Abstract
We have previously reported that insulin and osmotic shock stimulate an increase in glucose transport activity and translocation of the insulin-responsive glucose transporter isoform GLUT4 to the plasma membrane through distinct pathways in 3T3L1 adipocytes (D. Chen, J. S. Elmendorf, A. L. Olson, X. Li, H. S. Earp, and J. E. Pessin, J. Biol. Chem. 272:27401–27410, 1997). In investigations of the relationships between these two signaling pathways, we have now observed that these two stimuli are not additive, and, in fact, osmotic shock pretreatment was found to completely prevent any further insulin stimulation of glucose transport activity and GLUT4 protein translocation. In addition, osmotic shock inhibited the insulin stimulation of lipogenesis and glycogen synthesis. This inhibition of insulin-stimulated downstream signaling occurred without any significant effect on insulin receptor autophosphorylation or tyrosine phosphorylation of insulin receptor substrate 1 (IRS1). Furthermore, there was no effect on either the insulin-stimulated association of the p85 type I phosphatidylinositol (PI) 3-kinase regulatory subunit with IRS1 or phosphotyrosine antibody-immunoprecipitated PI 3-kinase activity. In contrast, osmotic shock pretreatment markedly inhibited the insulin stimulation of protein kinase B (PKB) and p70S6 kinase activities. In addition, the dephosphorylation of PKB was prevented by pretreatment with the phosphatase inhibitors okadaic acid and calyculin A. These data support a model in which osmotic shock-induced insulin resistance of downstream biological responses results from an inhibition of insulin-stimulated PKB activation.
It is well established that in striated muscle and adipose tissue, insulin predominantly stimulates glucose uptake by inducing the translocation of the insulin-responsive glucose transporter isoform GLUT4 from its intracellular storage sites to the plasma membrane (24, 25, 27, 43). Although the molecular pathways and specific protein interactions leading to GLUT4 translocation have not yet been fully elucidated, recent studies have identified several of the proximal insulin-dependent signaling events. Initially, the binding of insulin to the cell surface insulin receptor triggers the autophosphorylation and activation of the intrinsic protein tyrosine kinase activity of the insulin receptor β subunit (10). In turn, the activated insulin receptor can then tyrosine phosphorylate a variety of intracellular substrates, including insulin receptor substrate 1 (IRS1), IRS2, IRS3, IRS4, Gab1, signal regulatory proteins (SIRPs), and Shc (10, 23, 28, 36, 37, 57). In particular, the tyrosine phosphorylation of the IRS proteins generates multisubunit docking sites for a variety of Src homology 2 domain-containing effector molecules which are necessary to sort and transmit mitogenic and metabolic signals (10, 59).
Several studies examining the signaling pathways regulating the insulin stimulation of GLUT4 translocation, glucose uptake, and glycogen and protein synthesis have strongly indicated a role for the activation and/or appropriate targeting of the type I phosphatidylinositol (PI) 3-kinase (2, 15, 42, 48, 49). The phospholipid product of the PI 3-kinase (PI-3,4,5-P3) has been observed to function as an upstream regulator of the atypical protein kinase C isoforms lambda and zeta and the serine/threonine kinase protein kinase B (PKB) (3, 35, 38, 50, 51). In the case of PKB, the interaction of its amino-terminal pleckstrin homology (PH) domain with this phosphoinositide triphosphate induces a conformational change in PKB, releasing an inhibitory constraint and thereby making it a more efficient substrate for the phosphatidylinositide-dependent kinase (PDK) PDK1 (3, 4, 51). The insulin-stimulated phosphorylation of PKB on threonine 308 by PDK1 and on serine 473 by PDK2 is required for maximal activation of PKB activity (1, 3, 4, 51).
Currently, several potential PKB targets leading to specific downstream biological responses have been identified. These include mTOR and p70S6 kinase, which are directly involved in the regulation of protein synthesis, and glycogen synthesis kinase 3 (GSK3), which has been implicated in the regulation of glycogen synthesis (13, 14, 55). Although an essential role for PKB in the insulin-stimulated translocation of GLUT4 has recently become controversial (30, 35), stable or inducible expression of a constitutively active membrane-bound form of PKB results in increased glucose transport activity and the persistent plasma membrane localization of GLUT4 (20, 32, 34, 54). Consistent with this apparent PKB-dependent translocation of GLUT4, expression of a dominant-interfering PKB mutant inhibited insulin-stimulated GLUT4 translocation (12).
In addition to the insulin-stimulated IRS–PI 3-kinase–PKB pathway leading to GLUT4 translocation, several studies have observed that insulinomimetic agents, such as guanosine 5′-O-(3-thiotriphosphate) and osmotic shock, stimulate GLUT4 translocation through a novel tyrosine kinase pathway independent of PI 3-kinase and PKB (11, 16, 21, 45, 61, 63). Furthermore, it has recently been reported that hyperosmotic stress prevents vanadate- and platelet-derived growth factor-stimulated PKB activation by maintaining PKB in a dephosphorylated inactive state (39). Since insulin and osmotic shock apparently utilize distinct signals to activate GLUT4 translocation and glucose transport, we have examined the molecular interaction between these two pathways. In the present study we have determined that osmotic shock markedly attenuates the insulin stimulation of GLUT4 translocation and glucose transport activity without any significant effect on insulin receptor autophosphorylation, IRS1 tyrosine phosphorylation, or activation of PI 3-kinase activity. Similarly, osmotic shock also inhibited the insulin stimulation of lipogenesis and glycogen synthesis. However, osmotic shock resulted in a dephosphorylation of PKB at both serine 473 and threonine 308, which markedly attenuated PKB activity. In addition, the osmotic shock-induced dephosphorylation of PKB activation was prevented by pretreatment with the phosphatase inhibitors okadaic acid and calyculin A. Thus, these data suggest that at least one mechanism for osmotic shock-induced insulin resistance results from the stimulation of a PKB phosphatase that maintains PKB in an inactive state.
MATERIALS AND METHODS
Cell culture.
3T3L1 preadipocytes were maintained in Dulbecco’s modified Eagle’s medium containing 25 mM glucose (DMEM) plus 10% calf serum in an 8% CO2 atmosphere and were differentiated to adipocytes by standard procedures as previously described (41). Briefly, following confluency, the cells were placed in differentiation medium (DMEM, 10% fetal bovine serum, 200 nM insulin, 0.25 μM dexamethasone, 500 μM isobutylmethylxanthine) for 4 days. The medium was then changed to DMEM containing 10% fetal bovine serum and 200 nM insulin. After an additional 4 days, the cells were placed in DMEM containing 10% fetal bovine serum without any additives and were used at between 8 and 14 days after initiation of the differentiation protocol.
Plasma membrane sheet immunoblotting.
Plasma membrane sheets were prepared by the method of Robinson et al. (44). After stimulation, differentiated 3T3L1 adipocytes were washed twice with ice-cold phosphate-buffered saline (PBS). Cells were then treated with 0.5 mg of poly-d-lysine per ml in PBS for 30 s, followed by three washes with hypotonic buffer (23 mM KCl, 10 mM HEPES [pH 7.5], 1.7 mM MgCl2, 1 mM EGTA). The cells were subsequently covered with sonication buffer (3× hypotonic buffer containing 1 mM dithiothreitol and 0.1 mM phenylmethylsulfonyl fluoride) and sonicated with a Fisher probe membrane disrupter. Following sonication, the unsonicated intact cells were removed with a cotton swab, and the plasma membrane sheets were washed five times with the sonication buffer, resuspended in sucrose buffer (250 mM sucrose, 20 mM HEPES [pH 7.4], 2 mM EDTA), and centrifuged at 190,000 × g for 1 h at 4°C. The pellets were then resuspended in Laemmli sample buffer, and aliquots were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis. Upon electrophoretic transfer to nitrocellulose membrane, immunoblotting was performed with a polyclonal GLUT4 antibody.
Glucose transport activity.
3T3L1 adipocytes were placed in DMEM containing 25 mM glucose plus 0.5% bovine serum albumin (BSA) for 2 h at 37°C. The cells were washed with KRPH buffer (5 mM Na2HPO4, 20 mM HEPES [pH 7.4], 1 mM MgSO4, 1 mM CaCl2, 136 mM NaCl, 4.7 mM KCl, 1% BSA) and either not treated or stimulated as described in the figure legends. Glucose uptake was determined at 37°C by incubation with 50 μM 2-deoxyglucose containing 0.5 μCi of 2-[3H]deoxyglucose in the absence or presence of 10 μM cytochalasin B. The reaction was stopped after 5 min by washing the cells three times with ice-cold PBS. The cells were then solubilized in 1% Triton X-100 at 37°C for 30 min, and aliquots were subject to scintillation counting and Bradford protein assay.
Lipogenesis and glycogen synthesis.
Lipogenesis and glycogen synthesis assays were performed as previously described (60). Briefly, 3T3L1 adipocytes (six-well culture dishes) were serum starved in Krebs-Ringer bicarbonate-HEPES buffer (KRBH) (30 mM HEPES [pH 7.4], 134 mM NaCl, 3.5 mM KCl, 1.2 mM KH2PO4, 0.5 mM MgSO4, 1.5 mM CaCl2, 5 mM NaHCO3) supplemented with 0.5% BSA and 2.5 mM glucose. The cells were washed once with PBS and incubated for another 15 min in KRBH–0.5% BSA without glucose, after which they were either not treated or stimulated as described in the figure legends. The assay was initiated by the addition of [U-14C]glucose (0.125 μCi/sample) and glucose (final concentration, 5 mM) and terminated 1 h later by washing the cells three times with ice-cold PBS. The cells were then harvested in 1 ml of PBS and added to 5 ml of Betafluor scintillant (National Diagnostics). Following an overnight extraction, the radioactivity incorporated into lipid was determined by counting the radioactivity in 4 ml of the organic phase. For the glycogen synthesis assay, 3T3L1 adipocytes were serum starved, glucose deprived, and stimulated as described for the lipogenesis assay. The glycogen synthesis assay was initiated by adding [U-14C]glucose (2 μCi/sample) and glucose (final concentration, 5 mM) and terminated 1 h later by solubilizing the cells with 0.7 ml of 30% KOH. The radioactivity incorporated into glycogen was determined by precipitation as previously described (22).
