Abstract
The COVID‐19 pandemic, which has ravaged our world for more than a year, still shapes our agenda with a scale of intensity that fluctuates over time. In our study, we aimed to determine the correlation between serum migration inhibitory factor (MIF) level and disease severity in COVID‐19 with different prognoses. Between 15 October 2020 and 20 January 2021, 110 patients over the age of 18 who were diagnosed with COVID‐19 and 40 volunteer healthcare personnel were included in our study. MIF levels were measured by enzyme‐linked immunosorbent assay. In the comparison of serum MIF values in the patient and control group, it was observed that the MIF level was significantly higher in patients with both moderate and severe COVID‐19 levels compared to the control group (p = 0.001, 0.001). In the comparison of serum MIF values of moderate to severe COVID‐19 patients, it was observed that MIF level was higher in severe patients (p = 0.001). In the receiver operating characteristic curve analysis performed to differentiate between severe and moderate COVID‐19 patients with MIF levels, the area under the curve was observed as 0.78. When the cutoff value of the MIF level was taken as 4.455 ng/ml, the sensitivity was 83% and the specificity was 62%. Failure to adequately balance the pro‐inflammatory cytokines synthesized in COVID‐19 with anti‐inflammatory effect is the most important reason for the aggravation of the disease course. Playing a role in pro‐inflammatory cytokine synthesis, MIF can provide important information about the disease prognosis in the early period.
Keywords: COVID‐19, macrophage activation syndrome, macrophage migration inhibitory factor
Highlights
Failure to adequately balance the proinflammatory cytokines synthesized in COVID‐19 with anti‐inflammatory effect is the most important reason for the aggravation of the disease course. Playing a role in proinflammatory cytokine synthesis, MIF can provide important information about the disease prognosis in the early period.
1. INTRODUCTION
Coronaviruses are enveloped single‐stranded RNA viruses. Although coronavirus infections usually cause mild respiratory illness, in recent years coronaviruses have caused epidemics that threaten humanity. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is a novel coronavirus isolated from the respiratory epithelium of patients with pneumonia in Wuhan, China, in December 2019. CoV‐2, named SARS‐Coronavirus disease 2019 (COVID‐19) as of September 17, 2020, reached pandemic rates, affected more than 100 million people, and caused more than 4 million deaths worldwide. 1
COVID‐19 related macrophage activation syndrome (MAS) is an immune system condition in which excessive cytokines are produced as a result of excessive activation of immune system cells and causes systemic hyperinflammation in its later stages. 2 , 3 It usually leads to multiple organ failure and a high mortality rate. MAS is characterized by increased expression of pro‐inflammatory cytokines. Without any therapeutic intervention, it can cause strong inflammation, severe tissue damage, and even death of the patient. Many studies have found that cytokines, such as tumor necrosis factor‐α (TNF‐α), interleukin‐6 (IL‐6), and IL‐1, play an important role in MAS. 2 , 4 , 5
Macrophage migration inhibitory factor (MIF) is a pleiotropic pro‐inflammatory cytokine first isolated from T lymphocytes and inhibits the random migration of macrophages. 6 , 7 Synthesis of MIF, whose synthesis decreases to a minimal level under low inflammatory activity, increases in its synthesis with high inflammatory activity, and besides its inflammatory activity, it carries out the apoptosis of the cells that play a role in inflammatory activity by inhibiting p53. MIF is recognized as a multifunctional molecule that activates the production of inflammatory cytokines such as tumor necrosis factor‐α (TNF‐α), interleukin‐1β (IL‐1β), interleukin‐6 (IL‐6), and interferon (IFN). 8 MIF, which is constitutively expressed from various cells, is found in almost every tissue. 6 The fact that a strong relationship has been found between the increasing level of MIF level in sepsis and autoimmune diseases and the clinical course and prognosis in the studies conducted has been a hope that it can be used for therapeutic purposes in the future.
In our study, we aimed to compare the serum MIF level in patients with MAS, which is one of the most important causes of morbidity and mortality in COVID‐19 patients and to determine the relationship with the clinical course.
2. MATERIALS AND METHODS
2.1. Study design
Between 15 October 2020 and 20 January 2021, 110 patients over the age of 18 were diagnosed with COVID‐19 by the real‐time polymerase chain reaction (PCR) method, and 40 volunteer healthcare personnel over the age of 18 who were asymptomatic and who were PCR negative after nasopharyngeal swab were included in our study.
High‐resolution computed tomography (HRCT) was performed in a standardized manner in patients at high risk for COVID‐19. According to the HRCT results, patients with bilateral ground‐glass opacity, subsegmental consolidation or linear opacities, paving stone appearance, and inverted halo sign with peripheral localization were evaluated as typical findings, while patients with radiologically atypical findings were admitted as patients with compatible clinical complaints. After the patients were admitted to the clinic, their hematological parameters, biochemical parameters including liver and kidney function tests, coagulation parameters, ferritin, d‐Dimer, troponin‐I, CRP, and arterial blood gas parameters were measured. The current parameters of the patients were repeated daily.
