Abstract
Objective
The presence of primary hyperparathyroidism (PHPT) and Klinefelter syndrome (KS) is rare, and its association with KS mosaicism is even rarer. We report an unusual combination of these entities with a mild phenotype of KS.
Methods
The patient was a 44-year-old male with a history of PHPT who had recurrent urolithiasis despite being treated with a successful parathyroidectomy. On examination, he had axillary hair growth, bilateral gynecomastia, a large port-wine stain at the right hemithorax and upper right limb, and genitalia and pubic hair corresponding to Tanner IV classification with small, normal consistency testicles.
Results
Laboratory findings were unremarkable except for a slightly elevated luteinizing hormone, which was normal on repeat testing. Because of the picture of unexplained gynecomastia, laboratory findings, and low-volume testis, a diagnosis of KS was considered. Chromosomal analysis revealed a rare 45,X/46,XY/47,XXY/48,XXYY/48,XXXY KS mosaic.
Conclusions
KS phenotypes are largely variable, and their association with PHPT remains to be elucidated.
Key words: hyperparathyroidism, Klinefelter syndrome, mosaicism, nephrolithiasis
Abbreviations: KS, Klinefelter syndrome; LH, luteinizing hormone; PHPT, primary hyperparathyroidism; PTH, parathyroid hormone
Introduction
Despite being described in 1942,1 the diagnosis of Klinefelter syndrome (KS) remains a significant challenge. Nearly 75% of the cases will never obtain the correct diagnosis,2 resulting in unfavorable repercussions in morbidity and mortality, but this percentage could be higher in countries with less favorable health care services. Small testes, gynecomastia, hypergonadotropic hypogonadism, and infertility are the classic features of KS; however, the absence of apparent signs complicates the identification of affected individuals.3
KS is the most frequent cause of male primary hypergonadotropic hypogonadism.4 A 47,XXY karyotype occurs in 90% of cases, whereas the remaining cases comprise various grades of mosaicism or abnormal structure of the X chromosome.3,5 On rare occasions, KS variants can present as higher-grade X chromosome aneuploidies (48,XXYY or 48,XXYY polysomies) and can be associated with severe phenotypes, including cognitive and behavioral disorders.6
Primary hyperparathyroidism (PHPT) is a common endocrine derangement with a prevalence of up to 1 to 4 per 1000 in the general population.7 It is characterized by hypercalcemia and inappropriately normal or elevated levels of parathyroid hormone (PTH). The vast majority of PHPT cases are caused by a single parathyroid adenoma; the remaining causes include 4-gland hyperplasia, multiple adenomas, and parathyroid cancer.7 Only 20% to 30% of patients with PHPT have symptoms, nephrolithiasis being the most common.8
The presence of PHPT and KS have not been reported in the same patient very often,9 and its association with KS mosaicism is even rarer. We report an unusual case of a young Hispanic male with a mosaic KS and PHPT.
Case Report
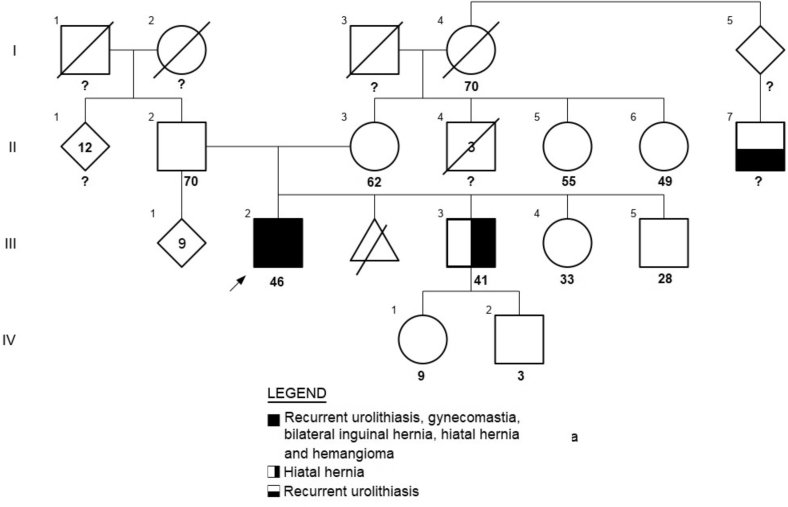
A Mexican mestizo man aged 44 years was referred to our endocrinology department for management of recurrent urolithiasis. He presented with complaints of episodic pain bilaterally in the lumbar region since age 19 and had been using nonsteroidal analgesic drugs to reduce his pain for the past 20 years. He had undergone subcutaneous mastectomy at age 15 for gynecomastia but was not evaluated for its cause. At age 39, he underwent transurethral lithotripsy with the removal of a 2-cm stone from the left ureter, and he was reportedly diagnosed with primary PHPT. He did not receive any treatment, and further evaluation was not pursued. At age 43, a partial parathyroidectomy (3 and a half glands) was done (histologic examination reported parathyroid hyperplasia), nephrolithiasis was persistent, and intact PTH was within the reference range (Table 1). A 99mTc-sestamibi SPECT/CT was performed 1 month after the surgery and did not reveal abnormalities. He affirmed that he has several uncles on both the paternal and maternal sides of his family with urinary calculi but could not provide any details (Fig. 1).