Immunoblotting.
Whole-cell detergent extracts of 3T3L1 adipocytes were prepared as previously described (56). Briefly, following stimulation, the cells were solubilized in lysis buffer (50 mM HEPES [pH 7.4], 1% Triton X-100, 100 mM sodium fluoride, 2.5 mM EDTA, 10 mM sodium pyrophosphate, 2 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride, 2 μM pepstatin A, 10 μg of aprotinin per ml, 10 μM leupeptin). The extracts were then microcentrifuged at 13,000 × g for 10 min, and 40 μg of the supernatant was resolved by SDS-polyacrylamide gel electrophoresis. In order to visualize the changes in PKB mobility induced by phosphorylation, the samples were resolved on 1.5-mm by 10-cm SDS–10% low-cross-linking (acrylamide/bisacrylamide ratio, 30:0.1) polyacrylamide gels. Under most other conditions, a 10% regular cross-linking (30:0.8 acrylamide/bisacrylamide ratio) polyacrylamide gel was used. In either case, the whole-cell detergent extracts were then subjected to immunoblotting with either a polyclonal PKB antibody that was raised against Akt2/PKBβ (kindly provided by Morris Birnbaum), the polyclonal phosphoserine 473-specific and phosphothreonine 308-specific PKBα antibodies (New England Biolabs), or the polyclonal p70S6 kinase antibody (Upstate Biotechnology Inc.). The immunoblots were visualized by using the enhanced chemiluminescence detection system (Amersham).
Immunoprecipitations.
Whole-cell detergent extracts were prepared as described above and incubated for 2 h at 4°C with 5 μg of the polyclonal insulin receptor antibody (Santa Cruz), the polyclonal IRS1 antibody (Upstate Biotechnology Inc.), or the monoclonal 3F10 hemagglutinin (HA) antibody (Boehringer Mannheim). The samples were then precipitated with protein A- or protein G-Sepharose and were immunoblotted with the horseradish peroxidase-conjugated monoclonal antiphosphotyrosine antibody PY20 (Transducation Laboratories), the polyclonal insulin receptor antibody, the polyclonal IRS1 antibody, the monoclonal p85 antibody (Transduction Laboratories), or the phosphospecific PKB antibodies.
Enzymatic activity of PKB.
PKB kinase activity was determined as described by Moule et al. (40) with minor modification. Briefly, 600 μg of 3T3L1 adipocyte cell detergent extracts were immunoprecipitated with 5 μg of PKBα antibody (Upstate Biotechnology Inc.) and protein G-Sepharose beads for 2 h at 4°C. The protein G-Sepharose beads were washed and resuspended in 40 μl of assay buffer (20 mM MOPS [morpholinepropanesulfonic acid] [pH 7.0], 1 mM EDTA, 1 mM EGTA, 0.01% Brij 35, 5% glycerol) containing 0.1% 2-mercaptoethanol, 2.5 μM cyclic AMP-dependent protein kinase inhibitor peptide, and 0.5 mg of histone H2B per ml. The reaction was initiated by the addition of 100 μM ATP containing 10 μCi of [γ-32P]ATP at room temperature and was terminated after 20 min by the addition of Laemmli sample buffer.
p70S6 protein kinase activity.
The p70S6 protein kinase assay was conducted as described by Band and Posner (6) with minor modifications. 3T3L1 adipocytes were solubilized in a lysis buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 10 mM sodium pyrophosphate, 100 mM sodium fluoride, 1.5 mM MgCl2, 1 mM EGTA, 200 μM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml, 10% glycerol, 1% Triton X-100). The extracts were then microcentrifuged at 13,000 × g for 15 min, and the supernatant containing 700 μg of protein was incubated with 5 μg of the polyclonal p70S6 kinase antibody (Upstate Biotechnology Inc.) and protein G-Sepharose beads for 3 h at 4°C. The immune complexes were subsequently washed three times with the lysis buffer and twice with assay dilution buffer (20 mM MOPS [pH 7.2], 25 mM β-glycerol phosphate, 5 mM EGTA, 1 mM sodium vanadate, 1 mM dithiothreitol). Following washing, the p70S6 kinase activity was measured with a p70S6 kinase assay kit (Upstate Biotechnology Inc.) by using an S6 kinase peptide (AKRRRLSSLRA) as the substrate in accordance with the manufacturer’s instructions.
PI-3 kinase activity.
PI-3 kinase activity was determined as previously described (47, 58). Briefly, 1 mg of 3T3L1 adipocyte detergent cell extracts were incubated with 5 μg of the antiphosphotyrosine antibody PY20 and protein A-Sepharose beads for 4 h at 4°C. The beads were then washed sequentially three times with wash buffer A (1% Nonidet P-40 in PBS), wash buffer B (100 mM Tris [pH 7.5], 5 mM LiCl, 100 μM sodium vanadate), and wash buffer C (10 mM Tris [pH 7.5], 100 mM NaCl, 1 mM EDTA, 100 μM sodium vanadate), with each buffer containing 100 mM sodium fluoride, 10 mM sodium pyrophosphate, and 1 mM phenylmethylsulfonyl fluoride. The beads were then resuspended in 60 μl of kinase assay buffer (10 mM Tris [pH 7.4], 150 mM NaCl, 5 mM EDTA, 20 mM MgCl2), and the kinase reaction was initiated by the addition of 20 μg of phosphatidylinositol and 50 μM ATP containing 30 μCi of [γ-32P]ATP. The samples were incubated for 10 min at room temperature, and the reactions were terminated by the addition of 20 μl of 8 N HCl. The samples were then extracted with 160 μl of chloroform-methanol (1:1), and the organic phase was concentrated by evaporation. The resultant lipid fractions were resolved by thin-layer chromatography in chloroform-methanol-water-ammonium hydroxide (60:47:11.3:2). The phosphorylated products were then visualized by autoradiography.
Transfection of CHO/IR cells.
Chinese hamster ovary cells expressing the insulin receptor (CHO/IR cells) were transfected with the mammalian expression plasmid pcDNA3 encoding HA epitope-tagged PKBα in which the PH domain was deleted (ΔPH-PKB), threonine 308 was replaced with aspartic acid (T308D), or serine 473 was replaced with aspartic acid (S473D). Briefly, CHO/IR cells were incubated in minimal Eagle’s medium supplemented with 10% fetal bovine serum at 37°C and 5% CO2. Fully confluent CHO/IR cells were then transiently transfected by electroporation (0.34 kV and 960 μF) with 2 μg of plasmid DNA per cuvette as described previously (62). Following electroporation, the cells were allowed to adhere to 10-cm-diameter tissue culture dishes for 30 h and were then serum starved for 6 h prior to stimulation with 100 nM insulin and/or 600 mM sorbitol at 37°C.
RESULTS
Osmotic shock stimulates glucose transport and GLUT4 translocation but completely prevents any further increase by insulin.
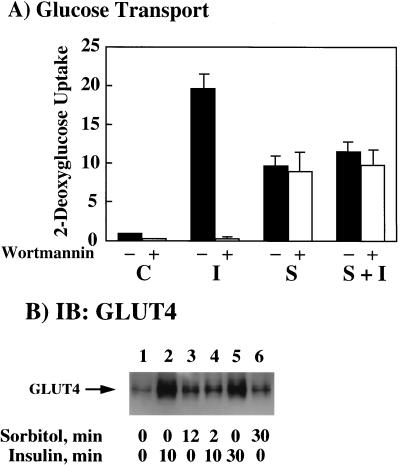
Recently, we and others have reported that osmotic shock can activate glucose transport and GLUT4 translocation in 3T3L1 adipocytes through a novel protein tyrosine kinase pathway that is independent of the insulin receptor, IRS proteins, PI 3-kinase, and PKB (11, 45). To examine the interrelationship between these two pathways, the insulin stimulation of glucose transport activity and GLUT4 translocation in the absence and presence of osmotic shock was determined (Fig. 1). As expected, insulin stimulation resulted in approximately a 20-fold increase in 2-deoxyglucose uptake compared to that in the control unstimulated cells (Fig. 1A, solid bars C and I). As previously observed (11), osmotic shock increased glucose transport 10-fold, which was approximately 50% of that induced by insulin stimulation (Fig. 1A, solid bars I and S). However, osmotic shock pretreatment followed by insulin stimulation did not result in any further increase in glucose transport activity (Fig. 1A, solid bar S+I). In addition, the selective PI 3-kinase inhibitor wortmannin completely inhibited the insulin stimulation of glucose transport but was without effect on either the osmotic shock or osmotic shock-plus-insulin stimulation (Fig. 1A, open bars). Thus, these data demonstrate that the stimulation of glucose transport by osmotic shock is not additive to that by insulin. Rather, osmotic shock induces a relative state of insulin resistance.
FIG. 1.
