Abstract
Objective
Malignant struma ovarii diagnosis is challenging due to its benign histologic appearance and rarity. We present a case of struma ovarii determined malignant after pulmonary metastases were incidentally discovered.
Methods
A 29-year-old female with a history of benign struma ovarii presented to the emergency room with right lower abdominal pain. Abdomen and pelvis computed tomography showed multiple bilateral pulmonary nodules, which demonstrated well-differentiated thyroid tissue on biopsy. Review of prior ovarian pathology identified features of highly differentiated thyroid carcinoma. Laboratory studies were negative for thyroglobulin (TG) antibodies, thyrotropin was 0.713 mIU/L, and TG was 169 ng/mL. The patient underwent total thyroidectomy, revealing a 0.3 cm follicular variant papillary thyroid microcarcinoma without lymphovascular invasion. An I-123 whole-body scan revealed bilateral metastases in the thigh muscles.
Results
I-123 whole-body scan after receiving I-131 therapy demonstrated uptake in the lungs, thyroid bed, and bilateral thighs. A computed tomography scan 5 months later revealed a decreased size of the pulmonary nodules.
Conclusions
Careful histologic examination is key in making an early diagnosis of malignant struma ovarii. It requires a high index of suspicion and close histologic examination to identify malignant features, mainly the presence of cytologic overlapping ground-glass nuclei and mitotic activity or vascular invasion. Additionally, a thorough review of the imaging is needed to identify any abnormal findings suggestive of metastases. Our case demonstrates that this diagnosis may be made retrospectively after the discovery of metastases and patients can have excellent response to I-131 therapy despite a relatively low TG level.
Key words: malignant struma ovarii, pulmonary metastasis, thyroglobulin
Abbreviations: TG, thyroglobulin; TSH, thyrotropin
Introduction
Struma ovarii is a rare ovarian germ cell tumor that contains >50% thyroid tissue.1 The majority (90%-95%) of struma ovarii are benign.2 Although rare, malignant struma ovarii is an important diagnosis to consider because subtle metastasis can occur at a later time. Malignancy is more common in larger tumors, and almost 75% of malignancies occur in tumors >16 cm and more rarely in tumors <5 cm.3 Malignant struma ovarii shares histologic features of differentiated thyroid cancer and can be classified as a papillary or follicular subtype.4 Because of its benign histologic appearance, malignant struma ovarii, particularly of the follicular subtype, poses a diagnostic challenge and is sometimes not diagnosed until the neoplasm spreads beyond the ovary.5 While metastases are uncommon, the predominant site of metastatic spread is within the adjacent pelvis. Distant metastases to lungs, bones, liver, skin, and brain are exceedingly rare.6 We report a case of malignant struma ovarii with distant metastasis that was retrospectively diagnosed after the incidental discovery of pulmonary metastases on an imaging study.
Case Report
A 29-year-old woman presented to the emergency room with right lower abdominal pain. A diagnosis of right ovarian torsion was made, and she underwent right salpingo-oophorectomy, with the removal of a 12.7 × 9.5 × 10.5 cm multiloculated cystic ovarian mass. Pathology reported “…small focus of mature cystic teratoma of ovary with struma ovarii. The majority of the multicystic mass consists of mature thyroid tissue with colloid admixed with areas of fetal-type follicular tissue. No atypia is observed. No immature component is present.” No additional study for tumor markers or immunohistochemical staining was done.
Two years later, the patient was incidentally noted to have bilateral pulmonary nodules that had increased in size and number compared with the cross-sectional imaging done during her prior admission. The patient underwent video-assisted thoracoscopic surgery for biopsy of the lung nodules. The pathology report stated “…lung tissue with multiple thyroid nodules consistent with metastases from right ovarian mature cystic teratoma with struma ovarii. No carcinoma or significant atypia is present in metastatic thyroid tissue.” A nonblinded review of the right ovarian pathology was done at 2 outside institutions. The first examiner reported that “…the ovarian tumor in isolation does not show overt features of malignancy. However, in the context of an extraovarian similar-appearing lesion in the lung, it has been proposed that this scenario would be consistent with ovarian highly differentiated follicular carcinoma. It has also been suggested in the literature that the latter can only be diagnosed retrospectively after the detection of a metastasis.” The second examiner reported that “…the follicular cells show predominantly solid growth with rare papillary structure and characteristic nuclear features of papillary thyroid carcinoma including enlarged and overlapping nuclei, clear and open chromatin, scattered nuclear grooves, and rare mitotic figures.” (Figure).
