Abstract
Introduction
Post stroke management has moved into the focus as it represents the only way to secure acute treatment effects in the long term. Due to individual courses, post stroke management appears rather challenging and is hindered by existing barriers between treatment sectors. As a novel concept, the PostStroke-Manager combines digital and sensor-based technology with personal assistance to enable intersectoral cooperation, best possible reduction of stroke-related disability, optimal secondary prevention, and detection of physical and psychological comorbidities.
Methods
This prospective single-center observational study aims to investigate the feasibility of the PostStroke-Manager concept in an outpatient setting. Ninety patients who have suffered an ischemic or hemorrhagic stroke or transient ischemic attack will be equipped with a tablet and mobile devices recording physical activity, blood pressure, and electrocardiographic signals. Through a server-based platform, patients will be connected with the primary care physician, a stroke pilot and, if necessary, other specialists who will use web-based platforms. Via the tablet, patients will have access to an application with 10 newly designed components including, for instance, a communication tool, medication schedule, medical records platform, and psychometric screenings (e.g., depression, anxiety symptoms, quality of life, adherence, cognitive impairment). During the 1-year follow-up period, clinical visits are scheduled at three-month intervals. In the interim, communication will be secured by an appropriate tool that includes text messenger, audio, and video telephony. As the primary endpoint, feasibility will be measured by a 14-item questionnaire that addresses digital components, technical support, and personal assistance. The PostStroke-Manager will be judged feasible if at least 50% of these aspects are rated positively by at least 75% of patients. Secondary endpoints include feedback from professionals and longitudinal analyses on clinical and psychometric parameters.
Perspective
This study will answer the question of whether combined digital and personal support is a feasible approach to post stroke management. Furthermore, the patient perspective gained regarding digital support may help to specify future applications. This study will also provide information regarding the potential use of remote therapies and mobile devices in situations with limited face-to-face contacts.
Trial registration
German Register for Clinical Trials (DRKS00023213.), registered 27 April 2021.
Keywords: Stroke, Post stroke management, Secondary prevention, Digital health, Mobile devices
Introduction
Stroke treatment has traditionally focused on recanalization strategies and the establishment of specialized treatment centers [1]. These interventions have resulted in significantly reduced short-term mortality and improved functional outcome [2]. Recently, post stroke management has come into focus as stroke is increasingly understood as a chronic condition with long-lasting impairments and individual burdens [3]. Socio-economic implications arise from a recent epidemiologic projection indicating a 27% increase in stroke prevalence over the next 30 years within the European Union [4].
The complexity of post stroke management with individual courses, impairments, and needs, impedes the development of overarching concepts [5, 6]. Goals include, first, the best possible reduction of stroke-related impairments to alleviate the individual burden and enable social participation or return to work wherever possible. In this regard, barrier-free access to disease-related information and cross-sectoral communications between health care professionals could help to avoid misinformation and to tailor medical therapies to individual needs [7]. Second, the rate of secondary events, which, after cerebral ischemia, typically affect 16.8% of patients within the first 5 years [8], should be kept as low as possible. To achieve this goal, control of risk factors, e.g., by close monitoring of blood pressure and lipids, and detection of atrial fibrillation is essential, along with adequate treatment by pharmacological or lifestyle interventions [9]. Third, physical and psychological comorbidities, also occurring at later stages, should be assessed to enable early interventions. In particular, depression and anxiety symptoms were described in a relevant proportion of stroke patients [10], and are believed to impact the quality of life. In addition to these requirements contributing to a comprehensive post stroke management with a patient-centered perspective, modern strategies also include mobilizing individual skills and resources to enhance patient empowerment.
In recent years, initiatives have been launched to establish post stroke programs. Focusing on structured pathways, these programs are characterized by clinical visits with supportive elements addressing patient motivation and physical activity, risk factors and their treatment [11–14]. Unexpectedly, one such program has recently failed to show a beneficial effect regarding secondary vascular events within a follow-up period of 3.6 years [13]. However, this study has shown positive effects in secondary outcomes such as the degree of blood pressure and lipid control as also seen in another study [11]. Other programs have integrated “stroke pilots”, specially trained personnel with various supportive roles [15–18]. Final evaluations on this personal support are still pending, but practical experiences revealed signals indicating that stroke pilots are beneficial in the field of post stroke management [19].
As a first attempt to incorporate technological innovations in post stroke management, digital elements have been implemented in pilot projects as documentation tools for stroke pilots [20] or patients [15], and a communication tool between treatment sectors [21]. The beneficial effects of more complex digital interventions in other conditions [22, 23] suggest that such technology could also be a powerful tool for post stroke management [24]. Against this background, we designed the PostStroke-Manager as a novel concept combining multiple innovations based on digital and personal assistance. This concept is designed to significantly improve management after stroke with a patient-centered perspective. Using an application (app) along with mobile sensors for physical activity, blood pressure, and electrocardiographic (ECG) signals, in combination with an intensified personal assistance, this concept accompanies patients during their path after stroke. By enabling communication between disconnected healthcare sectors and free availability of individualized information to professionals, providing optimal control of risk factors independent of the patients’ location, and allowing psychometry-based screening of psychological comorbidities and impaired quality of life, such a concept would address many currently existing challenges in the post stroke management.