Osmotic shock pretreatment inhibits insulin-stimulated glucose transport activity and GLUT4 translocation. (A) Differentiated 3T3L1 adipocytes were either left untreated (−, solid bars) or treated with 100 nM wortmannin (+, open bars) for 15 min at 37°C. The cells were then either left untreated (C), stimulated with 100 nM insulin for 15 min (I), incubated with 600 mM sorbitol for 45 min (S), or preincubated with 600 mM sorbitol for 30 min and then stimulated with insulin for another 15 min (S+I). The initial rate of 2-[3H]deoxyglucose uptake was then determined as described in Materials and Methods. The basal rate of 2-[3H]deoxyglucose uptake activity was normalized to 1. Error bars indicate standard deviations. (B) Differentiated 3T3L1 adipocytes were either left untreated (lane 1) or stimulated with 100 nM insulin for 10 (lane 2) or 30 (lane 5) min at 37°C. The 3T3L1 adipocytes were also pretreated with 600 mM sorbitol for 12 (lane 3) and 30 (lane 6) min at 37°C. In parallel, the cells were also pretreated with 600 mM sorbitol for 2 min and then incubated for an additional 10 min with 100 nM insulin (lane 4). Purified plasma membrane sheets were then prepared as described in Materials and Methods. The isolated plasma membranes were then immunoblotted (IB) with the GLUT4 antibody.
Since the translocation of the GLUT4 glucose transporter isoform from intracellular storage sites to the plasma membrane is the primary event responsible for both insulin- and osmotic shock-stimulated glucose transport, we directly examined the translocation of GLUT4 by immunoblotting of isolated plasma membrane sheets (Fig. 1B). Insulin stimulation for 10 min resulted in the typical increase of immunoreactive GLUT4 protein at the plasma membrane indicative of GLUT4 translocation (Fig. 1B, lanes 1 and 2). Similar to the case for glucose transport, brief osmotic shock treatment also resulted in GLUT4 translocation, although to a smaller extent than that by insulin (Fig. 1B, lane 3). As observed for glucose transport, osmotic shock pretreatment prevented insulin from inducing any further increase in GLUT4 translocation (Fig. 1B, lane 4). Furthermore, the effect of osmotic shock was also relatively rapid, with similar extents of GLUT4 translocation occurring by 12 or 30 min (Fig. 1B, lanes 3 and 6). Similarly, the full extent of insulin-stimulated GLUT4 translocation occurred by 10 min and did not significantly increase following 30 min of insulin stimulation (Fig. 1B, lanes 2 and 5). Thus, these data demonstrate that osmotic shock pretreatment inhibits the insulin stimulation of GLUT4 translocation and glucose transport activity in 3T3L1 adipocytes.
Osmotic shock inhibits the insulin stimulation of lipogenesis and glycogen synthesis.
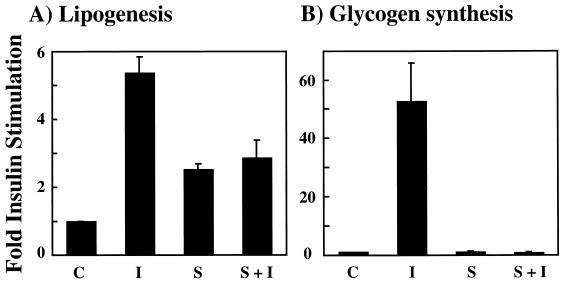
In addition to glucose transport, insulin acutely stimulates other metabolic responses in adipocytes, such as lipogenesis and glycogen synthesis (Fig. 2). In these cells, insulin treatment resulted in approximately a 5-fold increase in lipogenesis, whereas osmotic shock was only partially effective, displaying approximately a 2.5-fold stimulation (Fig. 2A, compare bar C with bars I and S). Similar to the case for GLUT4 translocation, osmotic shock pretreatment completely prevented any further insulin-stimulated increase in lipogenesis (Fig. 2A, solid bar S+I). In contrast, insulin induced a 50-fold increase in glycogen synthesis (Fig. 2B, bars C and I). However, in this case osmotic shock itself had no effect on glycogen synthesis but still completely inhibited any insulin stimulation (Fig. 2B, bars S and S+I). The inability of osmotic shock to stimulate glycogen synthesis is consistent with the reported role of a PKB-dependent inactivation of GSK3 as a required event for glycogen synthase activation (13, 14).
FIG. 2.
Osmotic shock pretreatment inhibits the insulin stimulation of lipogenesis and glycogen synthesis. Differentiated 3T3L1 adipocytes were either left untreated (C), stimulated with 100 nM insulin for 15 min (I), incubated with 600 mM sorbitol for 15 min (S), or preincubated with 600 mM sorbitol for 15 min and then stimulated with insulin for another 15 min (S+I). The initial rate of [14C]glucose incorporation into lipid (A) or glycogen (B) was determined as described in Materials and Methods. The basal rates of lipogenesis and glycogen synthesis were normalized to 1. Error bars indicate standard deviations.
Osmotic shock does not affect insulin-stimulated insulin receptor autophosphorylation or tyrosine phosphorylation of IRS1.
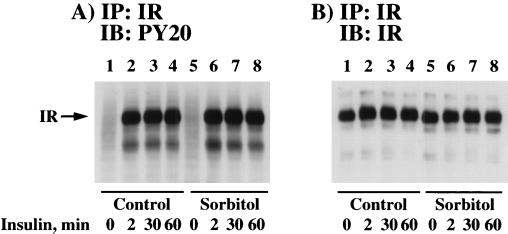
The first detectable event following insulin binding to the insulin receptor is the tyrosine autophosphorylation of the insulin receptor β subunit and subsequent activation of its substrate tyrosine kinase activity (10). Therefore, to determine whether osmotic shock pretreatment had any effect on insulin-stimulated β subunit autophosphorylation, 3T3L1 adipocytes were either left untreated or osmotically shocked for 30 min prior to the addition of insulin (Fig. 3). In the control cells, insulin stimulation for 2 to 60 min resulted in a marked increase in insulin receptor autophosphorylation as determined by phosphotyrosine immunoblots of insulin receptor immunoprecipitates (Fig. 3A, lanes 1 to 4). Similarly, the time course and extent of insulin receptor autophosphorylation were not affected by prior osmotic shock treatment of the 3T3L1 adipocytes (Fig. 3A, lanes 5 to 8). To ensure equal immunoprecipitation of the insulin receptor under these conditions, the insulin receptor immunoprecipitates were also subjected to immunoblotting with a β subunit-specific antibody (Fig. 3B).
FIG. 3.
Osmotic shock pretreatment does not affect insulin-induced tyrosine phosphorylation of the insulin receptor. Differentiated 3T3L1 adipocytes were either left untreated (lanes 1 to 4) or pretreated with 600 mM sorbitol (lanes 5 to 8) for 30 min at 37°C. The cells were then incubated in the absence of insulin (lanes 1 and 5) or in the presence of 100 nM insulin for 2 (lanes 2 and 6), 30 (lanes 3 and 7), or 60 (lanes 4 and 8) min. Whole-cell extracts were then prepared, and the insulin receptor (IR) was immunoprecipitated (IP) with an insulin receptor antibody as described in Materials and Methods. The immunoprecipitates were then immunoblotted (IB) with the PY20 phosphotyrosine antibody (A) or the insulin receptor antibody (B).
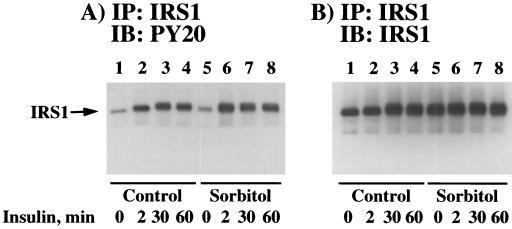
In parallel, we next determined the effect of osmotic shock on the insulin stimulation of IRS1 tyrosine phosphorylation (Fig. 4). In control cells, insulin stimulation resulted in a time-dependent increase in IRS1 tyrosine phosphorylation which persisted for at least 60 min (Fig. 4A, lanes 1 to 4). As observed for insulin receptor autophosphorylation (Fig. 3), pretreatment by osmotic shock did not have any significant effect on the subsequent insulin stimulation of IRS1 tyrosine phosphorylation (Fig. 4A, lanes 5 to 8). The small apparent increase in IRS1 protein following both insulin stimulation (Fig. 4B, lanes 2 to 4) and osmotic shock treatment (Fig. 4B, lanes 5 to 8) reflects a broadening of the IRS1 band due to serine/threonine phosphorylation (references 52 and 53 and unpublished results). Thus, under these conditions, the amount of immunoprecipitated IRS1 protein remained relatively constant (Fig. 4B). Similar to the case for 3T3L1 adipocytes, we have also observed that osmotic shock treatment of CHO/IR cells had no effect on insulin-stimulated β subunit or IRS1 tyrosine phosphorylation (data not shown). Together, these data demonstrate that osmotic shock does not impair either the time dependence or extent of insulin-stimulated insulin receptor autophosphorylation and IRS1 tyrosine phosphorylation.
FIG. 4.
Osmotic shock pretreatment does not affect insulin-induced tyrosine phosphorylation of IRS1. Differentiated 3T3L1 adipocytes were either left untreated (lanes 1 to 4) or pretreated with 600 mM sorbitol (lanes 5 to 8) for 30 min at 37°C. The cells were then incubated in the absence of insulin (lanes 1 and 5) or in the presence of 100 nM insulin for 2 (lanes 2 and 6), 30 (lanes 3 and 7), or 60 (lanes 4 and 8) min. Whole-cell extracts were then prepared, and IRS1 was immunoprecipitated with an IRS1 antibody as described in Materials and Methods. The immunoprecipitates were then immunoblotted with the PY20 phosphotyrosine antibody (A) or the IRS1 antibody (B).
Osmotic shock pretreatment does not affect the insulin-stimulated IRS1 association and activation of the PI 3-kinase.