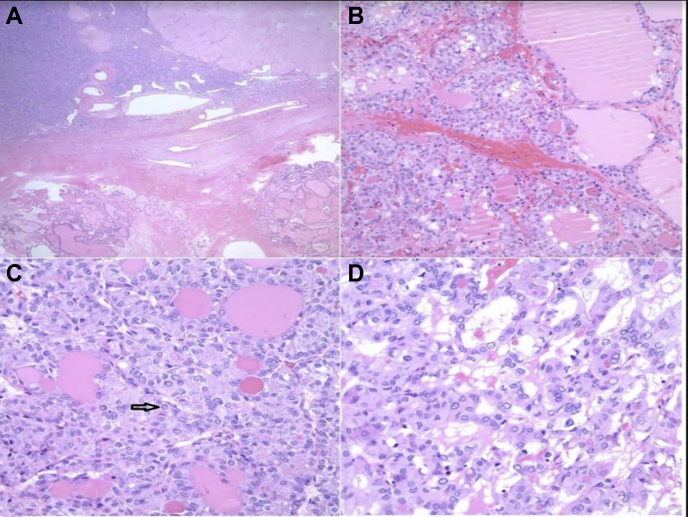
Fig.
Histologic features of the ovarian mass. A, Ovary (low power). Ovarian stroma with thick-walled blood vessels and corpora albicantia (top). Nodules of thyroid-type tissue composed of variable-sized follicles filled with colloid and lined by low cuboidal to columnar follicular epithelial cells (bottom). B, Ovary (high power). There is a hypercellular focus within the thyroid tissue showing a follicular and trabecular pattern of growth and decreased colloid. Note that the follicular cells are larger and have crowded oval to irregular nuclei with pale chromatin. C, Ovary (high power). Cells with pale to vesicular nuclear chromatin, elongated nuclei, and longitudinal nuclear grooves. A nuclear pseudoinclusion is visible (arrow). The cells have moderate amounts of eosinophilic cytoplasm. D, Ovary (high power). Follicular growth of intermediate-sized low cuboidal cells with a moderate amount of dense eosinophilic cytoplasm. The nuclei show crowding and overlapping. Note the pale nuclear chromatin and scant dense colloid.
The patient underwent a total thyroidectomy, revealing a 0.3 × 0.3 cm follicular variant papillary thyroid microcarcinoma without lymphovascular invasion. Preoperatively, the patient was negative for thyroglobulin (TG) antibodies, thyrotropin (TSH) was 0.713 mIU/L (reference range: 0.5-5 mIU/L), and TG was 169 ng/mL (reference range: 3-40 ng/mL). Postoperatively, the patient was started on levothyroxine with subsequent TSH suppression (TSH, 0.058 mIU/L), and TG levels decreased to 80 ng/mL, fluctuating between 56 to 252 ng/mL thereafter.
The patient underwent an I-123 whole-body scan that revealed uptake in the thyroid bed, bilateral pulmonary nodules, and bilateral thigh muscles. Ultrasound of the lower extremities was negative for metastatic findings. After dosimetric studies, 200 mCi of I-131 was administered, and a posttherapy scan showed uptake in the thyroid bed, bilateral lung nodules, and thigh computed tomography of the chest 5 months later demonstrated a decrease in size of the lung nodules, the largest of which decreased from 0.5 to 0.3 cm. One year post radioactive iodine therapy, the TG level is undetectable (TSH- and TG-antibody negative), and the patient remains clinically stable.
Discussion
We have described an interesting case of a young woman who initially presented with an ovarian torsion due to a presumed benign struma ovarii and was subsequently found to have thyroid tissue within lung nodules suggestive of metastatic disease. As is recommended, the patient underwent staging, followed by thyroidectomy, revealing a 3 mm follicular variant of papillary carcinoma. She was treated with levothyroxine to suppress TSH, underwent dosimetry, and was treated with 200 mCi of I-131. Her posttreatment scan showed metastatic lesions in the lungs, thyroid bed, and bilateral thighs. Interestingly, her TG levels remained <252 ng/mL at all times despite the high burden of metastatic disease but still showed an excellent response to I-131 therapy.
