelength photoacoustic system. IEEE J. Sel. Top. 2018;25(1):1–8. [Google Scholar]
- 23.Feng T., Kozloff K.M., Tian C. Bone assessment via thermal photo-acoustic measurements. Opt. Lett. 2015;40:1721–1724. doi: 10.1364/OL.40.001721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng T., Perosky J.E., Kozloff K.M. Characterization of bone microstructure using photoacoustic spectrum analysis. Opt. Express. 2015;23:25217–25224. doi: 10.1364/OE.23.025217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng T., Zhu Y., Wang X. Functional photoacoustic and ultrasonic assessment of osteoporosis--a clinical feasibility study. BME Front. 2020;2020 doi: 10.34133/2020/1081540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Regensburger A.P., Fonteyne L.M., Jüngert J., Wagner A.L., Knieling F. Detection of collagens by multispectral optoacoustic tomography as an imaging biomarker for Duchenne muscular dystrophy. Nat. Med. 2019;25:1905–1915. doi: 10.1038/s41591-019-0669-y. [DOI] [PubMed] [Google Scholar]
- 27.Park E., Lee Y.-J., Lee C., Eom T.J. Effective photoacoustic absorption spectrum for collagen-based tissue imaging. J. Biomed. Opt. 2020;25 doi: 10.1117/1.JBO.25.5.056002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu G., Meng Z.X., Lin J.D. High resolution physio-chemical tissue analysis: towards non-invasive in vivo biopsy. Sci. Rep. 2016;6(1):1–14. doi: 10.1038/srep16937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sekar S.K.V., Bargigia I., Mora A.D. Diffuse optical characterization of collagen absorption from 500 to 1700 nm. J. Biomed. Opt. 2017;22 doi: 10.1117/1.JBO.22.1.015006. [DOI] [PubMed] [Google Scholar]
- 30.Liu C., Ta D., Bo H., Le L.H., Wang W. The analysis and compensation of cortical thickness effect on ultrasonic backscatter signals in cancellous bone. J. Appl. Phys. 2014;116 124903.124901–124903.124906. [Google Scholar]
- 31.Liu C., Han H., Ta D., Wang W. Effect of selected signals of interest on ultrasonic backscattering measurement in cancellous bones. Sci. China Phys. Mech. Astro. 2013;56:1310–1316. [Google Scholar]
- 32.Jacques S.L. Optical properties of biological tissues: a review. Phys. Med. Biol. 2013;58:R37. doi: 10.1088/0031-9155/58/11/R37. [DOI] [PubMed] [Google Scholar]
- 33.Boskey A.L., Robey P.G. In: Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. eighth ed. Rosen C.J., editor. Wiley-Blackwell; 2013. The composition of bone; pp. 49–58. [Google Scholar]
- 34.Abdulsamee N. Erbium family laser: silent revolution in dentistry, Review. EC Dental Science. 2017;13:168–190. [Google Scholar]
- 35.Deán-Ben X., Gottschalk S., Mc Larney B., Shoham S., Razansky D. Advanced optoacoustic methods for multiscale imaging of in vivo dynamics. Chem. Soc. Rev. 2017;46:2158–2198. doi: 10.1039/c6cs00765a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fantarella D., Kotlow L. The 9.3-μm CO2 dental laser: technical development and early clinical experiences. J. Laser Dent. 2014;22(1):10–26. [Google Scholar]
- 37.Featherstone J., Nelson D. Laser effects on dental hard tissues. Adv. Dent. Res. 1987;1:21–26. doi: 10.1177/08959374870010010701. [DOI] [PubMed] [Google Scholar]
- 38.Hkatov A.N., Genina E.A., Kochubey V.I., Tuchin V.V. Saratov Fall Meeting 2005: Optical Technologies in Biophysics and Medicine VII. 2006. [Google Scholar]
- 39.Saito M., Marumo K. Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos. Int. 2010;21:195–214. doi: 10.1007/s00198-009-1066-z. [DOI] [PubMed] [Google Scholar]
- 40.Di Pietro G. Bone marrow lipid profiles from peripheral skeleton as potential biomarkers for osteoporosis: a 1H-MR spectroscopy study. Acad. Radiol. 2016;23:273–283. doi: 10.1016/j.acra.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 41.Wang B. A convex cone method for accurate blood oxygenation photoacoustic imaging. Proc. SPIE 11550, Optoelectronic Imaging and Multimedia Technology VII, 1155006 (10 October 2020) 2020 doi: 10.1117/12.2574835. [DOI] [Google Scholar]
- 42.Kim M. Real-time interleaved spectroscopic photoacoustic and ultrasound (PAUS) scanning with simultaneous fluence compensation and motion correction. Nat. Commun. 2021;12:716. doi: 10.1038/s41467-021-20947-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stangl S. Eigenspectra optoacoustic tomography achieves quantitative blood oxygenation imaging deep in tissues. Nat. Commun. 2016;7:1–10. doi: 10.1038/ncomms12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bench C., Cox B. Quantitative photoacoustic estimates of intervascular blood oxygenation differences using linear unmixing. Journal of Physics: Conference Series; IOP Publishing; 2021. p. 012001. [Google Scholar]
- 45.Alijabbari N. Model-based optical and acoustical compensation for photoacoustic tomography of heterogeneous mediums. Photoacoustics. 2021;23 doi: 10.1016/j.pacs.2021.100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grohl J., Schellenberg M., Dreher K., Maier-Hein L. Deep learning for biomedical photoacoustic imaging: a review. Photoacoustics. 2021;22 doi: 10.1016/j.pacs.2021.100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim M.W., Jeng G.S., O’Donnell M., Pelivanov I. Correction of wavelength-dependent laser fluence in swept-beam spectroscopic photoacoustic imaging with a hand-held probe. Photoacoustics. 2020;19 doi: 10.1016/j.pacs.2020.100192. [DOI] [PMC free article] [PubMed] [Google Scholar]