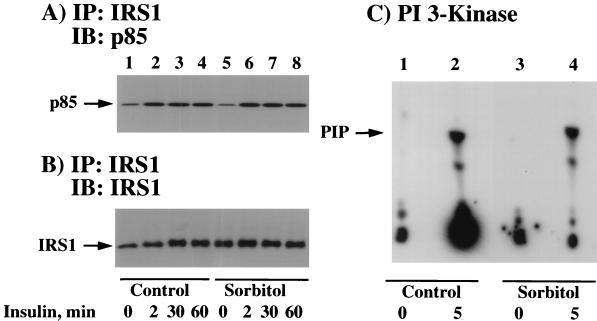
Numerous studies have documented that the type I PI 3-kinase associates with tyrosine-phosphorylated IRS1 and that PI 3-kinase activity is necessary for insulin-stimulated GLUT4 translocation and glucose transport (15, 48). Although we did not observe any overall change in insulin-stimulated IRS1 tyrosine phosphorylation as a result of osmotic shock pretreatment, it remained formally possible that osmotic shock affected the specific association of IRS1 with the PI 3-kinase. To assess this possibility, we next immunoprecipitated IRS1 and determined the amount of associated PI 3-kinase by immunoblotting for the p85 regulatory subunit (Fig. 5A). Insulin stimulation for various times resulted in a specific association of the p85 regulatory subunit with IRS1 (Fig. 5A, lanes 1 to 4). Similarly, osmotic shock pretreatment of the 3T3L1 adipocytes had no effect on either the extent or time dependence of insulin-stimulated association of IRS1 with the p85 protein (Fig. 5A, lanes 5 to 8). In parallel, the amount of IRS1 protein immunoprecipitated was essentially identical (Fig. 5B).
FIG. 5.
Osmotic shock pretreatment does not affect insulin-induced association of IRS1 with the p85 subunit of the PI-3 kinase or PI-3 kinase activation. (A) Differentiated 3T3L1 adipocytes were either left untreated (lanes 1 to 4) or pretreated with 600 mM sorbitol (lanes 5 to 8) for 30 min at 37°C. The cells were then incubated in the absence of insulin (lanes 1 and 5) or in the presence of 100 nM insulin for 2 (lanes 2 and 6), 30 (lanes 3 and 7), or 60 (lanes 4 and 8) min. Whole- cell extracts were then prepared, and IRS1 was immunoprecipitated (IP) with an IRS1 antibody as described in Materials and Methods. The immunoprecipitates were then immunoblotted (IB) with the p85 PI 3-kinase regulatory subunit. (B) In parallel, the IRS1 immunoprecipitates described above were immunoblotted with an IRS1 antibody. (C) Differentiated 3T3L1 adipocytes were either left untreated (lanes 1 and 2) or pretreated with 600 mM sorbitol (lanes 3 and 4) for 30 min at 37°C. The cells were then incubated in the absence (lanes 1 and 3) or presence (lanes 2 and 4) of 100 nM insulin for 5 min. Whole-cell extracts were then prepared, and total tyrosine-phosphorylated proteins were immunoprecipitated with the PY20 phosphotyrosine antibody. The amount of PY20-immunoprecipitated PI-3 kinase activity was determined by using the substrate phosphatidylinositol as described in Materials and Methods. PIP, phosphatidylinositol phosphate.
Although 3T3L1 adipocytes express primarily the IRS1 protein with relatively low levels of IRS2, it was also possible that osmotic shock might have affected other tyrosine-phosphorylated docking proteins which associate with the PI 3-kinase. To assess this possibility, we determined the PI 3-kinase activity in phosphotyrosine antibody immunoprecipitates (Fig. 5C). As expected, there was essentially no detectable PI 3-kinase activity in phosphotyrosine immunoprecipitates from control cells, whereas there was a marked increase following insulin stimulation (Fig. 5C, lanes 1 and 2). Similarly, the basal and insulin-stimulated increase in phosphotyrosine-immunoprecipitated PI 3-kinase activity were unchanged in cells pretreated by osmotic shock (Fig. 5C, lanes 3 and 4). In addition, osmotic shock treatment had no significant effect on the IRS1 association and/or activation of the PI 3-kinase in CHO/IR cells (data not shown). Thus, these data are consistent with the results of the coimmunoprecipitation of IRS1 with the p85 PI 3-kinase regulatory subunit and suggest that osmotic shock inhibits insulin-stimulated glucose transport by blocking a signaling step further downstream of PI-3 kinase.
Osmotic shock inhibits the insulin activation of PKB.
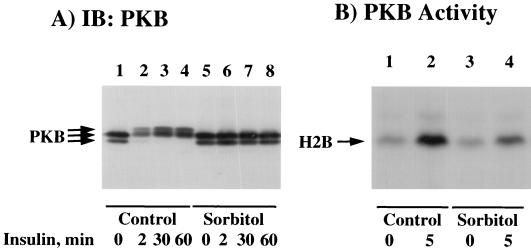
PKB is a serine/threonine kinase activated by insulin and other growth hormones in a PI-3 kinase-dependent manner (1, 5, 8, 18, 33). Recently, several studies have also implicated PKB in the pathway leading to the insulin stimulation of GLUT4 translocation, glucose transport, and glycogen synthesis (12–14, 20, 32, 34, 54). To investigate whether PKB was a target for osmotic shock inhibition, we compared the abilities of insulin to activate PKB in the presence and absence of osmotic shock (Fig. 6). Full activation of PKB requires serine/threonine phosphorylation, resulting in a decrease in SDS-polyacrylamide gel electrophoretic mobility (13, 33). As expected, insulin stimulation led to a rapid and persistent reduction in the electrophoretic mobility of PKB (Fig. 6A, lanes 1 to 4). Although osmotic shock itself had no effect on PKB electrophoretic mobility (Fig. 6A, lane 5), osmotic shock pretreatment completely prevented the insulin-stimulated PKB gel shift (Fig. 6A, lanes 6 to 8). Since previous studies have reported that changes in PKB phosphorylation, and hence SDS-polyacrylamide electrophoretic mobility, may not necessarily correlate with protein kinase activity (17, 19, 31, 40), we confirmed these data by using a PKB in vitro kinase assay (Fig. 6B). Under these conditions, insulin stimulation increased PKB activity approximately sevenfold (Fig. 6B, lanes 1 and 2). In contrast, osmotic shock pretreatment resulted in a 70% reduction of insulin-stimulated PKB activity (Fig. 6B, lane 4). This occurred without any significant effect of osmotic shock on basal PKB activity (Fig. 6B, lane 3). Similarly, osmotic shock pretreatment of CHO/IR cells also prevented the insulin-stimulated electrophoretic mobility shift and activation of PKB (data not shown).
FIG. 6.
Osmotic shock pretreatment inhibits the insulin stimulation of PKB mobility shift and kinase activity. (A) Differentiated 3T3L1 adipocytes were either left untreated (lanes 1 to 4) or pretreated with 600 mM sorbitol (lanes 5 to 8) for 30 min at 37°C. The cells were then incubated in the absence of insulin (lanes 1 and 5) or in the presence of 100 nM insulin for 2 (lanes 2 and 6), 30 (lanes 3 and 7), or 60 (lanes 4 and 8) min. Whole-cell extracts were then prepared and immunoblotted (IB) with the PKB antibody as described in Materials and Methods. (B) Differentiated 3T3L1 adipocytes were either left untreated (lanes 1 and 2) or pretreated with 600 mM sorbitol (lanes 3 and 4) for 30 min at 37°C. The cells were then incubated in the absence (lanes 1 and 3) or presence (lanes 2 and 4) of 100 nM insulin for 5 min. Whole-cell extracts were then prepared and immunoprecipitated with the PKB antibody. The amount of immunoprecipitated PKB activity was determined by using the substrate histone 2B (H2B) as described in Materials and Methods.
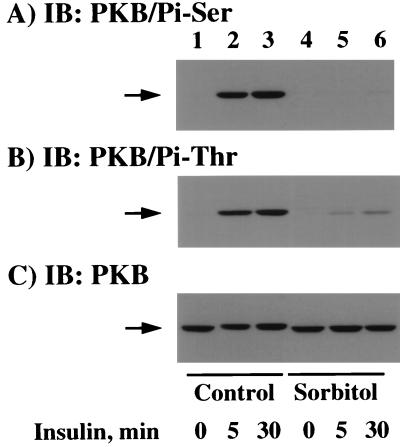
Recently, it has been established that the phosphorylation of two specific residues (threonine 308 and serine 473) is required for maximal PKB activation (1). We therefore examined the effect of osmotic shock on the insulin-stimulated phosphorylation of PKB by using both serine 473 and threonine 308 phospho-specific PKB antibodies (Fig. 7). In unstimulated cells, there was a relatively low level of serine 473 phospho-PKB antibody immunoreactivity (Fig. 7A, lane 1). As expected, insulin stimulation for 5 and 30 min resulted in an increase in PKB serine 473 phosphorylation (Fig. 7A, lanes 2 and 3). In contrast, pretreatment with osmotic shock completely prevented the insulin-stimulated increase in PKB serine 473 phosphorylation (Fig. 7A, lanes 5 and 6). Similarly, immunoblotting with a threonine 308 phospho-specific antibody demonstrated a low level of threonine phosphorylation in unstimulated cells which increased following insulin stimulation for 5 and 30 min (Fig. 7B, lanes 1 to 3). Although osmotic shock itself had no significant effect on threonine 308 phosphorylation, osmotic shock pretreatment resulted in a substantial attenuation of insulin-stimulated PKB threonine 308 phosphorylation (Fig. 7B, lanes 4 to 6). To ensure that these differences were not due to unequal PKB expression, the relative levels of PKB were determined by immunoblotting (Fig. 7C). It should also be noted that in these experiments the SDS-polyacrylamide gel electrophoresis conditions were not optimized to detect the PKB gel shift. Nevertheless, the reduction in PKB electrophoretic mobility was discernible following insulin stimulation (Fig. 7C, lanes 1 to 3) but not in the osmotic shock-treated cells (Fig. 7C, lanes 4 to 6). This apparent inhibition of the PKB electrophoretic gel shift is in agreement with a reduction in PKB phosphorylation. Together, these data directly demonstrate that osmotic shock can repress the insulin stimulation of PKB activity through the inhibition of threonine 308 and serine 473 phosphorylation.