Metastatic struma ovarii is rare and occurs in about 5% to 15% of all malignant struma ovarii cases.6 The median age at presentation is usually 40 to 60 years, although it has been reported in patients aged as young as 10 years.7 The most common presenting clinical symptoms are abdominal or pelvic pain, a palpable mass, and ascites, particularly when pelvic metastases are present. While uncommon, hyperthyroidism may occur in 5% to 8% of cases, where an autonomic nodule within the teratoma exists or the patient has a coexisting Graves disease.2,8
The distinction between benign and malignant struma ovarii is currently challenging for most experienced pathologists. A combination of nuclear features, which include the typical characteristics of well-differentiated thyroid carcinoma (nuclear grooves and overlapping ground-glass nuclei), mitoses, necrosis, vascular invasion, or extension of the tumor outside the ovarian capsule, with immunohistochemical stains and cross-sectional imaging studies documenting metastatic disease, aid in the differentiation between benign and malignant struma.9 However, sometimes these features are absent or very subtle and clinical features of metastases, for example, lung with normal-appearing thyroid tissue (as in our case) become critically important to complement the diagnosis.
Given the rarity of malignant struma ovarii, very limited data are available to describe the value of performing special stains or tumor markers on these tissues. Cancer antigen 125 is a common tumor marker for malignant struma ovarii but is of very low diagnostic value due to its nonspecificity.10 A broad panel of immunohistochemical stains, including paired-box gene 8, thyroid transcription factor 1, and TG, can be helpful to differentiate malignant struma ovarii from other ovarian malignancies, but it has not been reported if they can help to differentiate benign vs malignant struma ovarii.11 It remains to be seen if markers found to be useful in thyroid malignancies, such as HBME-1, specific cytokeratins (eg, cytokeratin 19), and RET, might be helpful in the diagnosis of malignant struma ovarii.12 The field of the genetic profiling of thyroid tumors is relatively new and needs further data to evaluate if these tools may be helpful in differentiating benign vs malignant struma.
There are no consistent guidelines for the management of malignant struma ovarii after initial surgical diagnosis.4 Most recommendations are based upon a review of available case reports and case series.13 The often-suggested treatment strategy includes debulking surgery (ie, unilateral or bilateral salpingo-oophorectomy with or without hysterectomy), followed by adjunctive therapies based on the presence of metastasis and the risk of recurrence. Patients with gross extraovarian extension, larger lesions (>4 cm), and the presence of BRAF mutations have a higher risk of recurrence.14 The practice for patients with high-risk features is to perform a total thyroidectomy followed by radioactive I-131 therapy.13,14 These patients also require thyroxine supplementation for TSH suppression after ablation.13 Following initial treatment, patients with malignant struma ovarii require long-term follow-up with regular monitoring of serum TG levels with or without whole-body I-131 scintigraphy.
A low TG level in thyroid cancer is often seen with tumors that may have dedifferentiated. Not much information is available regarding the dedifferentiation of metastatic struma ovarii. In our case, the histopathological findings of metastatic tissue very close in appearance to normal thyroid tissue and I-131 uptake within these lesions argue that the tumor remained differentiated, maintaining its capacity to concentrate I-131.10,15 The presence of thyroid microcarcinoma also raises the possibility of metastasis from primary thyroid malignancy; however, the thyroid carcinoma was 3 mm in size and had clear margins with no evidence of lymphovascular invasion, making metastasis from primary thyroid cancer less likely. Moreover, 2 of 3 pathologists agreed that the lung lesions were consistent with metastatic follicular carcinoma in the context of the diagnosis of struma ovarii, with follicular lesions similar in appearance to the lung. In the future, it is possible that the genomic signature of the tumor could be helpful in these diagnostically challenging cases.
Conclusion
Careful histologic examination of struma ovarii is key in making early diagnosis of malignant cases because benign-appearing histology or subtle histologic changes pose a diagnostic challenge. TG levels usually give guidance on disease burden and treatment response. However, in this case, TG levels were lower than reported in previous cases despite a well-differentiated appearance on pathology. Nonetheless, the patient had an excellent response to radioactive iodine at all metastatic sites, indicating a well-differentiated disease and a favorable prognosis. Thus, TG levels may not predict the response to I-131 therapy in such cases.