Table.
Laboratory Findings
| Values (normal range) | Before parathyroidectomy | After parathyroidectomy | On admission | At follow-up |
|---|---|---|---|---|
| Blood | … | … | … | … |
| Sodium (136-146 mmol/L) | … | … | 146 | 141 |
| Potassium (3.5-5.1 mmol/L) | … | … | 4.49 | 4.09 |
| Chloride (98-107 mmol/L) | … | … | 106.5 | 103 |
| Calcium (8.6-10.3 mg/dL) | 10.3 | … | 10.2 | 9.12 |
| Phosphorus (2.5-5 mg/dL) | 2.3 | … | 3.63 | 2.64 |
| Magnesium (1.9-2.7 mg/dL) | 2.29 | … | 2.20 | 2.00 |
| Creatinine (0.3-0.7 mg/dL) | 1.03 | … | 0.99 | 0.94 |
| Albumin (3.5-5.7 g/dL) | 5.3 | … | 4.75 | 5.20 |
| Intact parathyroid hormone (12-88 pg/mL) | 128.33 | 58.9 | 60.7 | 70.0 |
| 25 (OH) vitamin D (30-100 ng/mL) | 17.6 | … | 40.0 | 22.1 |
| 1, 25-dihydroxyvitamin D (19.6-54.3 pg/mL) | … | … | … | 45.7 |
| Follicle-stimulating hormone (1.27-19.26 mIU/mL) (men) | … | … | 13.09 | 7.98 |
| Luteinizing hormone (1.24-8.62 mIU/mL) (men) | … | … | 9.37 | 4.12 |
| Estradiol (<60 pg/mL) (men) | … | … | 19.0 | … |
| Testosterone (1.75-7.81 ng/mL) | … | … | 3.53 | 4.27 |
| Thyrotropin (0.3-5 mIU/L) | … | … | 0.98 | 1.75 |
| Prolactin (3.9-29.5 ng/mL) | … | … | 8.30 | 33.11 |
| Insulin-like growth factor 1 (78-230 ng/mL) | … | … | 264.1 | … |
| Growth hormone (0-3 ng/mL) | … | … | 0.03 | 0.031 |
| Corticotropin (10-100 pg/mL) | … | … | 70 | 35 |
| Free thyroxine (0.63-1.34 ng/dL) | … | … | 0.95 | 1.1 |
| Cortisol 8 am (6.7-22.6 mcg/dL) | … | … | 11.86 | 12.59 |
| Glycated hemoglobin (<5.7%) | … | … | 4.9 | 4.8 |
| Total cholesterol (<200 mg/dL) | … | … | 207 | 174 |
| High-density lipoprotein (40-60 mg/dL) | … | … | 49 | 45 |
| Low-density lipoprotein (<130 mg/dL) | … | … | 151 | 113 |
| Triglycerides (<150 mg/dL) | … | … | 97 | 113 |
| Fasting plasma glucose (70-105 mg/dL) | … | … | 104 | 81 |
| Alkaline phosphatase (34-104 U/L) | 105 | … | 69 | 73 |
| Alanine aminotransferase (7-52 U/L) | … | … | 18.5 | 25 |
| Aspartate aminotransferase (13-39 U/L) | … | … | 17.0 | 20 |
| 24-h Urine excretion | … | … | … | … |
| Sodium (mmol/vol) | … | … | 143 | 197 |
| Potassium (mmol/vol) | … | … | 40 | 32 |
| Calcium (<300 mg/vol) | 330.8 | … | 241 | 209 |
| Phosphorus (<1000 mg/vol) | … | … | 747 | 673 |
| Creatinine (mg/vol) | … | … | 1792 | 1497 |
| Citrate (250-1000 mg/vol) | 490.3 | … | 343 | 475 |
| Oxalate (7-44 mg/vol) | 20.2 | … | 21.52 | 23.22 |
Fig. 1.