Methods
Aim of the trial
This study aims to evaluate the feasibility of the PostStroke-Manager whose concept includes a newly developed mobile, digital and sensor-based technology combined with personal assistance in post stroke management.
Study description and study design
In a prospective single-center observational study, patients with an acute ischemic or hemorrhagic cerebral event and the ability to use mobile digital technologies, will be equipped with a commercially available tablet (Galaxy Tab S6lite, Samsung, Schwalbach, Germany) with installed PostStroke-Manager app, as well as a smartwatch with an activity tracker (TicWatch Pro 2020, Mobvoi, Hong Kong, China) and a blood pressure monitor also registering ECG signals (Veroval 2in1, Hartmann, Heidenheim, Germany). After a training phase under inpatient conditions and subsequent discharge from the hospital, patients will use the PostStroke-Manager app (see below for details) for a 1-year follow-up period, while the primary care physician, a stroke pilot, and, if necessary, specialists are connected with the patients via a web-based platform. Recruitment is planned for 1 year, beginning in fall 2021 after a brief period of technical verifications under inpatient conditions. Overall, recruitment and individual follow-up periods are expected to be completed in fall 2023 (last patient out).
Eligibility criteria
Patients with one of the following diagnoses will be considered for participation: Ischemic or hemorrhagic stroke, defined by a persistent or transient neurological deficit in combination with an ischemic lesion or an intracerebral hemorrhage, visualized by computed tomography or magnetic resonance imaging, or transient ischemic attack (TIA), defined by a transient neurological deficit without radiological evidence of an ischemic lesion [25].
Additional inclusion criteria are: Age of at least 18 years, no or only moderate functional impairment before the qualifying event (“(pre-[modified Rankin scale (mRS)] 0-2), stable course of clinical symptoms allowing discharge from hospital, residence in the area of Leipzig/Germany, and willingness of the patients’ primary care physician to participate in the study.
The main exclusion criteria are the following: Inability to use the digital system and mobile devices (e.g., severe neglect or aphasia and hemiplegia rate as disqualifying impairments), physical impairment that could interfere with the realization of the 1-year follow-up period (e.g., cancer with a life expectancy of less than 1 year), clinically relevant psychiatric disorder, known dementia, and existing or planned pregnancy.
Intervention
Using the PostStroke-Manager app, patients have continuous access to 10 components (Fig. 1) designed with the following intentions: (1) to summarize information about the individual risk profile, the qualifying event, its further course (including neurological sequelae, rehabilitations and follow-up examinations), which will prevent misinformation of professionals and care-providers regarding already identified risk factors, detailed disease-related impairments and treatment plans; (2) to provide a platform for medical records of the event and upcoming medical findings, that will allow barrier-free access to essential information; (3) to enable communication between the patient, the primary care physician, and the stroke pilot as well as other specialists if necessary, via a text messenger, audio or video telephony, to reduce communication barriers for optimal treatment planning; (4) to provide a calendar function as tool for planning individual healthcare services; (5) to enable psychometric measurements on depression, anxiety symptoms, quality of life, adherence and empowerment as well as cognitive performance, which allows early detection of comorbidities; (6) to provide a medication schedule with reminder function on a daily level, which is also linked to a commercially available drug library (MMI Pharmaindex, Medizinische Medien Informations GmbH, Neu-Isenburg, Germany); (7) to record blood pressure, heart rate and ECG signals on a daily basis, ensuring optimal control of risk factors; (8) to manage individual post stroke goals and actions with respect to the individual neurological impairments and risk factors; (9) to provide neuropsychological exercises based on established tests (Bells test and trail making test) and individual recommendations (e.g., exercise sheets provided by speech therapists); and (10) to provide an information portal with, for instance, pathophysiological details of the underlying event, treatment options and details on social and financial supports from the public health system, to enhance patients’ empowerment. The digital system also includes basic technical support by a database with frequently asked questions (FAQs) and a ticket system that allows electronic feedback.
Fig. 1.
Overview of the PostStroke-Manager concept with combined digital support and personal assistance. Using a tablet with a pre-installed app, 10 components will be available for the patient that cover different aspects of post stroke management. Personal assistance will be given by a nurse with special training in case management and typical issues arising in acute and later stages of stroke. The figure including icons was created with Microsoft PowerPoint and Word for Mac (version 16.47, 2021, Microsoft, Redmond, USA)
Personal assistance is provided by a stroke pilot who is an experienced nurse with special training in case management (acquired at the Dresden International University, Dresden, Germany) and additional training on stroke-related issues following the educational program of the German Stroke Foundation (Gütersloh, Germany). Specifically, the stroke pilot will help to navigate individual rehabilitation processes, which is inherently complex, particularly in the outpatient setting with varying providers of physical therapy, occupational therapy and speech therapy (Fig. 1). Furthermore, interactions with the patient are intended to evaluate individual disabilities with associated needs and goals, and also to screen for comorbidities (e.g., depression and anxiety symptoms). In addition to reviewing medication adherence and the use of physician-prescribed therapies, contacts will be used to cooperatively develop a biopsychological disease model based on individual risk factors, which plays a central role in patient empowerment. Regular visits are scheduled in 3-months-intervals with additional on-site contacts as demanded by the patient. In the meantime, support will be provided through the communication tool. To consider individual factors in terms of social and living environmental aspects (e.g., possible support by relatives, presence of an elevator in case of a higher floor level), the stroke pilot will conduct at least one home visit in the third month after study initiation. Regarding the tablet and mobile devices used by the patient, the stroke pilot provides instructions at the study entry and during the follow-up period.
