Abstract
Since the coronavirus disease 2019 (COVID‐19) outbreak, laboratory diagnosis has mainly been conducted using reverse‐transcription polymerase chain reaction (RT‐PCR). Detecting the presence of an infectious virus in the collected sample is essential to analyze if a person can transmit infectious severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). However, there have been no quantitative investigations conducted for infectious SARS‐CoV‐2 in clinical samples. Therefore, in the present study, a rapid and simple focus‐forming assay using the peroxidase‐antiperoxidase technique was developed to quantify infectious SARS‐CoV‐2 titers in 119 samples (n = 52, nasopharyngeal swabs [NPS]; n = 67, saliva) from patients with COVID‐19. Furthermore, the study findings were compared with the cycle threshold (Ct) values of real‐time RT‐PCR. The infectious virus titers in NPS samples and Ct values were inversely correlated, and no infectious virus could be detected when the Ct value exceeded 30. In contrast, a low correlation was observed between the infectious virus titers in saliva and Ct values (r = ‐0.261, p = 0.027). Furthermore, the infectious virus titers in the saliva were significantly lower than those in the NPS samples. Ten days after the onset of COVID‐19 symptoms, the infectious virus was undetectable, and Ct values were more than 30 in NSP and saliva samples. The results indicate that patients whose symptoms subsided 10 days after onset, with Ct values more than 30 in NSP and saliva samples, were less likely to infect others.
Keywords: COVID‐19, Ct value, infectious virus titer, nasopharyngeal swab, saliva, SARS‐CoV‐2
Highlights
A rapid and simple focus‐forming assay using the peroxidase‐antiperoxidase technique was developed to quantify infectious SARS‐CoV‐2 titers.
Infectious virus titers in NPS samples and Ct values were inversely correlated, and no infectious virus could be detected when the Ct value exceeded 30. In contract, a low correlation was observed between the infectious virus titers in saliva and Ct values.
Infectious virus titers in the saliva were significantly lower than that in the NPS samples.
Patients whose symptoms subsided 10 days after onset, with Ct values more than 30 in NSP and saliva samples, were less likely to infect others.
1. INTRODUCTION
The first case of coronavirus disease 2019 (COVID‐19) in Japan was reported in mid‐January 2020. The patient was a Japanese citizen who had returned to Japan from Wuhan City, China, on January 3, 2020. Subsequently, sporadic outbreaks in Japan were reported. The number of cases sharply increased in April, subsequently marking the first wave of the pandemic in mid‐April, 2020. The number of cases decreased substantially in June; however, a rise in cases was reported in July, 2020. By the end of 2020, the number of cases exceeded than that of initially reported during the first wave in 2020.
COVID‐19 is classified as The Designated Infectious Disease on February 1, 2020 under the Japanese Infectious Disease Control Law. Hence, it is now possible to implement hospitalization measures and employment restrictions to the patients. Individuals with confirmed COVID‐19 infection would be hospitalized regardless of their symptoms. Furthermore, discharge from the hospital requires confirmatory negative polymerase chain reaction (PCR) tests from at least two consecutive samples collected more than or equal to 24 h apart.1 However, many infected people test positive by repeated PCR tests, even if they are asymptomatic. These factors lead to hospital beds being occupied by asymptomatic patients as well as those with mild symptoms, thereby hindering the hospitalization of critically ill patients. Moreover, testing numerous negative samples during recovery phase imposes a heavy burden on laboratories that conduct PCR tests in terms of time and labor. In consideration of this situation, the Japanese government reviewed the discharge criteria in mid‐June 2020 and revised that it is not always necessary to have a negative PCR test.
Currently, the standard method for laboratory testing of COVID‐19 is quantitative real‐time reverse‐transcription polymerase chain reaction (RT‐PCR) that determines the cycle threshold (Ct) values of viral RNA as an index.2, 3, 4 High Ct values may indicate low infectious virus titers; however, there is little evidence to support this possibility. Moreover, it is important to confirm the presence of infectious virus in the clinical samples to determine whether a patient infected with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), belonging to the family Coronaviridae, genus Betacoronavirus, can infect others.5, 6, 7, 8, 9, 10, 11, 12 However, cell culture assays that detect infectious viruses require specialized skills and experience, must be safely performed, and are rarely conducted for SARS‐CoV‐2. A few studies have reported the detection of cytopathic effects (CPEs) of infectious viruses,13, 14, 15 whereas others used the focus‐forming assay.16 However, at present, there have been no quantitative investigations performed on infectiousness of SARS‐CoV‐2 in clinical samples. Therefore, in this study, a focus‐forming assay using the peroxidase‐antiperoxidase (PAP) staining technique, typically applied to numerous types of viruses, was developed.17, 18, 19, 20, 21
Here, infectious SARS‐CoV‐2 titers in patients with COVID‐19 and their corresponding Ct values by RT‐PCR were measured. The findings of the present study will significantly contribute toward the control of COVID‐19 transmission and consequently, reduce the number of patients requiring long‐term hospitalization.