Patient’s pedigree. The proband (III.2) is marked by a black arrow.
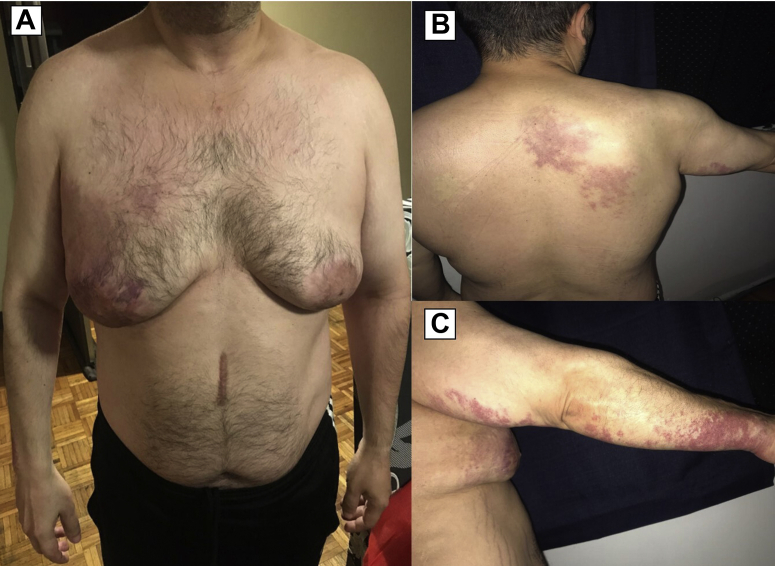
At the time of his visit, the patient appeared old for his age. He had a height of 164 cm, a weight of 77.3 kg (body mass index, 29 kg/m2), and an arm span of 158 cm. Physical examination was remarkable for bilateral gynecomastia (Grade IV), without masses or discharge, and a giant hemangioma on the right hemithorax and upper right limb (Fig. 2). Axillary hair growth was present. He had a Tanner stage of P5G4 with a penile length of 9.5 cm and circumference of 7 cm. The volume of the right testicle was approximately 4.5 x 2.5 x 2.5 cm, but the left testicle could not be palpated. There was no history of anosmia, visual disturbance, low libido, or erectile dysfunction. Serum calcium, intact PTH, albumin, phosphorus, vitamin D, and 24-h urinary calcium excretion were measured, and all were within the normal range. The levels of follicle-stimulating hormone, luteinizing hormone (LH), and testosterone were 13.09 (1.27-19.26 mIU/mL), 9.37 (1.24-8.62 mIU/mL), and 3.53 (1.75-7.81 ng/mL), respectively. Other test results are shown in Table 1. Bone mineral density evaluation revealed a femoral Z-score of 0.6 and a lumbar Z-score of 2.6. The dorsal and lumbar spine morphometric study did not show vertebral fractures. The spectroscopic analysis of the kidney stones identified mono- and dihydrate calcium oxalate. Additional work-up tests included a urine amino acid panel, which did not reveal any abnormalities. Considering recurrent urolithiasis despite the absence of biochemical etiology and under the patient’s consent, a nephrolithiasis multigene panel (Invitae)10 was performed, which reported 2 variants of uncertain significance: c.569 T>C (p.Met190Thr) in the SLC3A1 gene and c.2359C>T (p.Arg787Trp) in the XDH gene, both in the heterozygote state.
Fig. 2.
Clinical manifestations in the propositus. A, Prominent gynecomastia and a violaceous spot (10 cm at its greatest dimension) in the right pectoral area with well-defined boundaries. B, A large port-wine stain was also observed in the right scapular area, with well-defined irregular borders of vascular origin. C, Vascular spots with the same characteristics were observed on the right arm and forearm. Note that it is a dermatosis that only affects the right hemibody and does not cross the midline.