Because the PostStroke-Manager app is not yet certified as a medical device, the medical aspects of individual post stroke management, such as checks of blood pressure and ECG as well as prescription of medication and physical therapies, will be provided by the primary care physician. As part of the regular care, consultations are planned in intervals of three months, which will also be used for blood-based analyses of lipids and glucose metabolism, and reassessment of current medication and needs for additional therapies.
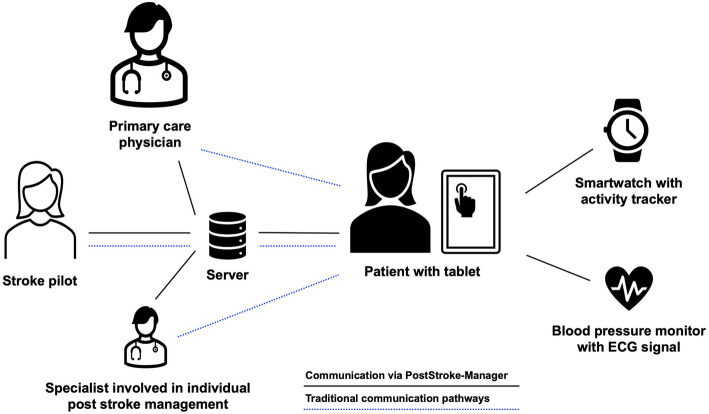
To enable communication between involved persons and to ensure stable registration of data obtained from the mobile devices, both the smartwatch and the blood pressure monitor are connected to a personalized tablet via Bluetooth, while the tablet is connected to a central server either via WLAN or mobile data usage (SIM card) (Fig. 2). Using web-based access to the system, stroke pilot, primary care physician and other specialists are connected to the server and have access to the individual patient data. To ensure highest levels of data protection, patients will have the central role in granting access to individual data as they voluntarily initiate connections to other persons. Thereby, connections will follow an informed consent and will technically be realized by a (QR) code.
Fig. 2.
Conceptualization of the PostStroke-Manager with a central position of the patient, equipped with a tablet and mobile devices for registration of physical activity, blood pressure, and electrocardiographic signals. Using WLAN or SIM cards, the tablet will be connected to a server allowing interactions with the primary care physician, the stroke pilot as well as specialists if indicated. Thereby, the system will mimic traditional communication pathways and at the same time will add a lot of technological advances for post stroke management. Abbreviation: ECG: electrocardiography. The figure including icons was created with Microsoft PowerPoint and Word for Mac (version 16.47, 2021, Microsoft, Redmond, USA)
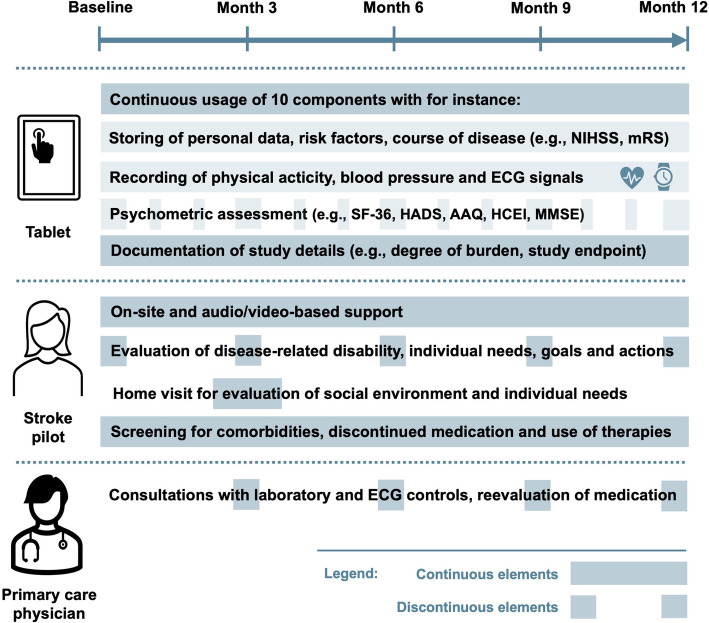
During the 1-year follow-up period, the combined digital-based support and personal assistance will be accompanied by quarterly arranged clinical visits performed by a study physician together with the stroke pilot (Fig. 3). Measurements for the clinical course and the psychometric testing are spaced at different intervals (Table 1). All data will be collected digitally as inputs are made entirely in the app- or web-based platform. For safety, clinical visits will also be used to check the correct usage of the mobile devices, and patients will be asked about any burden they have experienced from using the system. For this purpose, a 4-point scale was designed with the response opinions “no burden”, “low burden”, “relevant burden” and “severe burden”. In the case of a “relevant burden,” the patient will be asked to stop the study, whereas in the case of a “severe burden” participation of the patient will be terminated by study staff.