2. MATERIALS AND METHODS
2.1. Ethics statement
The Ethics Review Board of Osaka Institute of Public Health (OIPH) (approval number: 2006‐01) and Institutional Review Board of the Research Institute for Microbial Diseases, Osaka University (approval number: 2020‐6‐1) approved this study. Informed consent was obtained in the form of opt‐out on the website.
2.2. Samples
Samples were handled in a biosafety level (BSL)−3 facility at the Research Institute for Microbial Diseases of Osaka University, with the approval of the Safety Management Committee on Pathogens of Osaka University. From mid‐July to mid‐December 2020, 119 samples (n = 52, nasopharyngeal swabs [NPSs]; n = 67, saliva) were collected from patients with COVID‐19 in Osaka Prefecture. The patients included 62 women and 57 men, in the age range of 1–99 years. The samples were submitted to OIPH from hospitals through public health centers and tested by RT‐PCR within 24 h. The remaining PCR‐positive samples were stored at 4°C, following which, they were used to measure the infectious virus titer in the sample. The testing was conducted at the Department of Viral Infection, Research Institute for Microbial Diseases of Osaka University within 4 days of the initial RT‐PCR assay.
2.3. Real‐time RT‐PCR
The samples were centrifuged at 15,000 rpm for 5 min. Viral RNA was extracted from 200 µl of the supernatant using the MagDEA Dx SV Kit (Precision System Science) or from 140 µl of the supernatant using the QIAamp Viral RNA Mini Kit (QIAGEN) according to the manufacturer's instructions. Real‐time quantitative reverse transcription PCR (RT‐qPCR) assays targeting the viral N gene using the QuantiTect Probe RT‐PCR Kit (QIAGEN) and N2 sets of primers and probes were designed according to the method published by the National Institute of Infectious Diseases, Japan (https://www.niid.go.jp/niid/en/examination.html). The components of the RT‐qPCR reaction mixture were as follows: 10.0 µl of 2× Master mix, 1.0 µl of forward primer (10 µM), 1.4 µl of reverse primer (10 µM), 0.8 µl of probe (5 µM), 0.2 µl of Quantitect RT mix, 1.6 µl of deionized water, and 5 µl of template RNA. Reverse transcription was performed at 50°C for 30 min, followed by denaturation at 95°C for 15 min, and 45 amplification cycles at 95°C for 15 s and 60°C for 60 s. A Step‐One Plus Real‐Time PCR System and a Quant Studio 5 Real‐Time PCR System (Life Technologies) were used for the analysis.
2.4. Focus‐forming assay for viral infectivity
Vero‐E6/TMPRSS2 (JCRB 1819) cells were seeded in 24‐well plates (2 × 105 cells/well) and incubated in an atmosphere containing 5% CO2 at 37°C. On the following day, the medium in each well‐containing cell monolayers was removed. The cell monolayers were then inoculated with 100 µl of serially diluted (10‐fold) clinical samples under BSL‐3 containment. After 30 min of absorption, the diluted samples were aspirated and washed three times with serum‐free minimum essential medium (MEM). The cells were covered with 0.5 ml of Dulbecco's Modified Eagle Medium (DMEM) containing 1% carboxymethyl cellulose, 2% fetal bovine serum, and antibiotics (200 units/ml penicillin, 200 µg/ml streptomycin, and 0.25 µg/ml amphotericin B). Eighteen hours after inoculation, 20% formalin (0.5 ml) (10% formalin) was added to the cells, and they were incubated for 30 min at room temperature. The formalin‐containing medium was removed, and the cells were washed three times with phosphate‐buffered saline (PBS). The cells were then fixed in absolute ethanol for 5 min at room temperature under BSL‐2 containment. Four immunological reactions using the PAP staining technique were subsequently performed for 40 min at room temperature. Each well contained 400 µl of each antibody, including anti‐SARS/SARS‐2 NP mouse monoclonal antibody (EastCoast Bio) diluted to 1 µg/ml, antimouse rabbit IgG antibody (MP Biomedicals) diluted at 1:1000, antirabbit goat IgG antibody (MP Biomedicals) diluted at 1:500, and rabbit PAP (Jackson Immuno Research) diluted at 1:200. After each reaction, the cells were washed three times with PBS. In the final step of the PAP staining technique, the peroxidase reaction was developed for approximately 5 min according to a previously published method22 using 0.01% H2O2 and 0.3 mg/ml of 3ʹ‐3ʹ‐diaminobenzidine tetrahydrochloride in PBS. The wells were then washed with tap water and dried, and the stained foci were macroscopically counted. Virus infectivity is expressed as focus forming units (FFUs).