FIG. 7.
Osmotic shock pretreatment inhibits the insulin-stimulated phosphorylation of PKB on threonine 308 and serine 473. Differentiated 3T3L1 adipocytes were either left untreated (lanes 1 to 3) or pretreated with 600 mM sorbitol (lanes 4 to 6) for 30 min at 37°C. The cells were then incubated in the absence of insulin (lanes 1 and 4) or in the presence of 100 nM insulin for 5 (lanes 2 and 5) or 30 (lanes 3 and 6) min. Whole-cell extracts were then prepared and immunoblotted (IB) with the serine 473 phospho-specific PKB antibody (A), the threonine 308 phospho-specific PKB antibody (B), or the PKB antibody (C) as described in Materials and Methods.
Osmotic shock inhibits the insulin activation of p70S6 kinase.
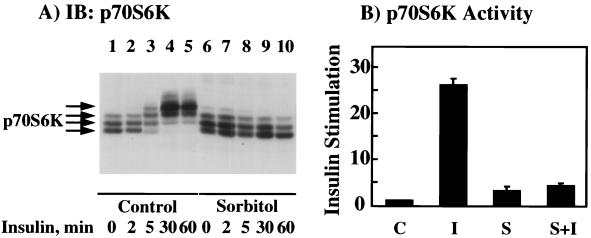
One downstream target of PKB is the p70S6 kinase, which is an important signaling molecule involved in the regulation of mRNA translation (8, 26). Similar to that of PKB, activation of p70S6 kinase is dependent upon its phosphorylation on multiple serine and threonine residues, also resulting in a characteristic reduction in SDS-polyacrylamide gel electrophoretic mobility (7, 29). We therefore examined the effect of osmotic shock on p70S6 kinase activation as a measure of the in vivo stimulation of PKB activity (Fig. 8). As typically observed, insulin stimulation resulted in a marked reduction in p70S6 kinase gel mobility (Fig. 8A, lanes 1 to 5). The time course of the insulin-stimulated decrease in p70S6 kinase mobility was slightly slower than that for PKB, which is consistent with PKB functioning upstream of p70S6 kinase (compare Fig. 6A and 8). In any case, not only did osmotic shock fail to induce any mobility shift of p70S6 kinase (Fig. 8A, lane 6), but it also completely prevented the insulin-stimulated p70S6 kinase mobility shift (Fig. 8A, lanes 7 to 10). Consistent with these data, insulin stimulated p70S6 kinase activity approximately 25-fold compared to that in unstimulated cells (Fig. 8B). In contrast, osmotic shock increased p70S6 kinase activity approximately threefold, whereas osmotic shock pretreatment completely prevented any insulin-stimulated increase of p70S6 kinase activity (Fig. 8B).
FIG. 8.
Osmotic shock pretreatment inhibits the insulin stimulation of p70S6 kinase mobility shift and kinase activity. (A) Differentiated 3T3L1 adipocytes were either left untreated (lanes 1 to 5) or pretreated with 600 mM sorbitol (lanes 6 to 10) for 30 min at 37°C. The cells were then incubated in the absence of insulin (lanes 1 and 6) or in the presence of 100 nM insulin for 2 (lanes 2 and 7), 5 (lanes 3 and 8), 30 (lanes 4 and 9), or 60 (lanes 5 and 10) min. Whole-cell extracts were then prepared and immunoblotted (IB) with the p70S6 kinase antibody as described in Materials and Methods. (B) Differentiated 3T3L1 adipocytes were either untreated (C), stimulated with 100 nM insulin for 10 min (I), incubated with 600 mM sorbitol for 40 min (S), or preincubated with 600 mM sorbitol for 30 min and then stimulated with insulin for another 10 min (S+I). Whole-cell extracts were then prepared and immunoprecipitated with the p70S6 kinase antibody. The amount of immunoprecipitated p70S6 kinase activity was determined by using the peptide substrate AKRRRLSSLRA as described in Materials and Methods. The basal p70S6 kinase activity was normalized to 1. Error bars indicate standard deviations.
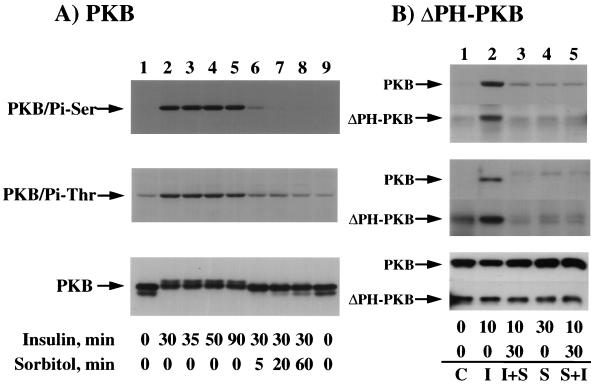
Osmotic shock is dominant over insulin and induces the dephosphorylation of ΔPH-PKB.
There are several possible mechanisms that could potentially account for the osmotic shock inhibition of insulin-stimulated PKB activity. These include inhibition of PDK1 and PDK2 activities and/or activation of a PKB phosphatase(s). To distinguish between these possibilities, we first determined whether the effect of osmotic shock was dominant over that of insulin (Fig. 9). Insulin stimulation resulted in the persistent phosphorylation of PKB on serine 473 and threonine 308 (Fig. 9A, top and middle panels, lanes 1 to 5). In addition, insulin stimulation for 30 to 90 min resulted in a maximal and persistent mobility shift of PKB (Fig. 9A, bottom panel, lanes 1 to 5). However, following 30 min of insulin stimulation, brief osmotic shock exposure resulted in both a rapid dephosphorylation of PKB on both threonine 308 and serine 473 (Fig. 9A, top and middle panels, lanes 6 to 9) and increased PKB mobility (Fig. 9A, bottom panel, lanes 6 to 9).
FIG. 9.
The osmotic shock-induced dephosphorylation of endogenous PKB and expressed ΔPH-PKB is dominant over the effect of insulin. (A) Differentiated 3T3L1 adipocytes were either not treated (lanes 1 and 9) or stimulated with 100 nM insulin for 30 (lanes 2, 6, 7, and 8), 35 (lane 3), 50 (lane 4), or 90 (lane 5) min at 37°C. The cells incubated with insulin for 30 min were then treated with 600 mM sorbitol for an additional 5 (lane 6), 20 (lane 7), or 60 (lane 8) min. Whole-cell extracts were then prepared and immunoblotted with the serine 473 phospho-specific PKB antibody (top panel), the threonine 308 phospho-specific PKB antibody (middle panel), and the PKB antibody (bottom panel) as described in Materials and Methods. (B) CHO/IR cells were electroporated with 2 μg of ΔPH-PKBα, and 24 h later the cells were either left untreated (C, lane 1), stimulated with 100 nM insulin for 10 min (I, lane 2), stimulated with insulin for 10 min and then subjected to a 30-min treatment with 600 mM sorbitol (I+S, lane 3), stimulated with 600 mM sorbitol for 30 min (S, lane 4), or stimulated with 600 mM sorbitol and then subjected to a 10-min treatment with insulin (S+I, lane 5). Whole-cell extracts were prepared and immunoblotted with the serine 473 phospho-specific PKB antibody (top panel) or the threonine 308 phospho-specific PKB antibody for the endogenous PKB (middle panel) or immunoprecipitated with the HA monoclonal antibody for the expressed ΔPH-PKB followed by immunoblotting with the threonine 308 phospho-specific PKB antibody (middle panel). The amounts of endogenous and expressed ΔPH-PKB protein were also determined by immunoblotting with the PKB antibody (bottom panel) as described in Materials and Methods.
To examine whether osmotic shock inhibited the function of PKB upstream activators, we also examined the dephosphorylation of an expressed ΔPH-PKBα protein in CHO/IR cells (Fig. 9B). Since the binding of the PH domain to phosphoinositide triphosphate relieves an inhibitory constraint for constitutively active PDK1 (3, 4, 51), the phosphorylation of ΔPH-PKB on threonine 308 should be independent of PI-3,4,5-P3 formation. In the basal state, there was a low level of serine 473 phosphorylation in both the endogenous PKB and the expressed ΔPH-PKB protein, whereas insulin stimulation resulted in a marked increase (Fig. 9B, top panel, lanes 1 and 2). In contrast, the expressed ΔPH-PKB protein displayed a significant level of threonine 308 phosphorylation compared to endogenous PKB in the basal state (Fig. 9B, middle panel, lane 1). The increased basal phosphorylation on threonine 308 for the ΔPH-PKB protein is consistent with the derepression of an inhibitory constraint imposed by the PH domain for this substrate site. However, insulin stimulation still resulted in increased threonine 308 phosphorylation (Fig. 9B, middle panel, lane 2). Nevertheless, osmotic shock resulted in a substantial dephosphorylation of serine 473 and threonine 308 in the ΔPH-PKB protein (Fig. 9B, top and middle panels, lane 3). The ability of osmotic shock to maintain ΔPH-PKB in a dephosphorylated state was independent of whether insulin was added prior or subsequent to osmotic shock treatment (Fig. 9B, top and middle panels, lanes 4 and 5). Under these conditions, immunoblotting also demonstrated equal expression of endogenous PKB and the ΔPH-PKB construct (Fig. 9B, bottom panel). These data suggest that the osmotic shock inactivation of PKB does not result from an inhibition of phosphatidylinositide production.