Fig. 3.
Overview of the PostStroke-Manager feasibility study. During a follow-up period of one year, visits are planned in 3-months-intervals, allowing direct interactions between the stroke pilot and the patients, while in the meantime communications will typically be arranged via the digital system. Abbreviations: NIHSS: National institutes of health stroke Scale, mRS: modified Rankin scale, SF-36: Short form (36) health survey, HADS: Hospital anxiety and depression scale, AAQ: Adherence assessment questionnaire, HCEI: Healthcare empowerment inventory, MMSE: Mini mental status examination, ECG: electrocardiography. The figure including icons was created with Microsoft PowerPoint and Word for Mac (version 16.47, 2021, Microsoft, Redmond, USA)
Table 1.
Overview of clinical and psychometric measurements
| Abbreviation | Full name and reference | Timepoint |
|---|---|---|
| Neurological deficits and functional impairment | ||
| NIHSS | National Institute of Health stroke scale [26] | Baseline, months 3, 6, 9 and 12 |
| mRS | modified Rankin scale [27] | Baseline, months 3, 6, 9 and 12 |
| BI | Barthel index [28] | Baseline, months 3, 6, 9 and 12 |
| Depression and anxiety symptoms | ||
| PHQ-9 | Patient health questionnaire 9 items [29] | Every month |
| HADS | Hospital anxiety and depression scale [30] | Baseline, months 3, 6, 9 and 12 |
| Quality of life | ||
| EQ-5D | EuroQoL 5 dimensions [31] | Every month |
| SF-36 | Short form (36) health survey [32] | Baseline, months 3, 6, 9 and 12 |
| Adherence | ||
| AAQ | Adherence assessment questionnaire [33] | Baseline, months 6 and 12 |
| ABQ | Adherence barriers questionnaire [34] | Baseline, months 6 and 12 |
| Empowerment | ||
| HCEI | Healthcare empowerment inventory [35] | Baseline, months 3, 6, 9 and 12 |
| Neuropsychological and cognitive impairment | ||
| BT | Bells test [36] | Irregular |
| TMT | Trail making test [37] | Irregular |
| MMSE | Mini mental status examination [38] | Baseline, months 3, 6, 9 and 12 |
In case of restrictions for face-to-face contacts that may occur in the context of the SARS-CoV-2 pandemic, critical visits are planned digitally. For this purpose, the communication platform is available for video-based interactions, while data collected with the tablet and mobile devices will generally be transferred to the server and thus become available without physical contacts.
Outcome measures
The primary endpoint is feasibility of the PostStroke-Manager with digital and personal support in a typical outpatient setting, starting very early after the qualifying event and thus during hospital stay. To use patient-reported outcome measures for evaluation, a questionnaire was designed that covers individual benefits regarding the digital system on the whole and the personal assistance provided by the stroke pilot (two questions), the digital components of the system except for the psychometric assessment (9 questions) and the technical support (3 questions related to the FAQ database, the ticket system and the contact with the stroke pilot). Patients will answer these questions at the end of the follow-up period, using a 6-point scale with the following options: “Very high benefit”, “high benefit”, “sufficient benefit”, “some benefit”, “rare benefit” and “no benefit”. The PostStroke-Manager concept will be judged feasible when at least 50% of the above 14 questions are rated as positive by at least 75% of patients. Here, the criterion “positive” corresponds to the answers “very high benefit”, “high benefit”, “sufficient benefit”, and “some benefit”. Furthermore, patients will be asked about their opinion regarding the assistance favored in the future, i.e., whether assistance should be provided by a digital system (app), or stroke pilots, or both (5-point scale: “surely pilots”, “rather pilots”, “both in a similar manner”, “rather app”, “surely app”).
Secondary endpoints include feasibility with regard to the three groups TIA, ischemic and hemorrhagic stroke. Furthermore, qualitative feedback from participating stroke pilots and primary care physicians on the feasibility of implementing the PostStroke-Manager concept will help to gain insight into the perspective of health care providers in addition to the patients’ perspective. Descriptive analyses are planned on secondary cerebrovascular events, the 1-year course of psychometric assessments (e.g., HADS, SF-36), laboratory, clinical variables and stroke-related impairments (NIHSS, mRS, BI) as well as blood pressure measurements, ECG signals, physical activity and medication adherence as recorded digitally by an automatically generated daily question and standardized questionnaires (AAQ, ABQ). Parameters will be evaluated in the overall sample as well as regarding the three groups of diagnoses. To consider economic aspects, days of hospitalization, details on rehabilitations and the frequency of medical services (e.g., physical therapies, consultations of the primary care physician) will be assessed. In addition to these analyses, technical problems will be recorded and signals originating from the activity tracker (including accelerometer and gyroscope) and the blood pressure monitor will be used for biomedical interpretations.