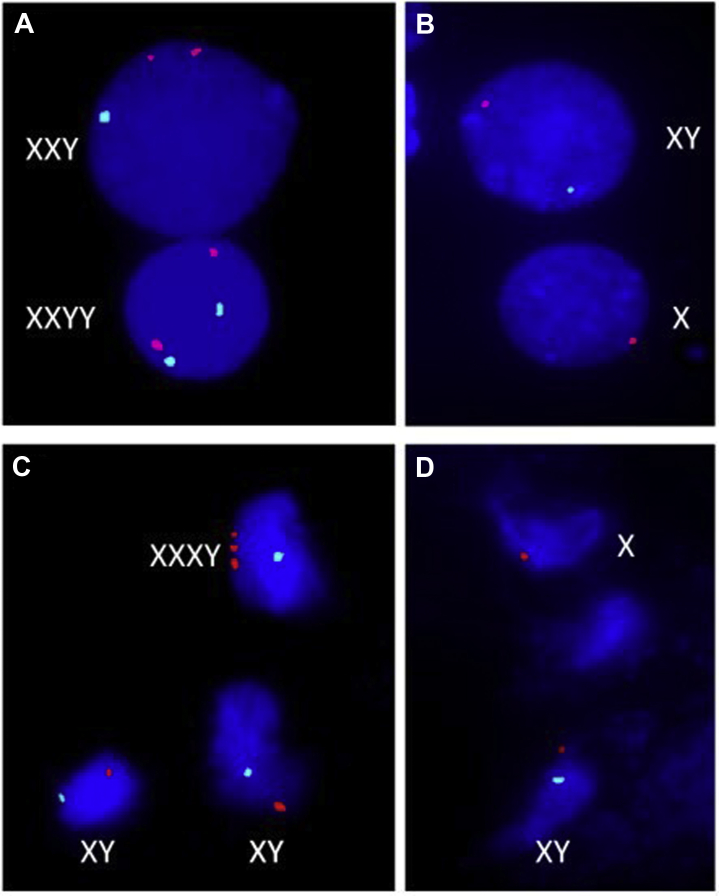
Because of the picture of unexplained gynecomastia, slightly high LH, and inability to palpate the left testicle, a diagnosis of KS was considered. A direct spermatobioscopy was carried out that revealed oligo-terato-asthenozoospermia (semen volume = 1.7 mL [normal >1.5 mL], total sperm number = 16 x 106/ejaculate [normal ≥39 x 106]). Ultrasonography of the testis showed the absence of both parenchymal alterations and hypervascularization; the right and left testicle volume was 8.1 mL and 10.4 mL, respectively. Chromosomal analysis was performed (GTG banding at 550 band resolution), which revealed low-grade mosaicism: mos 45,X,-Y[3]/48,XXXY[1]/46,XY[46]. Interphase fluorescence In Situ hybridization was performed on lymphocytes and buccal epithelial cells, using the Vysis-ABBOTT® LSI probes: CEPX (DXZ1) and LSI CEPY (DYZI1), which also revealed a low-grade mosaicism: nuc ish(DXZ1,DYZ1)x2[6/1000]/(DXZ1x3,DYZ1x1)[11/1000]/(DXZ1x1)[40/1000]/(DXZ1x2,DYZ1x1)[77/1000] and nuc ish(DXZ1,DYZ1)x2[6/1000]/(DXZ1x3,DYZ1x1)[8/1000]/(DXZ1x2,DYZ1x1)[61/1000]/(DXZ1x1)[128/1000]., respectively, according to the International System for Human Cytogenetic Nomenclature ISCN (2013)11 (Fig. 3).
Fig. 3.
Interphase fluorescence In Situ hybridization (FISH). Different cellular lines were found on lymphocytes (A and B) and buccal epithelial cells (C and D). Orange signal corresponds to X-chromosome centromere and aqua signal to Y-chromosome centromere.
At the present time, our patient continues to have recurrent episodes of urolithiasis and irregular compliance with the treatment recommendations (including low sodium intake and potassium citrate supplementation), despite our efforts.
Discussion
We report the occurrence of a rare case of mosaic KS associated with PHPT and a variant of uncertain significance in the SLC3A1 gene. Only 2 cases of the coexistence of KS and PHPT have been reported.9,12 Both cases had typical clinical and biochemical findings of KS. None of them were mosaic KS. To our knowledge, this is the first report of a mosaic KS case with normal gonadotropin levels at an adult age with mild clinical manifestations of KS, like gynecomastia, and with this unique pattern of mosaicism.