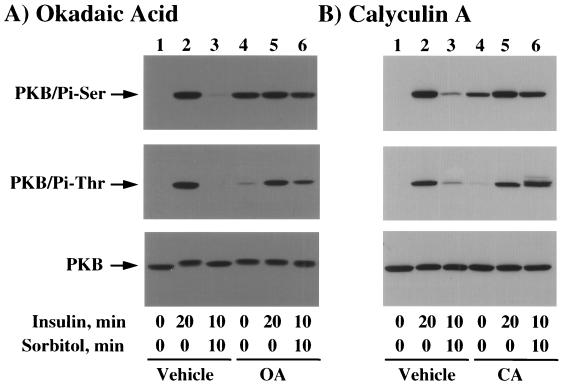
Inhibition of PP2A prevents the dephosphorylation of PKB by osmotic shock.
Previous studies have indicated that protein phosphatase 2A (PP2A) may be one protein phosphatase that is responsible for the dephosphorylation of PKB (5, 39). To further explore whether the osmotic shock-induced dephosphorylation of PKB was dependent upon protein phosphatase activity, we examined the effects of two distinct protein phosphatase inhibitors, okadaic acid and calyculin A. Insulin stimulation resulted in a marked increase in both serine 473 and threonine 308 phosphorylation concomitant with a decrease in PKB mobility (Fig. 10A, lanes 1 and 2). As expected, insulin pretreatment followed by osmotic shock resulted in a near-complete dephosphorylation of PKB back towards the basal state (Fig. 10A, lane 3). Interestingly, the PP1 and PP2A inhibitor okadaic acid induced an increase in serine 473 phosphorylation but had only a small effect on threonine 308 phosphorylation (Fig. 10A, lane 4). Since okadaic acid alone stimulated serine 473 phosphorylation of PKB, there was no additional effect of insulin (Fig. 10A, upper panel, lane 5). In contrast, insulin increased PKB threonine 308 phosphorylation in the presence of okadaic acid (Fig. 10A, middle panel, lane 5). Nevertheless, okadaic acid pretreatment substantially reduced the ability of osmotic shock to dephosphorylate PKB (Fig. 10A, lane 6). These alterations in the observed phosphorylation state of PKB were not a result of differential PKB expression and appeared to parallel the changes in PKB mobility (Fig. 10A, bottom panel).
FIG. 10.
Okadaic acid and calyculin A prevent the osmotic shock-induced dephosphorylation of PKB. (A) Differentiated 3T3L1 adipocytes were either left untreated (lanes 1 to 3) or pretreated with 1 μM okadaic acid (OA) (lanes 4 to 6) for 120 min at 37°C. The cells were then incubated in the absence of insulin (lanes 1 and 4) or in the presence of 100 nM insulin for 20 (lanes 2 and 5) or 10 (lanes 3 and 6) min. The latter insulin-stimulated cells were then incubated with 600 mM sorbitol (lanes 3 and 6) for an additional 10 min. Whole-cell extracts were then prepared and immunoblotted with the serine 473 phospho-specific PKB antibody (top panel), the threonine 308 phospho-specific PKB antibody (middle panel), or the PKB antibody (bottom panel) as described in Materials and Methods. (B) Differentiated 3T3L1 adipocytes were either left untreated (lanes 1 to 3) or pretreated with 25 nM calyculin A (CA) (lanes 4 to 6) for 15 min at 37°C. The cells were then incubated in the absence of insulin (lanes 1 and 4) or in the presence of 100 nM insulin for 20 (lanes 2 and 5) or 10 (lanes 3 and 6) min. The latter insulin-stimulated cells were then incubated with 600 mM sorbitol (lanes 3 and 6) for an additional 10 min. Whole-cell extracts were then prepared and immunoblotted with the serine 473 phospho-specific PKB antibody (top panel), the threonine 308 phospho-specific PKB antibody (middle panel), or the PKB antibody (bottom panel) as described in Materials and Methods.
Similarly, pretreatment of 3T3L1 adipocytes with the PP2A inhibitor calyculin A also resulted in increased serine 473 phosphorylation of PKB, with a substantially smaller effect on threonine 308 phosphorylation (Fig. 10B, lane 4). As expected, insulin stimulation increased both serine 473 and threonine 308 phosphorylation in the presence of calyculin A (Fig. 10B, lane 5). Consistent with the effect of okadaic acid, calyculin A also substantially reduced the ability of osmotic shock to dephosphorylate PKB on both serine 473 and threonine 308 (Fig. 10B, lane 6). Together, these data are consistent with an osmotic shock-induced dephosphorylation of PKB by a PP2A-like activity.
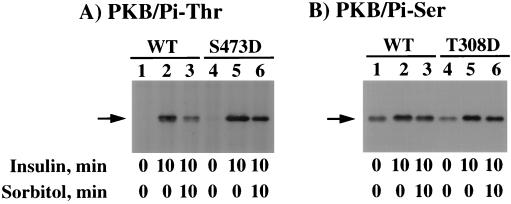
Osmotic shock preferentially stimulates the dephosphorylation of threonine 308.
Since the osmotic shock-stimulated dephosphorylation of endogenous PKB in 3T3L1 adipocytes was very rapid, we examined the effect of osmotic shock on overexpressed PKB in CHO/IR cells (Fig. 11). In these experiments, CHO/IR cells were transfected with the HA epitope-tagged wild-type PKB (PKB/WT), PKB in which threonine 308 was mutated to aspartic acid (PKB/T308D), and PKB in which serine 473 was mutated to aspartic acid (PKB/S473D). In unstimulated cells, immunoprecipitation of PKB/WT resulted in a relatively low level of threonine 308 phosphorylation as detected by immunoblotting with the threonine 308 phospho-PKB-specific antibody (Fig. 11A, lane 1). Insulin markedly increased the amount of threonine 308 phosphorylation (Fig. 11A, lane 2). Subsequent osmotic shock treatment for 10 min resulted in a substantial dephosphorylation of threonine 308 (Fig. 11A, lane 3). Similarly, the expression of the PKB/S473D mutant displayed a low basal phosphorylation of threonine 308 which was increased following insulin stimulation (Fig. 11A, lanes 4 and 5). Consistent with the expression of PKB/WT, subsequent osmotic shock treatment resulted in a dephosphorylation of threonine 308 (Fig. 11A, lane 6).
FIG. 11.
Osmotic shock preferentially induces the dephosphorylation of threonine 308 in CHO/IR cells. CHO/IR cells were electroporated with 2 μg of PKB/WT (WT), PKB/S473D (S473D), or PKB/T308D (T308D), and 24 h later the cells were either left untreated (lanes 1 and 4), stimulated with 100 nM insulin for 10 min (lanes 2 and 5), or stimulated with insulin for 10 min and then subjected to a 10-min treatment with 600 mM sorbitol (lanes 3 and 6). Whole-cell extracts were prepared and immunoprecipitated with the HA monoclonal antibody. The immunoprecipitates were then immunoblotted with the threonine 308 phospho-specific PKB antibody (A) and the serine 473 phospho-specific PKB antibody (B) as described in Materials and Methods.
In contrast, expression of PKB/WT in CHO/IR cells resulted in a relative high basal level of serine 473 phosphorylation (Fig. 11B, lane 1). Due to the high basal serine 473 phosphorylation, insulin stimulation caused a relatively modest increase (Fig. 11B, lane 2). More importantly, osmotic shock treatment did not significantly affect the prior insulin-stimulated increase of serine 473 phosphorylation (Fig. 11B, lane 3). Furthermore, expression of the PKB/T308D mutant also resulted in a relatively high basal serine 473 phosphorylation which was still increased following insulin stimulation (Fig. 11B, lanes 4 and 5). However, as observed for PKB/WT, subsequent osmotic shock treatment had little effect on the insulin-stimulated phosphorylation of serine 473. In addition, osmotic shock inhibited the insulin stimulation of PKB/WT and PKB/S473D protein kinase activities but had only a small effect on PKB/T308D (data not shown). Taken together, these data suggest that in CHO/IR cells osmotic shock preferentially induces the selective dephosphorylation of threonine 308 by a PP2A-like phosphatase activity.
DISCUSSION
Insulin is a pleiotropic hormone that elicits both mitogenic and metabolic responses essential for normal growth and development (46). Of particular importance is the indispensable role for insulin in the regulation of glucose homeostasis, which is achieved in part by increased glucose uptake and glycogen and lipid biosynthesis. To date, substantial progress has been made in elucidating the distinct pathways and insulin-dependent signal transduction events leading to peripheral tissue regulation of glucose metabolism. In efforts to identify the regulated pathways leading to GLUT4 translocation in 3T3L1 adipocytes, we and others have previously reported that osmotic shock is an insulinomimetic agent that increases glucose transport and GLUT4 translocation through an alternative signaling pathway independent of PI 3-kinase activation (11, 45).
Thus, based upon the established paradigms of insulin action, we sought to identify the common signal transduction events utilized by both insulin and osmotic shock to induce GLUT4 translocation. Surprisingly, however, we observed that osmotic shock pretreatment completely prevented insulin from inducing any further GLUT4 translocation. To identify which potential step in insulin signaling could be reasonable for this inhibition, we undertook a systemic examination of several proximal insulin receptor effector proteins. We have found that osmotic shock had no significant effect on insulin receptor autophosphorylation, IRS1 tyrosine phosphorylation, or IRS1-associated PI 3-kinase or PI 3-kinase activity. However, the insulin stimulation of PKB activation was markedly reduced, and the effect of osmotic shock was dominant over that of insulin.