Sample size calculation
Due to the observational nature of the study and the primary endpoint, as assessed by a questionnaire presented to all patients, typical sample size calculations focusing on group comparisons are not applicable. For secondary endpoints, such as feasibility regarding the three included diagnoses, an estimated sample size calculation was performed with an effect size ranging between of 0.35 and 0.65, an α-error of 0.05 and a power (1-β-error) of 0.85 using the statistical toolbox “G*Power” (version 3.1.9.6 [39];). This calculation revealed that statistically significant differences between the groups TIA, ischemic and hemorrhagic stroke, can be found when using a sample size of 30 patients each group, resulting in an overall sample size of 90 patients.
Contacts
This study is part of a joint project between the Department of Neurology and Innovation Center Computer Assisted Surgery (ICCAS), both affiliated with Leipzig University, Leipzig, Germany. The medical content of the study has been designed and will be conducted by the Department of Neurology at the University of Leipzig, while the technical environment has been implemented and will be managed by ICCAS. Within the study, contractual arrangements are used to implement collaboration with primary care physicians, including study- and patient-related communication and data entry into the system. Additional contractual arrangements will be used for the collaboration with rehabilitation facilities. The evaluation of the study will be performed by the PostStroke-Manager consortium.
Perspective
By combining a digital health care app that includes 10 components addressing the complex requirements in the setting of post stroke management, and intensive personal assistance by specially trained stroke pilots, the PostStroke-Manager concept represents a novel approach for patient-centered care.
This comprehensive approach integrates new developments in the field of medical care, personal interactions, mobile devices, data handling, and protection, all applied in a typical outpatient setting. Although all mobile devices fulfill the standard of an already existing CE certification, their uncontrolled use together with a newly created app in an outpatient setting, which would directly impact on medical decisions, is excluded due to regulatory requirements. Therefore, this study aims to demonstrate feasibility of such an approach as a pre-condition for further developments. In this light, the primary endpoint was intentionally chosen to not include efficacy elements such as a reduction of secondary vascular events. Instead, patient-reported outcomes [40] are used to capture the patients’ perspective on the combined digital and personal support. To obtain valid information on this topic, this study will include patients capable of using an app and mobile devices. In case of a positive evaluation of the PostStroke-Manager concept, efficacy and the usability by a broader spectrum of patients will need to be evaluated in a subsequent study. Methodologically, this approach will require the inclusion of a control group to allow comparison with usual care, a larger number of patients, with statistical power calculations that can build on the present study, and a follow-up period of several years to allow realistic detection of secondary events. This subsequent study should also include a group of patients with limited ability to use mobile devices and must grant access to caregivers. This will help to provide insight into the extent to which family and caregiver support is needed in the use of digital technologies.
To address different needs and priorities that may arise in secondary stroke prevention with reference to the first cerebrovascular event, this feasibility study includes patients with ischemic stroke,TIA, and cerebral hemorrhage. For the planned secondary endpoints, this stratification may help to figure out which patients may experience the combined digital and personal support as most beneficial.
More generally, this study will provide information on the critical patient-centered perspective regarding digital-based supports, which will ultimately help to improve conceptual aspects of future digital applications. Further, this study will generate insights into the potential use of mobile devices and digital technology for remote care as an option that has recently gained importance to maintain stroke care in situations with limited face-to-face contacts [41, 42].
Acknowledgments
The authors thank Dr. Katrin Rothmaler, René Martin, Davide Iacovazzi, and Lars Kollmann (Institute for Applied Informatics, Leipzig, Germany) for their contribution to the PostStroke-Manager project in earlier stages. Further, Dr. Michael Brinkmeier and the team of the German Stroke Foundation (Gütersloh, Germany) are kindly acknowledged for providing valuable input as well as methodological and financial support in the training of stroke pilots. The authors further thank Prof. Peter Schlattmann (Institute of Medical Statistics, Computer Science and Data Sciences, University Hospital Jena, Jena, Germany) for assistance in sample size calculation.
Abbreviations
- AAQ
Adherence assessment questionnaire
- ABQ
Adherence barriers questionnaire
- App
Application
- BI
Barthel index
- BT
Bells test
- DRKS
German Register for Clinical Trials
- ECG
electrocardiography
- EQ-5D
EuroQoL 5 dimensions
- FAQs
Frequently asked questions
- HADS
Hospital anxiety and depression scale
- HCEI
Healthcare empowerment inventory
- ICCAS
Innovation Center Computer Assisted Surgery
- MMSE
Mini mental status examination
- mRS
modified Rankin scale
- NIHSS
National institutes of health stroke Scale
- PHQ
Patient health questionnaire 9 items
- pre-mRS
modified Rankin scale before the event
- QR
Quick response
- SF36
Short form (36) health survey
- TMT
Trail making test
Authors’ contributions
DM wrote the manuscript and created the figures with assistance from AP and JC. DM and JC managed regulatory affairs of the clinical study. TH, MS, JBT, and GI conceptualized the technical part of the study, while the medical aspects were conceptualized by DM, AP, JC, DU, DG, and SS with a neurological perspective as well as JG and SL with a primary care physician’s perspective. DM, JC, and GI realized the overall acquisition of the PostStroke-Manager project. DM and AP managed the acquisition of mobile devices with assistance from TH, MS, and GI, who also enabled the server application. All authors are involved in basic conceptualizations and the development of the PostStroke-Manager concept, had full access to the manuscript and figures, and agreed with the submission for publication. The author(s) read and approved the final manuscript.