2.5. Virus isolation
VeroE6/TMPRSS2 cells were seeded in 24‐well plates (2 × 105 cells/well) one day before the experiment. The medium was removed from the plates and 100 µl/well of clinical samples were added. After 30 min, the samples were aspirated, and the cells were washed three times with serum‐free MEM. Cells were then cultured in 1 ml of DMEM containing 2% fetal bovine serum, 200 units/ml of penicillin, 200 µg/ml streptomycin, and 0.25 µg/ml amphotericin B in an atmosphere containing 5% CO2 at 37°C for up to 5 days. CPEs were macroscopically observed each day. The culture supernatant was collected when the appearance of CPEs was detected. Virus isolation is a qualitative test method that can detect even a small amount of virus, so it was considered to complement the focus counting method.
3. RESULTS
3.1. Focus‐forming assay for SARS‐CoV‐2 infectivity
Figure 1 shows a representative 24‐well plate with foci of SARS‐CoV‐2 in samples obtained from patients with COVID‐19, which were stained using the PAP technique. The number of foci was inversely proportional to sample dilution (Figure 1B). Higher magnification of the foci revealed aggregates of clear cells (Figure 1C).
Figure 1.
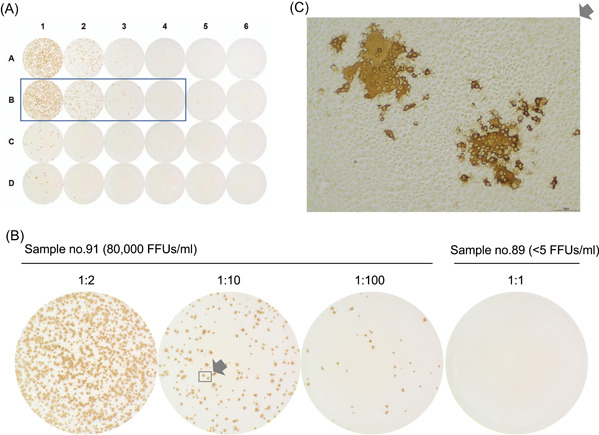
Focus‐forming infectivity assay of samples from patients with coronavirus disease 2019 using the peroxidase‐antiperoxidase (PAP) staining technique. (A) and (B) Representative images of a 24‐well plate (A), and a serially diluted (10‐fold) sample (no. 91) and a control sample (no. 81) (B). (C) Higher magnification of sample no. 91 at 1:10 dilution
3.2. Comparison of SARS‐CoV‐2 Ct values and infectious virus titers
The infectious virus titers of RT‐PCR‐positive samples were measured and correlated with the Ct values by Pearson's product moment correlation coefficient (Figure 2). The infectious virus titers in the NPS samples were inversely correlated with the Ct values (r = −0.506, p = 0.001) and difficult to measure when Ct values were more than 30 (Figure 2). In contrast, there was a low correlation between the infectious virus titers in saliva and the Ct values (r = −0.261, p = 0.027). The infectious virus was not detected in most of the samples with Ct values more than 30. However, despite the high Ct value (39.4) for a single saliva sample, it showed a high infectious virus titer (800 FFUs/mL). The infectious virus was undetectable in the RT‐PCR‐negative samples of both NSP and saliva. The samples were then grouped according to the Ct values, and the geometric mean titers for each group were calculated (Table 1). The results confirmed the results of Fig. 2 more clearly in that samples with Ct value <20 showed high infectious virus titer (18,686 FFUs/mL) and 100% virus isolation rate in NPS, and those with Ct values >25 showed low infectious virus titers and virus isolation rates. The infectious virus titers of NPS samples were uniformly higher than those of saliva samples. The results of virus isolation were consistent with those of the infectivity assay, which was performed in parallel (Table 1).