Although the role of PKB in the regulation of GLUT4 translocation remains controversial (30, 35), PKB also appears to be a central effector in other metabolic actions of insulin. For example, PKB has been reported to phosphorylate and activate mTOR and p70S6 kinase, leading to increased protein synthesis (9, 30). Expression of a constitutively active PKB mutant results in an insulin-independent stimulation of lipogenesis (34). In the case of glycogen synthesis, PKB can phosphorylate and inactivate GSK3, which is one important kinase that maintains glycogen synthase in an inactive phosphorylated state (13, 14). In this regard, we have also observed that osmotic shock prevents the insulin-stimulated increase in both lipogenesis and glycogen synthesis. Interestingly, although osmotic shock alone could partially activate both glucose transport and lipogenesis, there was no effect on glycogen synthesis. This is consistent with osmotic shock inactivating PKB and thereby maintaining GSK3 in a nonphosphorylated active state. In any case, these data strongly suggest that the osmotic shock inhibition of PKB activation is at least one effector resulting in a desensitization of insulin-mediated downstream responsiveness.
Based upon the known mechanisms for the regulation of PKB activity, there are several possible mechanisms by which osmotic shock might inhibit the insulin stimulation of PKB activation. First, osmotic shock could potentially prevent the formation of PI-3,4,5-P3 and/or the activation of PDK1 and -2. However, our data demonstrated no significant effect on the IRS1 association with and/or activation of the PI 3-kinase. In addition, osmotic shock induced the dephosphorylation of the ΔPH-PKB protein, which is phosphorylated by PDK1 in a PI-3,4,5-P3-independent manner (3). Consistent with these data, it was also recently reported that osmotic shock did not block the plasma membrane translocation of PKB in HEK293 cells (39). Thus, it is highly unlikely that osmotic shock prevented the in vivo formation of PI-3,4,5-P3 or activation of the upstream PKB activators.
Alternatively, osmotic shock appears to require the function of serine/threonine phosphatase activity to induce the dephosphorylation of PKB. This conclusion is supported by the observation that following the addition of the specific serine/threonine phosphatase inhibitors okadaic acid and calyculin A, there was a substantial inhibition of osmotic shock-induced PKB dephosphorylation. Although okadaic acid is a more selective inhibitor for PP2A than for PP1, calyculin A is highly specific for PP2A. These data suggest that the major protein phosphatase responsible for the dephosphorylation of PKB on both serine 473 and threonine 308 is PP2A. Furthermore, these observations are also in excellent agreement with recent results with fibroblast cell lines demonstrating that hyperosmotic stress inhibited both platelet-derived growth factor- and vanadate-stimulated PKB activation through the activation of a PP2A-like phosphatase (39).
In summary, osmotic shock, like several other insulinomimetic agents, not only partially activates several insulin-specific biological responses but also imparts a relative state of insulin resistance. The osmotic shock inhibition of insulin-stimulated downstream signaling events, including glucose uptake, GLUT4 translocation, lipogenesis, and glycogen synthesis, directly correlated with the inability of insulin to activate PKB under these conditions. In addition, the persistent inactivation of PKB by osmotic shock appears to result from the induction of phosphatase activity without any significant effect on the insulin receptor kinase, IRS1 tyrosine phosphorylation, or activation of the PI 3-kinase. Thus, additional studies are needed to directly identify the specific phosphatase(s) responsible for PKB dephosphorylation and its mechanisms of activation. Although we have examined the effect of acute hyperosmotic stress on insulin signaling only in culture, these findings may underlie the chronic effect of prolonged hyperglycemia to induce states of insulin resistance in vivo.
ACKNOWLEDGMENTS
This work was supported by research grants DK33823, DK49012, and DK25925 from the National Institutes of Health.
REFERENCES
- 1.Alessi D R, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings B A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi D R, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8:55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 3.Alessi D R, Deak M, Casamayor A, Caudwell F B, Morrice N, Norman D G, Gaffney P, Reese C B, MacDougall C N, Harbison D, Ashworth A, Bownes M. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol. 1997;7:776–789. doi: 10.1016/s0960-9822(06)00336-8. [DOI] [PubMed] [Google Scholar]
- 4.Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R, Reese C B, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase B alpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 5.Andjelkovic M, Jakubowicz T, Cron P, Ming X F, Han J W, Hemmings B A. Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proc Natl Acad Sci USA. 1996;93:5699–5704. doi: 10.1073/pnas.93.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Band C J, Posner B I. Phosphatidylinositol 3′-kinase and p70s6k are required for insulin but not bisperoxovanadium 1,10-phenanthroline (bpV(phen)) inhibition of insulin-like growth factor binding protein gene expression. Evidence for MEK-independent activation of mitogen-activated protein kinase by bpV(phen) J Biol Chem. 1997;272:138–145. doi: 10.1074/jbc.272.1.138. [DOI] [PubMed] [Google Scholar]
- 7.Beamish H, Williams R, Chen P, Khanna K K, Hobson K, Watters D, Shiloh Y, Lavin M. Rapamycin resistance in ataxia-telangiectasia. Oncogene. 1996;13:963–970. [PubMed] [Google Scholar]
- 8.Burgering B M, Coffer P J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 9.Burgering B M, Coffer P J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 10.Cheatham B, Kahn C R. Insulin action and the insulin signaling network. Endocrinol Rev. 1995;16:117–142. doi: 10.1210/edrv-16-2-117. [DOI] [PubMed] [Google Scholar]
- 11.Chen D, Elmendorf J S, Olson A L, Li X, Earp H S, Pessin J E. Osmotic shock stimulates GLUT4 translocation in 3T3L1 adipocytes by a novel tyrosine kinase pathway. J Biol Chem. 1997;272:27401–27410. doi: 10.1074/jbc.272.43.27401. [DOI] [PubMed] [Google Scholar]
- 12.Cong L N, Chen H, Li Y, Zhou L, McGibbon M A, Taylor S I, Quon M J. Physiological role of Akt in insulin-stimulated translocation of GLUT4 in transfected rat adipose cells. Mol Endocrinol. 1997;11:1881–1890. doi: 10.1210/mend.11.13.0027. [DOI] [PubMed] [Google Scholar]
- 13.Cross D A, Alessi D R, Cohen P, Andjelkovich M, Hemmings B A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 14.Cross D A, Watt P W, Shaw M, van der Kaay J, Downes C P, Holder J C, Cohen P. Insulin activates protein kinase B, inhibits glycogen synthase kinase-3 and activates glycogen synthase by rapamycin-insensitive pathways in skeletal muscle and adipose tissue. FEBS Lett. 1997;406:211–215. doi: 10.1016/s0014-5793(97)00240-8. [DOI] [PubMed] [Google Scholar]
- 15.Czech M P. Molecular actions of insulin on glucose transport. Annu Rev Nutr. 1995;15:441–471. doi: 10.1146/annurev.nu.15.070195.002301. [DOI] [PubMed] [Google Scholar]
- 16.Elmendorf J S, Chen D, Pessin J E. Guanosine 5′-O-(3-thiotriphosphate) (GTP-Gamma-S) stimulation of GLUT4 translocation is tyrosine kinase-dependent. J Biol Chem. 1998;273:13289–13296. doi: 10.1074/jbc.273.21.13289. [DOI] [PubMed] [Google Scholar]
- 17.Franke T F, Kaplan D R, Cantley L C, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 18.Franke T F, Yang S I, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 19.Frech M, Andjelkovic M, Ingley E, Reddy K K, Falck J R, Hemmings B A. High affinity binding of inositol phosphates and phosphoinositides to the pleckstrin homology domain of RAC/protein kinase B and their influence on kinase activity. J Biol Chem. 1997;272:8474–8481. doi: 10.1074/jbc.272.13.8474. [DOI] [PubMed] [Google Scholar]
- 20.Hajduch E, Alessi D R, Hemmings B A, Hundal H S. Constitutive activation of protein kinase B alpha by membrane targeting promotes glucose and system A amino acid transport, protein synthesis, and inactivation of glycogen synthase kinase 3 in L6 muscle cells. Diabetes. 1998;47:1006–1013. doi: 10.2337/diabetes.47.7.1006. [DOI] [PubMed] [Google Scholar]
- 21.Haruta T, Morris A J, Vollenweider P, Nelson J G, Rose D W, Mueckler M, Olefsky J M. Ligand-independent Glut4 translocation induced by guanosine 5′-O-(3-thiotriphosphate) involves tyrosine phosphorylation. Endocrinology. 1998;139:358–364. doi: 10.1210/endo.139.1.5698. [DOI] [PubMed] [Google Scholar]
- 22.Hess S L, Suchin C R, Saltiel A R. The specific protein phosphatase inhibitor okadaic acid differentially modulates insulin action. J Cell Biochem. 1991;45:374–380. doi: 10.1002/jcb.240450411. [DOI] [PubMed] [Google Scholar]
- 23.Holgado-Madruga M, Emlet D R, Moscatello D K, Godwin A K, Wong A J. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature. 1996;379:560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- 24.Holman G D, Cushman S W. 1994. Subcellular localization and trafficking of the GLUT4 glucose transporter isoform in insulin-responsive cells. Bioessays. 1994;16:753–759. doi: 10.1002/bies.950161010. [DOI] [PubMed] [Google Scholar]