Funding
This study is funded by the state government of Saxony (directive eHealthSax, grant no. 100334901, to DM, JC, and GI).
Availability of data and materials
The dataset generated and analyzed during the study is not publicly available due to the included personal data of patients. For scientific analyses, pseudonymized and anonymized data will be used by the PostStroke-Manager consortium. Generally, data will be stored for at least 10 years. Anonymized data may also be used for meta-analyses upon reasonable request according to the recommendation of the International Committee of Medical Journal Editors (ICMJE).
Declarations
Ethics approval and consent to participate
The presented study was approved by the local ethics committee at the Medical Faculty of the University of Leipzig (reference number: 504/20-ek). All participating patients will give informed consent by personal signature or verbally in the presence of a witness.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Joseph Classen and Galina Ivanova contributed equally to this work.
Contributor Information
Dominik Michalski, Email: dominik.michalski@medizin.uni-leipzig.de.
Alexander Prost, Email: alexander.prost@icloud.com.
Till Handel, Email: till.handel@uni-leipzig.de.
Max Schreiber, Email: max.schreiber@uni-leipzig.de.
Jean-Baptiste Tylcz, Email: jean-baptiste.tylcz@uni-leipzig.de.
Daniela Geisler, Email: daniela.geisler@medizin.uni-leipzig.de.
Daniela Urban, Email: daniela.urban@medizin.uni-leipzig.de.
Stephanie Schramm, Email: stephanie.schramm@medizin.uni-leipzig.de.
Stefan Lippmann, Email: stefan.lippmann@medizin.uni-leipzig.de.
Jenny Gullnick, Email: jenny.gullnick@medizin.uni-leipzig.de.
Thomas Neumuth, Email: thomas.neumuth@medizin.uni-leipzig.de.
Joseph Classen, Email: joseph.classen@medizin.uni-leipzig.de.
Galina Ivanova, Email: galina.ivanova@uni-leipzig.de.
References
- 1.Hankey GJ. Stroke. Lancet. 2017;389(10069):641–654. doi: 10.1016/S0140-6736(16)30962-X. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CB, van der Lugt A, de Miquel MA, Donnan GA, Roos YB, Bonafe A, Jahan R, Diener HC, van den Berg LA, Levy EI, Berkhemer OA, Pereira VM, Rempel J, Millán M, Davis SM, Roy D, Thornton J, Román LS, Ribó M, Beumer D, Stouch B, Brown S, Campbell BC, van Oostenbrugge RJ, Saver JL, Hill MD, Jovin TG, HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–1731. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 3.Hotter B, Padberg I, Liebenau A, Knispel P, Heel S, Steube D, Wissel J, Wellwood I, Meisel A. Identifying unmet needs in long-term stroke care using in-depth assessment and the post-Stroke checklist - the managing aftercare for Stroke (MAS-I) study. Eur Stroke J. 2018;3(3):237–245. doi: 10.1177/2396987318771174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wafa HA, Wolfe C, Emmett E, Roth GA, Johnson CO, Wang Y. Burden of Stroke in Europe: Thirty-year projections of incidence, prevalence, deaths, and disability-adjusted life years. Stroke. 2020;51(8):2418–2427. doi: 10.1161/STROKEAHA.120.029606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartford W, Lear S, Nimmon L. Stroke survivors' experiences of team support along their recovery continuum. BMC Health Serv Res. 2019;19(1):723. doi: 10.1186/s12913-019-4533-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehnerer S, Hotter B, Padberg I, Knispel P, Remstedt D, Liebenau A, Grittner U, Wellwood I, Meisel A, BSA Long Term Care Study Group Social work support and unmet social needs in life after stroke: A cross-sectional exploratory study. BMC Neurol. 2019;19(1):220. doi: 10.1186/s12883-019-1451-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reeves S, Pelone F, Harrison R, Goldman J, Zwarenstein M. Interprofessional collaboration to improve professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2017;6(6):CD000072. doi: 10.1002/14651858.CD000072.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amarenco P, Lavallée PC, Monteiro Tavares L, Labreuche J, Albers GW, Abboud H, Anticoli S, Audebert H, Bornstein NM, Caplan LR, Correia M, Donnan GA, Ferro JM, Gongora-Rivera F, Heide W, Hennerici MG, Kelly PJ, Král M, Lin HF, Molina C, Park JM, Purroy F, Rothwell PM, Segura T, Školoudík D, Steg PG, Touboul PJ, Uchiyama S, Vicaut É, Wang Y, Wong LKS, TIAregistry.org Investigators Five-year risk of Stroke after TIA or minor ischemic Stroke. New Engl J Med. 2018;378(23):2182–2190. doi: 10.1056/NEJMoa1802712. [DOI] [PubMed] [Google Scholar]