Figure 2.
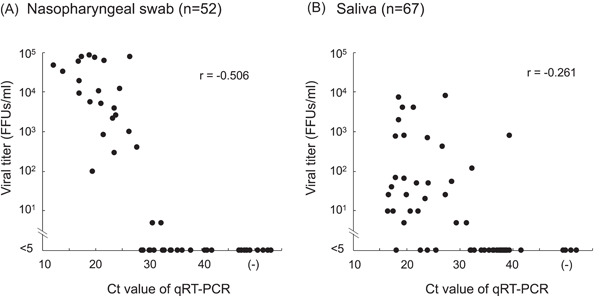
(A) and (B) Correlation between cycle threshold (Ct) values and infectious virus titers in nasopharyngeal swab (NPS) (A) and saliva (B) samples
Table 1.
Geometric mean virus titers and virus isolation rates in NSP and saliva grouped by Ct values
| Ct value of qPCR | Nasopharyngeal swab | Saliva | ||
|---|---|---|---|---|
| Geometric mean of viral titer (FFU/ml) | Virus isolation rates (%) | Geometric mean of viral titer (FFUs/ml) | Virus isolation rates (%) | |
| <20 | 18,686 (n = 10) | 100.0 (10/10) | 156 (n = 13) | 84.6 (11/13) |
| 20–25 | 3983 (n = 9) | 100.0 (9/9) | 29 (n = 10) | 90.0 (9/10) |
| 25–30 | 126 (n = 5) | 60.0 (3/5) | 54 (n = 6) | 83.3 (5/6) |
| 30–35 | <5 (n = 11) | 16.7 (2/12) | <5 (n = 7) | 14.3 (1/7) |
| 35–40 | <5 (n = 5) | 0.0 (0/4) | <5 (n = 23) | 8.7 (2/23) |
| >40 | <5 (n = 12) | 0.0 (0/12) | <5 (n = 8) | 0.0 (0/6) |
3.3. Ct values and infectious virus titers on the day of sample collection after the onset of COVID‐19 symptoms
For some samples with known dates of COVID‐19 symptom onset, the Ct values and infectious virus titers were investigated depending on the difference in the number of days between the date of onset and that of sample collection (Figure 3). Samples that were taken a few days after onset had low Ct values and high infectious titers. However, in samples collected 10 days post the onset, Ct values were high with >30 and the infectious virus was undetectable in both NPS and saliva samples.
Figure 3.
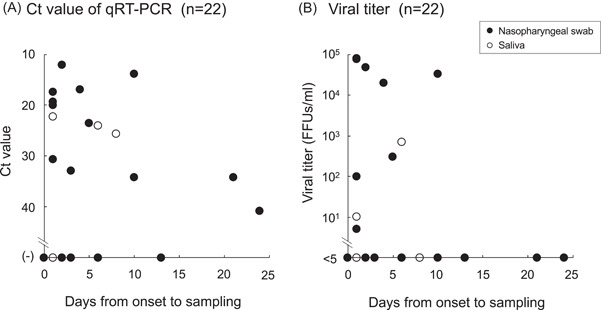
(A) and (B) Cycle threshold (Ct) values (A) and infectious virus titers (B) in nasopharyngeal swab (NPS) (closed circle) and saliva (open circle) samples on the day of sample collection after the onset of COVID‐19 symptoms. COVID‐19, coronavirus disease 2019; qRT‐PCR, quantitative reverse‐transcription polymerase chain reaction
4. DISCUSSION
OIPH initiated tests for suspected COVID‐19 infections in late January 2020. Samples were sent from hospitals and tested within 24 h using real‐time RT‐PCR. A Ct cutoff value of 40 was used to differentiate between infected and uninfected patients. Most of the initial samples were NPSs and sputum; however, testing for saliva samples started in late July, which in line with the notification from the Japanese government on June 2, 2020, that enables PCR tests using saliva for persons within 9 days of onset. Currently, the majority of samples comprise NPSs and saliva.