Unelevated serum gonadotropin levels in nonmosaic KS have been reported before.13 It has been associated with different grades of mosaicism,14 homogenous 47,XXY,13 and trisomy Xq,15 which indicate no obvious association between cytogenetic compositions and phenotypes. Typically, testosterone levels start to decline in late adolescence, and by early adulthood, overt hypergonadotropic hypogonadism ensues.16 In addition, testosterone levels start to decline as normal testicular tissue is destroyed.17 Because our patient had repeat PTH values that were normal with higher levels of vitamin D, we speculate that our patient could also have secondary hyperparathyroidism.
Our patient had gonadotropin levels that were virtually within normal ranges. Central hypogonadism is the result of the exhaustion of LH and follicle-stimulating hormone secondary to chronic stimulation.18, 19, 20 On the other hand, it is feasible that variable levels of gonadotropin in KS represent different phenotypes.21,22 Numerous findings on slight neuroendocrine variations have been reported in KS, including increased secretion of prolactin and growth hormone23 and increased levels of daily pulsatility of LH.24 Androgen sensitivity also has an important role in influencing the KS phenotype.3
The SLC3A1 gene (located at chromosome 2p16.3-p21) protein product is involved in the transportation of cystine and dibasic (ornithine, lysine, and arginine) and neutral amino acids.25,26 It encodes the neutral and basic amino acid transport protein rBAT, which forms a heterodimer with the gene product of SLC7A9, and its mutation results in cystinuria.26 Cystinuria caused by defects in SLC3A1 is inherited in a true autosomal recessive manner in which heterozygotes have normal urinary cystine excretion.27 The absence of cystine in the kidney stone analysis and the urine amino acid panel support this notion. However, there have been cases of type II and type III heterozygotes that suffer from calcium oxalate urolithiasis.28 This variant has a minor allele frequency of <0.01, and In-Silico predictors (MutationTaster) describe it as disease-causing. Nevertheless, the status remains unchanged.
Up to 30% of recurrence of nephrolithiasis after parathyroidectomy has been reported after an average follow-up of 5 years.29 The cause of recurrent urolithiasis despite been treated with successful parathyroidectomy remains unclear because hypercalciuria is the main lithogenic factor associated with stone formation in PHPT.30 Few studies have attempted to elucidate the increased risk of kidney stone formation despite successful parathyroidectomy. It has been hypothesized that young age;31 higher body mass index;32 persistent hypercalciuria;33 low urine citrate; and high urine phosphate, oxalate, and sodium may explain this occurrence.34 Our patient had none of these risk factors of kidney stone formation other than young age.
Conclusion
Until now, no syndromic or pathogenic connection has been established between PHPT and KS. As in the other 2 reported cases, the coexistence of these 2 entities may be coincidental. We are unaware of any reasons why the presence of KS should alter the prevalence of PHPT in patients with KS or why either of these entities should alter the features of the other. Because PHPT is detected earlier in life, mild symptoms, such as depression, fatigue, and mood disorder, can be easily overlooked, as is the case with hypogonadism.35 We hope that this case sheds light on the phenotypic variation of KS and contributes to the existing literature.
Acknowledgments
We thank the Department of Endocrinology and Metabolism and the Department of Dermatology of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
C.L., L.R., and J.A. acquired the data. R.R. contributed by performing the analysis of the conventional and molecular cytogenetic studies. C.L. wrote the manuscript. All authors contributed to the article and approved the submitted version.
Disclosure
The authors have no multiplicity of interest to disclose.