- 9.Esenwa C, Gutierrez J. Secondary stroke prevention: Challenges and solutions. Vasc Health Risk Manage. 2015;11:437–450. doi: 10.2147/VHRM.S63791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broomfield NM, Quinn TJ, Abdul-Rahim AH, Walters MR, Evans JJ. Depression and anxiety symptoms post-stroke/TIA: Prevalence and associations in cross-sectional data from a regional stroke registry. BMC Neurology. 2014;14(1):198. doi: 10.1186/s12883-014-0198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ögren J, Irewall AL, Söderström L, Mooe T. Long-term, telephone-based follow-up after stroke and TIA improves risk factors: 36-month results from the randomized controlled NAILED stroke risk factor trial. BMC Neurology. 2018;18(1):153. doi: 10.1186/s12883-018-1158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Towfighi A, Cheng EM, Ayala-Rivera M, Barry F, McCreath H, Ganz DA, Lee ML, Sanossian N, Mehta B, Dutta T, Razmara A, Bryg R, Song SS, Willis P, Wu S, Ramirez M, Richards A, Jackson N, Wacksman J, Mittman B, Tran J, Johnson RR, Ediss C, Sivers-Teixeira T, Shaby B, Montoya AL, Corrales M, Mojarro-Huang E, Castro M, Gomez P, Muñoz C, Garcia D, Moreno L, Fernandez M, Lopez E, Valdez S, Haber HR, Hill VA, Rao NM, Martinez B, Hudson L, Valle NP, Vickrey BG, Secondary Stroke Prevention by Uniting Community and Chronic Care Model Teams Early to End Disparities (SUCCEED) Investigators Effect of a coordinated community and chronic care model team intervention vs usual care on systolic blood pressure in patients with Stroke or transient ischemic attack: The SUCCEED randomized clinical trial. JAMA Network Open. 2021;4(2):e2036227. doi: 10.1001/jamanetworkopen.2020.36227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmadi M, Laumeier I, Ihl T, Steinicke M, Ferse C, Endres M, Grau A, Hastrup S, Poppert H, Palm F, Schoene M, Seifert CL, Kandil FI, Weber JE, von Weitzel-Mudersbach P, Wimmer M, Algra A, Amarenco P, Greving JP, Busse O, Köhler F, Marx P, Audebert HJ. A support programme for secondary prevention in patients with transient ischaemic attack and minor stroke (INSPiRE-TMS): An open-label, randomised controlled trial. Lancet Neurol. 2020;19(1):49–60. doi: 10.1016/S1474-4422(19)30369-2. [DOI] [PubMed] [Google Scholar]
- 14.Ihle-Hansen H, Langhammer B, Lydersen S, Gunnes M, Indredavik B, Askim T. A physical activity intervention to prevent cognitive decline after stroke: Secondary results from the life after STroke study, an 18-month randomized controlled trial. J Rehab Med. 2019;51(9):646–651. doi: 10.2340/16501977-2588. [DOI] [PubMed] [Google Scholar]
- 15.Kamoen O, Maqueda V, Yperzeele L, Pottel H, Cras P, Vanhooren G, Vanacker P. Stroke coach: A pilot study of a personal digital coaching program for patients after ischemic stroke. Acta Neurologica Belgica. 2020;120(1):91–97. doi: 10.1007/s13760-019-01218-z. [DOI] [PubMed] [Google Scholar]
- 16.SANO – Strukturierte ambulante Nachsorge nach Schlaganfall. Online: https://innovationsfonds.g-ba.de/projekte/neue-versorgungsformen/sano-strukturierte-ambulante-nachsorge-nach-schlaganfall.177 (Abfrage 19.04.2021)
- 17.Stroke OWL – Schlaganfall-Lotsen für Ostfalen-Lippe. Online: https://stroke-owl.de/de/startseite/ (Abfrage 19.04.2021)
- 18.SOS-Care: Schlaganfallnachsorge. Online: https://www.carusconsilium.de/de/ projekte/telemedizin/sos-care (Abfrage 19.04.2021)
- 19.Galle, G. & Brinkmeier, M. (2021) Mut zu echter Innovation: Die Einführung von Gesundheitslotsen in Deutschland. In: Scholz, S. & Engehausen, R. Innovationsfonds – Transfer in die regelversorgung. Verlag MedHochZwei, 148–164.