Although the viral RNA load is slightly higher in the sputum than in NPS samples,23, 24, 25, 26 NPSs are optimal for detecting viral shedding from the upper respiratory tract, which contributes to the transmission of infection.24, 25, 26, 27 It has been demonstrated that saliva contains a large copy number of viral genes and the burden of saliva collection is small. Consequently, medical personnel is at low risk of exposure to the virus.4, 28, 29, 30, 31, 32, 33 In the present study, Ct values and infectious virus titers were determined using NPS and saliva samples.
Detection of infectious SARS‐CoV‐2 has been mainly performed using viruses propagated in cultured cell lines.5, 10, 12, 13, 14, 15, 16, 29, 34 Although virus culture is a qualitative method, it has been applied to investigate the presence of infectious viruses over time.5, 8, 34 Examples of techniques to measure infectious viruses include plaque and focus‐forming assays to quantitatively employ a stock preparation of SARS‐CoV‐2.16 A study found a significant correlation between infectious virus titers obtained using plaque and focus‐forming assays.34 However, there exist no reports detailing quantitative infectious virus measurements using clinical COVID‐19 samples. Hence, in this study, a microplate immunoassay was developed, wherein the virus infection produced a clear, easily detectable focus.
The Ct values and infectious virus titers of NPS and saliva samples were measured, and the significance of the correlations was investigated (Figure 2). In the same Ct value groups, we found that infectious virus titers in the saliva were significantly lower than those in NPS samples (Table 1). Many studies report that the sensitivity of saliva for the diagnosis of SARS‐CoV‐2 infection is lesser than that of NPS samples.32, 33, 35 For example, RT‐PCR analyses of NPS and saliva samples from the same infected person revealed that if the Ct value for the NPS sample is less than or equal to 26, the saliva sample is 100% positive by RT‐PCR.27 However, if the Ct values for the NPS samples range from 26 to 33 or are more than 33, then the positivity rate of saliva samples is 48% or 14.6%, respectively.27 Another study using NPS and saliva samples collected from 622 patients who visited a COVID‐19 screening clinic found that the median Ct values for NPS samples are significantly lower than those for saliva samples, suggestive of a higher viral load in NPS samples.32
The present study demonstrated that when the Ct value was more than 30, the infectious virus was difficult to detect in NPS or saliva samples (Figure 2), which is consistent with the results of previous studies.5, 11, 12, 13, 32 One of the studies in which analysis of virus cultures prepared from 183 RT‐PCR‐positive specimens (n = 9, sputum; n = 174, NPS) found a significant correlation between the Ct value and the rate of positive cultures. Furthermore, all samples (100%) with Ct values ranging from 13 to 17 have been shown to yield the infectious virus.5 The positive culture rate, which has been found to decrease as the Ct value increases, is 12.5% at Ct = 33% and 0% when Ct was more than or equal to 34. An analysis of virus cultures of 47 specimens obtained from RT‐PCR‐positive residents of a nursing facility showed that when the Ct value is more than or equal to 30, the viral culture is mainly unsuccessful and the maximum Ct value of virus‐positive cultures is 34.3.36 However, during interpretation of such data, one must consider that virus titers vary with time and varied sampling methods and multiple tests may be required to accurately evaluate such patients.
The date of disease onset in some patients was known, and the Ct values and virus titers on the date of sample collection after disease onset were analyzed (Figure 3). The findings were consistent with those of previous studies, which indicated that the amount of infectious virus peaks immediately after the onset of symptoms and decreases subsequently.10, 13, 37 Further, the infectious virus is undetectable after 10 days of disease onset, and the Ct values of RT‐PCR samples after 10 days are more than or equal to 30.10, 13, 37 Therefore, it is unlikely that transmissible infectious virus is present in patients 10 days after the onset of symptoms.
In Japan, individuals infected with SARS‐CoV‐2 with a positive PCR test are eligible for admission to a designated medical institution, and this standard is strictly enforced when the number of patients is small. However, with an increase in the number of patients, hospital admission may not be possible, which significantly increases the number of patients who must wait elsewhere before they can be treated. The present study demonstrated that in patients with positive PCR test results, who were asymptomatic or mildly infected, the infectious virus produced was with very low titers when the Ct value was more than or equal to 30, which indicated that others were less likely to be infected. These findings will significantly aid efforts to mitigate the severe stress on the healthcare system in Japan, as well as in other countries, should the COVID‐19 pandemic continue at the present alarming pace.