References
- 1.Klinefelter H.F., Jr., Reifenstein E.C., Jr., Albright F., Jr. Syndrome characterized by gynecomastia, aspermatogenesis without a-leydigism, and increased excretion of follicle-stimulating hormone. J Clin Endocrinol Metab. 1942;2(11):615–627. [Google Scholar]
- 2.Herlihy A.S., Halliday J.L., Cock M.L., McLachlan R.I. The prevalence and diagnosis rates of Klinefelter syndrome: an Australian comparison. Med J Aust. 2011;194(1):24–28. doi: 10.5694/j.1326-5377.2011.tb04141.x. [DOI] [PubMed] [Google Scholar]
- 3.Gravholt C.H., Chang S., Wallentin M., Fedder J., Moore P., Skakkebæk A. Klinefelter syndrome: integrating genetics, neuropsychology, and endocrinology. Endocr Rev. 2018;39(4):389–423. doi: 10.1210/er.2017-00212. [DOI] [PubMed] [Google Scholar]
- 4.Kanakis G.A., Nieschlag E. Klinefelter syndrome: more than hypogonadism. Metabolism. 2018;86:135–144. doi: 10.1016/j.metabol.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 5.Frühmesser A., Kotzot D. Chromosomal variants in Klinefelter syndrome. Sex Dev. 2011;5(3):109–123. doi: 10.1159/000327324. [DOI] [PubMed] [Google Scholar]
- 6.Tartaglia N., Ayari N., Howell S., D’Epagnier C., Zeitler P. 48,XXYY, 48,XXXY and 49,XXXXY syndromes: not just variants of Klinefelter syndrome. Acta Paediatr. 2011;100(6):851–860. doi: 10.1111/j.1651-2227.2011.02235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bilezikian J.P., Cusano N.E., Khan A.A., Liu J.-M., Marcocci C., Bandeira F. Primary hyperparathyroidism. Nat Rev Dis Primers. 2016;2:16033. doi: 10.1038/nrdp.2016.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraser W.D. Hyperparathyroidism. Lancet. 2009;374(9684):145–158. doi: 10.1016/S0140-6736(09)60507-9. [DOI] [PubMed] [Google Scholar]
- 9.Castellano E., Pellegrino M., Attanasio R., Guarnieri V., Maffè A., Borretta G. Primary hyperparathyroidism and Klinefelter’s syndrome in a young man. Endocrinol Diabetes Metab Case Rep. 2015;2015:150019. doi: 10.1530/EDM-15-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Invitae Nephrolithiasis Panel Invitae Corporation. https://www.invitae.com/en/physician/tests/72037/
- 11.Shaffer L.G., McGowan-Jordan J., Schmid M., editors. ISCN 2013: an international system for human cytogenetic nomenclature. S. Karger; Basel, Switzerland: 2013. [Google Scholar]
- 12.Spalding J.A., Morrow G.W., Jr., Scholz D.A. Coexisting Klinefelter’s syndrome and primary hyperparathyroidism: report of case. Metabolism. 1962;11:732–734. [PubMed] [Google Scholar]
- 13.Cangiano B., Indirli R., Profka E. Central hypogonadism in Klinefelter syndrome: report of two cases and review of the literature. J Endocrinol Invest. 2021;44(3):459–470. doi: 10.1007/s40618-020-01324-3. [DOI] [PubMed] [Google Scholar]
- 14.Shirai M., Matsuda S., Mitsukawa S. A case of hypogonadotropic hypogonadism with an XY/XXY sex chromosome mosaicism. Tohoku J Exp Med. 1974;114(2):131–139. doi: 10.1620/tjem.114.131. [DOI] [PubMed] [Google Scholar]
- 15.Sabbaghian M., Meybodi A.M., Rahimian M., Sadighi Gilani M.A. Occurrence of 47,X,i(X)(q10),Y Klinefelter variant with hypogonadotropic hypogonadism. Fertil Steril. 2011;96(2):e115–e117. doi: 10.1016/j.fertnstert.2011.05.074. [DOI] [PubMed] [Google Scholar]
- 16.Aksglaede L., Skakkebaek N.E., Almstrup K., Juul A. Clinical and biological parameters in 166 boys, adolescents and adults with nonmosaic Klinefelter syndrome: a Copenhagen experience. Acta Paediatr. 2011;100(6):793–806. doi: 10.1111/j.1651-2227.2011.02246.x. [DOI] [PubMed] [Google Scholar]
- 17.Lanfranco F., Kamischke A., Zitzmann M., Nieschlag E. Klinefelter's syndrome. Lancet. 2004;364(9430):273–283. doi: 10.1016/S0140-6736(04)16678-6. [DOI] [PubMed] [Google Scholar]
- 18.Cherian K.E., Jebasingh F.K., Kapoor N., Paul T.V. Klinefelter syndrome with low gonadotropin levels. BMJ Case Rep. 2015;2015 doi: 10.1136/bcr-2015-213333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabinowitz D., Cohen M.M., Rosenmann E. Chromatin-positive Klinefelter’s syndrome with undetectable peripheral FSH levels. Am J Med. 1975;59(4):584–590. doi: 10.1016/0002-9343(75)90266-1. [DOI] [PubMed] [Google Scholar]
