In the current pandemic of SARS‐CoV‐2, the fast development of vaccines was mandatory due to many deaths and cases across the world since the beginning of the outbreak.
Questions about the potential immunogenicity of the vaccine and the safety in the short and long term have been raised.1, 2
Reports of cutaneous reactions have been made for other vaccines (as small case series following Moderna mRNA‐1273 vaccination).3
We report the case of a 37‐year‐old woman, with no medical history nor allergies, who developed a skin rash 4 days after the vaccination with the AstraZeneca AZD1222 COVID‐19 vaccine.
Rash was characterized by plaques of vesicular, vesicular–papular, partially confluent lesions with peripheral halo and necrotic center on thighs, feet, and face, without dermatomal distribution, nor hitching (Figure 1).
Figure 1.
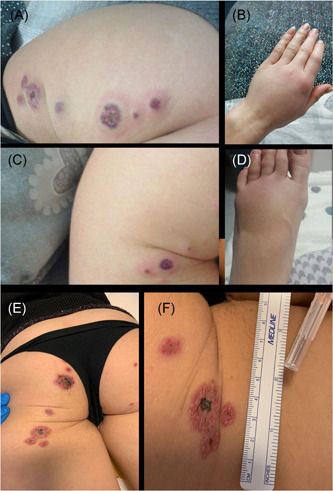
Different lesion types in (A), (C), (E), and (F). Pitting edema in (B) and (D). Arthritis in (B)
She also had arthritis of metacarpophalangeal joints (demonstrated with ultrasound), pitting edema of hands and feet, muscle weakness, and foot neuropathic pain.
We performed three different sites skin punch biopsies of different evolutive types of lesions, with specimens sent for histological, immunohistochemical, and immunofluorescence analysis.
We collected blood samples for ANA, ANCA, dsDNA antibodies, connective tissue disease immunoblot, cryoglobulins.
We started an empirical course of clarithromycin and valaciclovir before steroid infusion and for the following two weeks.
She received a 125 mg infusion of methylprednisone succinate and subsequent oral tapering (Prednisone 25 mg) in 3 weeks until complete suspension. The lesions rapidly disappeared in the first week.
On blood exams CRP elevation in a neutrophilic state (13.3 × 109/L leukocytes with 76.3% of neutrophils) was present. Procalcitonin and autoimmunity assay were negative.
Histology showed neutrophilic pustular dermatitis at different evolutive states sometimes with the presence of necrotizing neutrophilic infiltrate with nuclear debris (“nuclear dust”), without any demonstration of vasculitis. Immunohistochemistry showed a prevalence of neutrophils (MPO+) and histiocytes‐macrophages (CD68+) with rare T (CD3+) and B (CD20+) cells without plasma cells (Figure 2).
Figure 2.
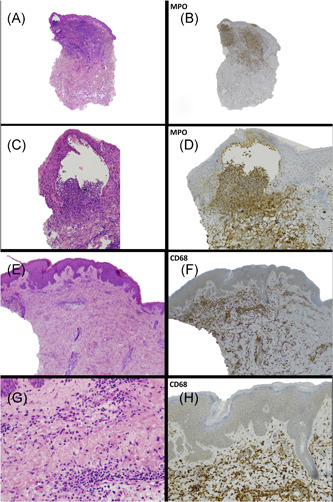
(A, C, E, and G) Different magnification of punch lesions with the demonstration of necrotizing neutrophilic infiltrate with nuclear debris (“nuclear dust”) without any demonstration of vasculitis. Immunohistochemistry: prevalence of neutrophils (MPO+) (B and D) and histiocytes‐macrophages (CD68+) (F and H)
The immunofluorescence was negative, so we classified the skin rash as a Sweet syndrome.4, 5
Four weeks after steroid discontinuation, the patient underwent an electromyographic study due to the persistence of foot neuropathic pain, muscle weakness, and fatigue.
Myositis in the tibialis anterior muscle was found without evidence of polyneuropathy.
We repeated auto‐antibodies: ANA was positive (1:160 Ac1, Ac 4.5) with borderline positivity of anti‐Pm/scl‐75 antibodies with aldolase raise (10,3 U/L NV < 7.6).
We repeated electromyography with the demonstration of moderate severity subacute myositis without muscle damage and polyneuropathy.
We made a diagnosis of polymyositis secondary to an autoimmune reaction following vaccination.
At present time, the patient is scheduled to be treated with IVIg (2 g/kg).
To our knowledge, this is the first reported case of adverse reaction to the AstraZeneca COVID vaccine that had studied in detail the skin and systemic autoimmune reaction.
Development of autoimmune reaction following SARS‐CoV‐2 infection has been described extensively6, 7; however, evidence of autoimmunity following vaccination seems to lack at present. Our case suggests that in predisposed subjects' vaccination could trigger an autoimmune reaction as the natural infection. Larger and accurate immunological studies are needed to elucidate this interrogative that is of fundamental relevance due to the future mass vaccination.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Drafting of the work, biopsy sampling, and patient management: Capassoni Marco. Histological examination and critical review: Ketabchi Sheyda and Cassisa Angelo. Electrophysiological study and critical review: Caramelli Riccardo. Critical review: Molinu A. Anna. Critical review, biopsy sampling, and patient management: Galluccio Felice. Coordinator and critical review: Guiducci Serena.
REFERENCES
- 1.Akinosoglou K, Tzivaki I, Marangos M. Covid‐19 vaccine and autoimmunity: Awakening the sleeping dragon. Clin Immunol. 2021;226:108721. 10.1016/j.clim.2021.108721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talotta R. Do COVID‐19 RNA‐based vaccines put at risk of immune‐mediated diseases? In reply to "potential antigenic cross‐reactivity between SARS‐CoV‐2 and human tissue with a possible link to an increase in autoimmune diseases". Clin Immunol. 2021;224:108665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumenthal KG, Freeman EE, Saff RR, et al. Delayed large local reactions to mRNA‐1273 vaccine against SARS‐CoV‐2. N Engl J Med. 2021;384(13):1273‐1277. 10.1056/NEJMc2102131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von den Driesch P. Sweet's syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol. 1994;31(4):535‐556. 10.1016/s0190-9622(94)70215-2 [DOI] [PubMed] [Google Scholar]
- 5.Su WP, Liu HN. Diagnostic criteria for Sweet's syndrome. Cutis. 1986;37(3):167‐174. [PubMed] [Google Scholar]
- 6.Caso F, Costa L, Ruscitti P, et al. Could Sars‐coronavirus‐2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev. 2020;19(5):102524. 10.1016/j.autrev.2020.102524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodríguez Y, Novelli L, Rojas M, et al. Autoinflammatory and autoimmune conditions at the crossroad of COVID‐19. J Autoimmun. 2020;114:102506. 10.1016/j.jaut.2020.102506 [DOI] [PMC free article] [PubMed] [Google Scholar]