CONFLICT OF INTERESTS
All authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Conception and design: Yoshinobu Okuno and T. Shioda, Investigation: Satoshi Hiroi, Ritsuko Kubota‐Koketsu, T. Sasaki, and Saeko Morikawa, Data analysis: Satoshi Hiroi, Ritsuko Kubota‐Koketsu, Kazushi Motomura, and Emi E. Nakayama, Writing‐Original draft preparation: Satoshi Hiroi, Ritsuko Kubota‐Koketsu, and Yoshinobu Okuno, Writing‐Review and editing: Satoshi Hiroi, Ritsuko Kubota‐Koketsu, T. Sasaki, Saeko Morikawa, Kazushi Motomura, Emi E. Nakayama, Yoshinobu Okuno, and T. Shioda. Satoshi Hiroi and Ritsuko Kubota‐Koketsu contributed equally to this study.
ACKNOWLEDGMENTS
We gratefully acknowledge the staff members of the Division of Microbiology, OIPH, for performing RT‐PCR on clinical samples from patients with COVID‐19. This study was supported by the MHLW Health Labor Sciences Research Grant Number 20CA2058 and the Project Promoting Support for Drug Discovery Grant (JP20nk0101602) from the Japan Agency for Medical Research and Development. We would like to thank Editage (www.editage.com) for English language editing.
Hiroi S, Kubota‐Koketsu R, Sasaki T, et al. Infectivity assay for detection of SARS‐CoV‐2 in samples from patients with COVID‐19. J Med Virol. 2021;93:5917‐5923. 10.1002/jmv.27145
REFERENCES
- 1.CDC . Discontinuation of transmission‐based precaution and disposition of patients with SARS‐CoV‐2 infection in healthcare settings (interim guidance). 2020.
- 2.Chu DKW, Pan Y, Cheng SMS, et al. Molecular diagnosis of a novel coronavirus (2019‐nCoV) causing an outbreak of pneumonia. Clin Chem. 2020;66(4):549‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveill. 2020;25(3):2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loeffelholz MJ, Tang YW. Laboratory diagnosis of emerging human coronavirus infections: the state of the art. Emerg Microbes Infect. 2020;9(1):747‐756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.La Scola B, Le Bideau M, Andreani J, et al. Viral RNA load as determined by cell culture as a management tool for discharge of SARS‐CoV‐2 patients from infectious disease wards. Eur J Clin Microbiol Infect Dis. 2020;39(6):1059‐1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romero‐Gómez MP, Gómez‐Sebastian S, Cendejas‐Bueno E, et al. Ct value is not enough to discriminate patients harbouring infective virus. J Infect. 2021;82(3):e35‐e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krupp K, Madhivanan P, Perez‐Velez CM. Should qualitative RT‐PCR be used to determine release from isolation of COVID‐19 patients? J Infect. 2020;81(3):452‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li N, Wang X, Lv T. Prolonged SARS‐CoV‐2 RNA shedding: not a rare phenomenon. J Med Virol. 2020;92(11):2286‐2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Widders A, Broom A, Broom J. SARS‐CoV‐2: the viral shedding vs infectivity dilemma. Infect Dis Health. 2020;25(3):210‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID‐2019. Nature. 2020;581(7809):465‐469. [DOI] [PubMed] [Google Scholar]
- 11.Basile K, McPhie K, Carter I, et al. Cell‐based culture of SARS‐CoV‐2 informs infectivity and safe de‐isolation assessments during COVID‐19. Clin Infect Dis. 2020:ciaa1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gniazdowski V, Morris CP, Wohl S, et al. Repeat COVID‐19 molecular testing: correlation of SARS‐CoV‐2 culture with molecular assays and cycle thresholds. Clin Infect Dis. 2020:ciaa1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singanayagam A, Patel M, Charlett A, et al. Duration of infectiousness and correlation with RT‐PCR cycle threshold values in cases of COVID‐19, England, January to May 2020. Euro Surveill. 2020;25(32):2001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bullard J, Dust K, Funk D, et al. Predicting infectious severe acute respiratory syndrome coronavirus 2 from diagnostic samples. Clin Infect Dis. 2020;71(10):2663‐2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang CG, Lee KM, Hsiao MJ, et al. Culture‐based virus isolation to evaluate potential infectivity of clinical specimens tested for COVID‐19. J Clin Microbiol. 2020;58(8):e01068‐e01020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Case JB, Bailey AL, Kim AS, Chen RE, Diamond MS. Growth, detection, quantification, and inactivation of SARS‐CoV‐2. Virology. 2020;548:39‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okuno Y, Fukunaga T, Srisupaluck S, Fukai K. A modified PAP (peroxidase‐anti‐peroxidase) staining technique using sera from patients with dengue hemorrhagic fever (DHF): 4 step PAP staining technique. Biken J. 1979;22(4):131‐135. [PubMed] [Google Scholar]