- 20.Carter J.N., Wiseman D.G., Lee H.B. Klinefelter’s syndrome with hypogonadotrophic hypogonadism. Br Med J. 1977;1(6055):212. doi: 10.1136/bmj.1.6055.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bojesen A., Juul S., Gravholt C.H. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J Clinical Endocrinol Metab. 2003;88(2):622–626. doi: 10.1210/jc.2002-021491. [DOI] [PubMed] [Google Scholar]
- 22.Bonomi M., Rochira V., Pasquali D. Klinefelter syndrome (KS): genetics, clinical phenotype and hypogonadism. J Endocrinol Invest. 2017;40(2):123–134. doi: 10.1007/s40618-016-0541-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giusti M., Mortara R., Bolognesi F., Mignone D., Giordano G. Sleep-wake behavior and integrated values of LH, FSH, PRL, FH, and TSH in Klinefelter’s syndrome. J Endocrinol Invest. 1979;2(4):385–393. doi: 10.1007/BF03349338. [DOI] [PubMed] [Google Scholar]
- 24.Aksglaede L., Jensen R.B., Carlsen E. Increased basal and pulsatile secretion of FSH and LH in young men with 47,XXY or 46,XX karyotypes. Eur J Endocrinol. 2008;158(6):803–810. doi: 10.1530/EJE-07-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pras E., Arber N., Aksentijevich I. Localization of a gene causing cystinuria to chromosome 2p. Nat Genet. 1994;6(4):415–419. doi: 10.1038/ng0494-415. [DOI] [PubMed] [Google Scholar]
- 26.Calonge M.J., Gasparini P., Chillarón J. Cystinuria caused by mutations in rBAT, a gene involved in the transport of cystine. Nat Genetics. 1994;6(4):420–425. doi: 10.1038/ng0494-420. [DOI] [PubMed] [Google Scholar]
- 27.Font-Llitjós M., Jiménez-Vidal M., Bisceglia L. New insights into cystinuria: 40 new mutations, genotype-phenotype correlation, and digenic inheritance causing partial phenotype. J Med Genet. 2005;42(1):58–68. doi: 10.1136/jmg.2004.022244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Resnick M.I., Goodman H.O., Boyce W.H. Heterozygous cystinuria and calcium oxalate urolithiasis. J Urol. 1979;122(1):52–54. doi: 10.1016/s0022-5347(17)56248-5. [DOI] [PubMed] [Google Scholar]
- 29.Deaconson T.F., Wilson S.D., Lemann J., Jr. The effect of parathyroidectomy on the recurrence of nephrolithiasis. Surgery. 1987;102(6):910–913. [PubMed] [Google Scholar]
- 30.Cong X., Shen L., Gu X. Current opinions on nephrolithiasis associated with primary hyperparathyroidism. Urolithiasis. 2018;46(5):453–457. doi: 10.1007/s00240-018-1038-x. [DOI] [PubMed] [Google Scholar]
- 31.Islam A.K., Holt S., Reisch J., Nwariaku F., Antonelli J., Maalouf N.M. What predicts recurrent kidney stone after parathyroidectomy in patients with primary hyperparathyroidism? J Am Coll Surg. 2020;231(1):74–82. doi: 10.1016/j.jamcollsurg.2020.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Tran H., Grange J.S., Adams-Huet B. The impact of obesity on the presentation of primary hyperparathyroidism. J Clin Endocrinol Metab. 2014;99(7):2359–2364. doi: 10.1210/jc.2013-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spivacow F.R., Negri A.L., del Valle E.E., Fradinger E., Martinez C., Polonsky A. Persistence of hypercalciuria after successful surgical treatment for primary hyperparathyroidism. Int Urol Nephrol. 2012;44(3):857–863. doi: 10.1007/s11255-011-9953-6. [DOI] [PubMed] [Google Scholar]
- 34.Marchini G.S., Faria K.V.M., Torricelli F.C.M. Sporadic primary hyperparathyroidism and stone disease: a comprehensive metabolic evaluation before and after parathyroidectomy. BJU Int. 2018;121(2):281–288. doi: 10.1111/bju.14072. [DOI] [PubMed] [Google Scholar]
- 35.Blanchard C., Mathonnet M., Sebag F. Surgery for ‘asymptomatic’ mild primary hyperparathyroidism improves some clinical symptoms postoperatively. Eur J Endocrinol. 2013;169(5):665–672. doi: 10.1530/EJE-13-0502. [DOI] [PubMed] [Google Scholar]