- 20.Michelsen T, Lins C, Hein A, Lüpkes C. Practical implementation of receiver-oriented encryption in STROKE OWL. Studies in Health Technology and Informatics. 2020;270:653–657. doi: 10.3233/SHTI200241. [DOI] [PubMed] [Google Scholar]
- 21.Rimmele DL, Schrage T, Brettschneider C, Engels A, Gerloff C, Härter M, Rosenkranz M, Schmidt H, Kriston L, Thomalla G. Rationale and design of an interventional study of cross-sectoral, coordinated treatment of stroke patients with patient-orientated outcome measurement (StroCare) Neurol Res Pract. 2021;3(1):7. doi: 10.1186/s42466-021-00107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnston N, Bodegard J, Jerström S, Åkesson J, Brorsson H, Alfredsson J, Albertsson PA, Karlsson JE, Varenhorst C. Effects of interactive patient smartphone support app on drug adherence and lifestyle changes in myocardial infarction patients: A randomized study. American Heart Journal. 2016;178:85–94. doi: 10.1016/j.ahj.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Lunde P, Nilsson BB, Bergland A, Kværner KJ, Bye A. The effectiveness of smartphone apps for lifestyle improvement in noncommunicable diseases: Systematic review and meta-analyses. Journal of Medical Internet Research. 2018;20(5):e162. doi: 10.2196/jmir.9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antonenko K, Paciaroni M, Sokolova L, Pezzella FR. Digital health in stroke medicine: What are the opportunities for stroke patients? Current Opinion in Neurology. 2021;34(1):27–37. doi: 10.1097/WCO.0000000000000891. [DOI] [PubMed] [Google Scholar]
- 25.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, Hoh BL, Janis LS, Kase CS, Kleindorfer DO, Lee JM, Moseley ME, Peterson ED, Turan TN, Valderrama AL, Vinters HV, American Heart Association Stroke Council. Council on Cardiovascular Surgery and Anesthesia, Council on Cardiovascular Radiology and Intervention, Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention, Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Institute of Health. National Institute of Neurological Disorders and Stroke. Online: https://www.stroke.nih.gov/documents/NIH_Stroke_Scale_508C.pdf (Abruf 18.04.2021)
- 27.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 28.Mahoney FI, Barthel DW. Functional evaluation: The Barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 29.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 31.Rabin R, de Charro F. EQ-5D: A measure of health status from the EuroQol group. Ann Med. 2001;33(5):337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 32.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Müller S, Gorasso V, Black KM, Murnane K, Wilke T. PRM208 - validation of the English version of the adherence assessment questionnaire (AAQ) Value Health. 2018;21:S392. doi: 10.1016/j.jval.2018.09.2326. [DOI] [Google Scholar]
- 34.Müller S, Kohlmann T, Wilke T. Validation of the adherence barriers questionnaire - an instrument for identifying potential risk factors associated with medication-related non-adherence. BMC Health Services Research. 2015;15(1):153. doi: 10.1186/s12913-015-0809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson, M.O., Rose, C.D., Dilworth, S.E., & Neilands, T.B. (2012). Advances in the conceptualization and measurement of health care empowerment: Development and validation of the health care empowerment inventory. PloS One, 7(9), e45692. Doi: 0.1371/journal.Pone.0045692. [DOI] [PMC free article] [PubMed]
- 36.Gauthier L, Dehaut F, Joanette Y. The bells test: A quantitative and qualitative test for visual neglect. Int J Clin Neuropsychol. 1989;11(2):49–54. [Google Scholar]
- 37.Tombaugh TN. Trail making test a and B: Normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 38.Folstein MF, Folstein SE, McHugh PR. "mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 39.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*power 3.1: Tests for correlation and regression analyses. Behavior Research Methods. 2009;41(4):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 40.Price-Haywood EG, Harden-Barrios J, Carr C, Reddy L, Bazzano LA, van Driel ML. Patient-reported outcomes in stroke clinical trials 2002-2016: A systematic review. Quality of Life Research. 2019;28(5):1119–1128. doi: 10.1007/s11136-018-2053-7. [DOI] [PubMed] [Google Scholar]
- 41.Aguiar de Sousa D, van der Worp HB, Caso V, Cordonnier C, Strbian D, Ntaios G, Schellinger PD, Sandset EC, European Stroke Organisation. Maintaining stroke care in Europe during the COVID-19 pandemic Results from an international survey of stroke professionals and practice recommendations from the European Stroke organisation. European Stroke Journal. 2000;5(3):230–236. doi: 10.1177/2396987320933746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iodice F, Romoli M, Giometto B, Clerico M, Tedeschi G, Bonavita S, Leocani L, Lavorgna L, Digital Technologies, Web and Social Media Study Group of the Italian Society of Neurology Stroke and digital technology: A wake-up call from COVID-19 pandemic. Neurol Sci. 2021;42(3):805–809. doi: 10.1007/s10072-020-04993-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset generated and analyzed during the study is not publicly available due to the included personal data of patients. For scientific analyses, pseudonymized and anonymized data will be used by the PostStroke-Manager consortium. Generally, data will be stored for at least 10 years. Anonymized data may also be used for meta-analyses upon reasonable request according to the recommendation of the International Committee of Medical Journal Editors (ICMJE).