- 18.Tanishita O, Takahashi Y, Okuno Y, Yamanishi K, Takahashi M. Evaluation of focus reduction neutralization test with peroxidase‐antiperoxidase staining technique for hemorrhagic fever with renal syndrome virus. J Clin Microbiol. 1984;20(6):1213‐1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okuno Y, Yamanishi K, Lwin S, Takahashi M. Micro‐neutralization test for mumps virus using the 96‐well tissue culture plate and PAP (peroxidase‐antiperoxidase) staining technique. Microbiol Immunol. 1985;29(4):327‐335. [DOI] [PubMed] [Google Scholar]
- 20.Okuno Y, Fukunaga T, Tadano M, Okamoto Y, Ohnishi T, Takagi M. Rapid focus reduction neutralization test of Japanese encephalitis virus in microtiter system: brief report. Arch Virol. 1985;86(1‐2):129‐135. [DOI] [PubMed] [Google Scholar]
- 21.Okuno Y, Tanaka K, Baba K, Maeda A, Kunita N, Ueda S. Rapid focus reduction neutralization test of influenza A and B viruses in microtiter system. J Clin Microbiol. 1990;28(6):1308‐1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.GrahamRC, Jr., Karnovsky MJ. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966;14(4):291‐302. [DOI] [PubMed] [Google Scholar]
- 23.Bwire GM, Majigo MV, Njiro BJ, Mawazo A. Detection profile of SARS‐CoV‐2 using RT‐PCR in different types of clinical specimens: a systematic review and meta‐analysis. J Med Virol. 2021;93(2):719‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan Y, Zhang D, Yang P, Poon LLM, Wang Q. Viral load of SARS‐CoV‐2 in clinical samples. Lancet Infect Dis. 2020;20(4):411‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W, Xu Y, Gao R, et al. Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA. 2020;323(18):1843‐1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torres M, Collins K, Corbit M, et al. Comparison of saliva and nasopharyngeal swab SARS‐CoV‐2 RT‐qPCR testing in a community setting. J Infect. 2020;82:84‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azzi L, Carcano G, Gianfagna F, et al. Saliva is a reliable tool to detect SARS‐CoV‐2. J Infect. 2020;81(1):e45‐e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.To KK, Tsang OT, Yip CC, et al. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020;71(15):841‐843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Comber L, Walsh KA, Jordan K, et al. Alternative clinical specimens for the detection of SARS‐CoV‐2: a rapid review. Rev Med Virol. 2020. [DOI] [PubMed] [Google Scholar]
- 31.Iwasaki S, Fujisawa S, Nakakubo S, et al. Comparison of SARS‐CoV‐2 detection in nasopharyngeal swab and saliva. J Infect. 2020;81(2):e145‐e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams E, Bond K, Zhang B, Putland M, Williamson DA. Saliva as a noninvasive specimen for detection of SARS‐CoV‐2. J Clin Microbiol. 2020;58(8):e00776‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yee R, Truong TT, Pannaraj PS, et al. Saliva is a promising alternative specimen for the detection of SARS‐CoV‐2 in children and adults. J Clin Microbiol. 2021;59(2):e02686‐02620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jureka AS, Silvas JA, Basler CF. Propagation, inactivation, and safety testing of SARS‐CoV‐2. Viruses. 2020;12(6):622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landry ML, Criscuolo J, Peaper DR. Challenges in use of saliva for detection of SARS CoV‐2 RNA in symptomatic outpatients. J Clin Virol. 2020;130:104567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS‐CoV‐2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382(22):2081‐2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walsh KA, Jordan K, Clyne B, et al. SARS‐CoV‐2 detection, viral load and infectivity over the course of an infection. J Infect. 2020;81(3):357‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]