Disclosure
This case report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Goffredo P., Sawka A.M., Pura J., Adam M.A., Roman S.A., Sosa J.A. Malignant struma ovarii: a population-level analysis of a large series of 68 patients. Thyroid. 2015;25(2):211–215. doi: 10.1089/thy.2014.0328. [DOI] [PubMed] [Google Scholar]
- 2.Dunzendorfer T., deLas Morenas A., Kalir T., Levin R.M. Struma ovarii and hyperthyroidism. Thyroid. 1999;9(5):499–502. doi: 10.1089/thy.1999.9.499. [DOI] [PubMed] [Google Scholar]
- 3.Young R.H. New and unusual aspects of ovarian germ cell tumors. Am J Surg Pathol. 1993;17(12):1210–1224. doi: 10.1097/00000478-199312000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Siegel M.R., Wolsky R.J., Alvarez E.A., Mengesha B.M. Struma ovarii with atypical features and synchronous primary thyroid cancer: a case report and review of the literature. Arch Gynecol Obstet. 2019;300(6):1693–1707. doi: 10.1007/s00404-019-05329-z. [DOI] [PubMed] [Google Scholar]
- 5.Roth L.M., Karseladze A.I. Highly differentiated follicular carcinoma arising from struma ovarii: a report of 3 cases, a review of the literature, and a reassessment of so-called peritoneal strumosis. Int J Gynecol Pathol. 2008;27(2):213–222. doi: 10.1097/PGP.0b013e318158e958. [DOI] [PubMed] [Google Scholar]
- 6.Ruel I.F., Fierrard H., Vercellino L. Pulmonary metastasis of struma ovarii: a case report. Clin Nucl Med. 2010;35(9):692–694. doi: 10.1097/RLU.0b013e3181e9fb1b. [DOI] [PubMed] [Google Scholar]
- 7.Iranparvar Alamdari M., Habibzadeh A., Pakrouy H., Chaichi P., Sheidaei S. An unusual presentation of a papillary thyroid carcinoma in the struma ovarii in a 10-year-old girl: a case report. Int J Surg Case Rep. 2018;51:218–220. doi: 10.1016/j.ijscr.2018.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teale E., Gouldesbrough D.R., Peacey S.R. Graves’ disease and coexisting struma ovarii: struma expression of thyrotropin receptors and the presence of thyrotropin receptor stimulating antibodies. Thyroid. 2006;16(8):791–793. doi: 10.1089/thy.2006.16.791. [DOI] [PubMed] [Google Scholar]
- 9.Devaney K., Snyder R., Norris H.J., Tavassoli F.A. Proliferative and histologically malignant struma ovarii: a clinicopathologic study of 54 cases. Int J Gynecol Pathol. 1993;12(4):333–343. doi: 10.1097/00004347-199310000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Lager C.J., Koenig R.J., Lieberman R.W., Avram A.M. Rare clinical entity: metastatic malignant struma ovarii diagnosed during pregnancy – lessons for management. Clin Diabetes Endocrinol. 2018;4:13. doi: 10.1186/s40842-018-0064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subhash Y., Santosh M., Vishwapriya G., Kedar D. Poorly differentiated thyroid carcinoma arising in struma ovarii- a report of two extremely rare cases. Hum Pathol (N Y) 2020;21:200393. [Google Scholar]
- 12.Cheung C.C., Ezzat S., Freeman J.L., Rosen I.B., Asa S.L. Immunohistochemical diagnosis of papillary thyroid carcinoma. Mod Pathol. 2001;14(4):338–342. doi: 10.1038/modpathol.3880312. [DOI] [PubMed] [Google Scholar]
- 13.Marti J.L., Clark V.E., Harper H., Chhieng D.C., Sosa J.A., Roman S.A. Optimal surgical management of well-differentiated thyroid cancer arising in struma ovarii: a series of 4 patients and a review of 53 reported cases. Thyroid. 2012;22(4):400–406. doi: 10.1089/thy.2011.0162. [DOI] [PubMed] [Google Scholar]
- 14.Wolff E.F., Hughes M., Merino M.J. Expression of benign and malignant thyroid tissue in ovarian teratomas and the importance of multimodal management as illustrated by a BRAF-positive follicular variant of papillary thyroid cancer. Thyroid. 2010;20(9):981–987. doi: 10.1089/thy.2009.0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rinaldi S., Plummer M., Biessy C. Thyroid-stimulating hormone, thyroglobulin, and thyroid hormones and risk of differentiated thyroid carcinoma: the EPIC study. J Natl Cancer Inst. 2014;106(6):dju097. doi: 10.1093/jnci/dju097. [DOI] [PubMed] [Google Scholar]