- 25.James D E, Piper R C. Insulin resistance, diabetes, and the insulin-regulated trafficking of GLUT-4. J Cell Biol. 1994;126:1123–1126. doi: 10.1083/jcb.126.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jefferies H B, Fumagalli S, Dennis P B, Reinhard C, Pearson R B, Thomas G. Rapamycin suppresses 5′TOP mRNA translation through inhibition of p70s6k. EMBO J. 1997;16:3693–3704. doi: 10.1093/emboj/16.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kandror K V, Pilch P F. Compartmentalization of protein traffic in insulin-sensitive cells. Am J Physiol. 1996;271:E1–E14. doi: 10.1152/ajpendo.1996.271.1.E1. [DOI] [PubMed] [Google Scholar]
- 28.Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature. 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- 29.Kim S J, Kahn C R. Insulin stimulates p70 S6 kinase in the nucleus of cells. Biochem Biophys Res Commun. 1997;234:681–685. doi: 10.1006/bbrc.1997.6699. [DOI] [PubMed] [Google Scholar]
- 30.Kitamura T, Ogawa W, Sakaue H, Hino Y, Kuroda S, Takata M, Matsumoto M, Maeda T, Konishi H, Kikkawa U, Kasuga M. Requirement for activation of the serine-threonine kinase Akt (protein kinase B) in insulin stimulation of protein synthesis but not of glucose transport. Mol Cell Biol. 1998;18:3708–3717. doi: 10.1128/mcb.18.7.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klippel A, Kavanaugh W M, Pot D, Williams L T. A specific product of phosphatidylinositol 3-kinase directly activates the protein kinase Akt through its pleckstrin homology domain. Mol Cell Biol. 1997;17:338–344. doi: 10.1128/mcb.17.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohn A D, Barthel A, Kovacina K S, Boge A, Wallach B, Summers S A, Birnbaum M J, Scott P H, Lawrence J C, Roth R A. Construction and characterization of a conditionally active version of the serine/threonine kinase Akt. J Biol Chem. 1998;273:11937–11943. doi: 10.1074/jbc.273.19.11937. [DOI] [PubMed] [Google Scholar]
- 33.Kohn A D, Kovacina K S, Roth R A. Insulin stimulates the kinase activity of RAC-PK, a pleckstrin homology domain containing ser/thr kinase. EMBO J. 1995;14:4288–4295. doi: 10.1002/j.1460-2075.1995.tb00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohn A D, Summers S A, Birnbaum M J, Roth R A. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 35.Kotani K, Ogawa W, Matsumoto M, Kitamura T, Sakaue H, Hino Y, Miyake K, Sano W, Akimoto K, Ohno S, Kasuga M. Requirement of atypical protein kinase clambda for insulin stimulation of glucose uptake but not for Akt activation in 3T3-L1 adipocytes. Mol Cell Biol. 1998;18:6971–6982. doi: 10.1128/mcb.18.12.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lavan B E, Fantin V R, Chang E T, Lane W S, Keller S R, Lienhard G E. A novel 160-kDa phosphotyrosine protein in insulin-treated embryonic kidney cells is a new member of the insulin receptor substrate family. J Biol Chem. 1997;272:21403–21407. doi: 10.1074/jbc.272.34.21403. [DOI] [PubMed] [Google Scholar]
- 37.Lavan B E, Lane W S, Lienhard G E. The 60-kDa phosphotyrosine protein in insulin-treated adipocytes is a new member of the insulin receptor substrate family. J Biol Chem. 1997;272:11439–11443. doi: 10.1074/jbc.272.17.11439. [DOI] [PubMed] [Google Scholar]
- 38.Le Good J A, Ziegler W H, Parekh D B, Alessi D R, Cohen P, Parker P J. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 39.Meier R, Thelen M, Hemmings B A. Inactivation and dephosphorylation of protein kinase B alpha (PKBalpha) promoted by hyperosmotic stress. EMBO J. 1998;17:7294–7303. doi: 10.1093/emboj/17.24.7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moule S K, Welsh G I, Edgell N J, Foulstone E J, Proud C G, Denton R M. Regulation of protein kinase B and glycogen synthase kinase-3 by insulin and beta-adrenergic agonists in rat epididymal fat cells. Activation of protein kinase B by wortmannin-sensitive and -insensitive mechanisms. J Biol Chem. 1997;272:7713–7719. doi: 10.1074/jbc.272.12.7713. [DOI] [PubMed] [Google Scholar]
- 41.Olson A L, Knight J B, Pessin J E. Syntaxin 4, VAMP2, and/or VAMP3/cellubrevin are functional target membrane and vesicle SNAP receptors for insulin-stimulated GLUT4 translocation in adipocytes. Mol Cell Biol. 1997;17:2425–2435. doi: 10.1128/mcb.17.5.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Proud C G, Denton R M. Molecular mechanisms for the control of translation by insulin. Biochem J. 1997;328:329–341. doi: 10.1042/bj3280329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rea S, James D E. Moving GLUT4: the biogenesis and trafficking of GLUT4 storage vesicles. Diabetes. 1997;46:1667–1677. doi: 10.2337/diab.46.11.1667. [DOI] [PubMed] [Google Scholar]
- 44.Robinson L J, Pang S, Harris D S, Heuser J, James D E. Translocation of the glucose transporter (GLUT4) to the cell surface in permeabilized 3T3-L1 adipocytes: effects of ATP insulin, and GTP gamma S and localization of GLUT4 to clathrin lattices. J Cell Biol. 1992;117:1181–1196. doi: 10.1083/jcb.117.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakaue H, Ogawa W, Takata M, Kuroda S, Kotani K, Matsumoto M, Sakaue M, Nishio S, Ueno H, Kasuga M. Phosphoinositide 3-kinase is required for insulin-induced but not for growth hormone- or hyperosmolarity-induced glucose uptake in 3T3-L1 adipocytes. Mol Endocrinol. 1997;11:1552–1562. doi: 10.1210/mend.11.10.9986. [DOI] [PubMed] [Google Scholar]
- 46.Saltiel A R. Diverse signaling pathways in the cellular actions of insulin. Am J Physiol. 1996;270:E375–E385. doi: 10.1152/ajpendo.1996.270.3.E375. [DOI] [PubMed] [Google Scholar]
- 47.Serunian L A, Auger K R, Cantley L C. Identification and quantification of polyphosphoinositides produced in response to platelet-derived growth factor stimulation. Methods Enzymol. 1991;198:78–87. doi: 10.1016/0076-6879(91)98010-4. [DOI] [PubMed] [Google Scholar]
- 48.Shepherd P R, Siddle K, Nave B T. Is stimulation of class-1 phosphatidylinositol 3-kinase activity by insulin sufficient to activate pathways involved in glucose metabolism. Biochem Soc Trans. 1997;25:978–981. doi: 10.1042/bst0250978. [DOI] [PubMed] [Google Scholar]
- 49.Shepherd P R, Withers D J, Siddle K. Phosphoinositide 3-kinase: the key switch mechanism in insulin signalling. Biochem J. 1998;333:471–490. doi: 10.1042/bj3330471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Standaert M L, Galloway L, Karnam P, Bandyopadhyay G, Moscat J, Farese R V. Protein kinase C-zeta as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes. Potential role in glucose transport. J Biol Chem. 1997;272:30075–30082. doi: 10.1074/jbc.272.48.30075. [DOI] [PubMed] [Google Scholar]
- 51.Stokoe D, Stephens L R, Copeland T, Gaffney P R, Reese C B, Painter G F, Holmes A B, McCormick F, Hawkins P T. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 52.Sun X J, Rothenberg P, Kahn C R, Backer J M, Araki E, Wilden P A, Cahill D A, Goldstein B J, White M F. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991;352:73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- 53.Tanasijevic M J, Myers M G, Jr, Thoma R S, Crimmins D L, White M F, Sacks D B. Phosphorylation of the insulin receptor substrate IRS-1 by casein kinase II. J Biol Chem. 1993;268:18157–18166. [PubMed] [Google Scholar]
- 54.Tanti J F, Grillo S, Gremeaux T, Coffer P J, Van Obberghen E, Le Marchand-Brustel Y. Potential role of protein kinase B in glucose transporter 4 translocation in adipocytes. Endocrinology. 1997;138:2005–2010. doi: 10.1210/endo.138.5.5136. [DOI] [PubMed] [Google Scholar]
- 55.Thomas G, Hall M N. TOR signalling and control of cell growth. Curr Opin Cell Biol. 1997;9:782–787. doi: 10.1016/s0955-0674(97)80078-6. [DOI] [PubMed] [Google Scholar]
- 56.Waters S B, Yamauchi K, Pessin J E. Insulin-stimulated disassociation of the SOS-Grb2 complex. Mol Cell Biol. 1995;15:2791–2799. doi: 10.1128/mcb.15.5.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.White M F. The insulin signalling system and the IRS proteins. Diabetologia. 1997;40:S2–S17. doi: 10.1007/s001250051387. [DOI] [PubMed] [Google Scholar]
- 58.White M F, Backer J M. Preparation and use of anti-phosphotyrosine antibodies to study structure and function of insulin receptor. Methods Enzymol. 1991;201:65–79. doi: 10.1016/0076-6879(91)01009-q. [DOI] [PubMed] [Google Scholar]
- 59.White M F, Kahn C R. The insulin signaling system. J Biol Chem. 1994;269:1–4. [PubMed] [Google Scholar]
- 60.Wiese R J, Mastick C C, Lazar D F, Saltiel A R. Activation of mitogen-activated protein kinase and phosphatidylinositol 3′-kinase is not sufficient for the hormonal stimulation of glucose uptake, lipogenesis, or glycogen synthesis in 3T3-L1 adipocytes. J Biol Chem. 1995;270:3442–3446. doi: 10.1074/jbc.270.7.3442. [DOI] [PubMed] [Google Scholar]
- 61.Wojtaszewski J F, Laustsen J L, Derave W, Richter E A. Hypoxia and contractions do not utilize the same signaling mechanism in stimulating skeletal muscle glucose transport. Biochim Biophys Acta. 1998;1380:396–404. doi: 10.1016/s0304-4165(98)00011-7. [DOI] [PubMed] [Google Scholar]
- 62.Yamauchi K, Pessin J. Insulin receptor substrate-1 (IRS1) and Shc compete for a limited pool of Grb2 in mediating insulin downstream signaling. J Biol Chem. 1994;269:31107–31114. [PubMed] [Google Scholar]
- 63.Yeh J I, Gulve E A, Rameh L, Birnbaum M J. The effects of wortmannin on rat skeletal muscle. Dissociation of signaling pathways for insulin- and contraction-activated hexose transport. J Biol Chem. 1995;270:2107–2111. doi: 10.1074/jbc.270.5.2107. [DOI] [PubMed] [Google Scholar]