2.2. Study group
The 150 people included in our study were divided into three groups. Group 1; Asymptomatic volunteer healthy workers (n: 40) who were negative as a result of the PCR performed for COVID‐19, Group 2; Moderately ill Patients with clinical signs of pneumonia with no signs of severe pneumonia: Pneumonia fitting any one of the following conditions: respiratory rate ≥ 30 breaths/min; SpO2 92%; patients with lung infiltration rate > 50%; n: 65), Group 3: Severe Illness Patients hospitalized with severe pneumonia and who developed MAS during their follow‐up (n: 45).
2.3. Exclusion criteria
Exclusion criteria include the presence of chronic or clinically significant infectious or inflammatory conditions in the past month, asthma, chronic obstructive pulmonary disease (COPD), malignancy, invasive surgery in the past month, uncontrolled hypertension, patients with high fasting blood glucose, diabetes cerebrovascular disease, kidney disease, and coronary artery disease. Anamnesis and laboratory parameters obtained during hospitalization were used to evaluate the patients in terms of exclusion criteria. In terms of coronary artery disease, asthma, COPD, and diabetes, consultations were made by cardiology, chest diseases, and internal medicine clinics.
2.4. Definitions and treatment
The temperature measured axillary in patients and above 37.3°C was defined as fever. In patients with fever under treatment for COVID‐19, blood, urine, and sputum cultures for possible bacterial and fungal superinfections were taken and empirically given antibiotherapy was revised according to the culture results. Acute respiratory failure was diagnosed and graded according to the Berlin 2015 diagnostic criteria. 9 If the daily cardiac‐specific troponin level of the patients was observed above normal, they were evaluated in terms of newly developed cardiac pathologies by echocardiography. As coagulopathy, prothrombin time was 3 s above normal and at partial thromboplastin level 5 s above normal. Three strategies to treat the patients according to their severity were implemented according to the Turkish Ministry of Health was COVID‐19 adult diagnosis and treatment guidelines. Patients with signs such as refractory fever, CRP and ferritin levels that remained high or continued to rise, d‐dimer elevation, cytopenia manifesting as thrombocytopenia or lymphopenia, abnormal liver function tests, hypofibrinogenemia, or elevated triglyceride levels in spite of treatment were monitored for MAS. As it is important to have a difference in consecutive measurements rather than a threshold value for laboratory findings, we have determined the diagnosis of MAS according to the successive follow‐up of clinical and laboratory data of patients. If these parameters continued to deteriorate during follow‐up with no apparent secondary bacterial infection, we treated patients with methylprednolone in doses of 250 mg/day or more for 3 days, however, if the patient did not respond to treatment, tocilizumab at a dose of 8 mg/kg (maximum 400 mg/day) was administered for MAS unless contraindicated. Clinical and laboratory response was evaluated after 24 h. If an adequate response was not observed, treatment was repeated at the same dose.
2.5. Measurement of biochemical markers
After 15 min of semi‐supine rest, blood samples were obtained from an antecubital vein into tubes containing ethylenediaminetetraacetic acid (EDTA) to prevent coagulation. Troponin I concentrations were measured by chemiluminescent immunoassay using an Immulite 2500 (Siemens Medical Solutions). MIF levels were measured by enzyme‐linked immunosorbent assay (BT Laboratory Co. Human Elisa Kit, Catalog no: E0141Hu).
2.6. Statistical analysis
The data were analyzed using IBM SPSS Statistics for Windows version 20.0 (IBM Corp.). Pearson's chi‐square test and Mann–Whitney U test were used for intergroup comparisons of parametric data and nonnormally distributed numerical data, respectively. Independent‐samples t‐test was used to compare demographic data and laboratory parameters between the groups. Wilcoxon analysis was used for intragroup comparisons of laboratory values during follow‐up. Pearson correlation analysis was used to evaluate relationships between MIF levels and age, CRP, LDH, lymphocyte, and neutrophil/lymphocyte ratio (NLR). Receiver operating characteristic (ROC) curve analysis was used to evaluate sensitivity and specificity in patients with severe and moderate COVID‐19. A p value less than 0.05 was considered statistically significant.
3. RESULTS
The mean age of 150 patients included in our study was 55.8 ± 14.6 years. The mean age of the patients in the control group was 56.1 ± 18.4 years. No statistically significant difference was observed between the patient and control groups (p = 0.44). 66 of the patients were male and 44 were female. The mean age of male patients was 55.7 ± 15.4, while the mean age of female patients was 55.9 ± 13.6. It was observed that there was no significant difference in the statistical analysis performed between the mean age of the patients according to gender (p = 0.84).
The evaluation of laboratory parameters according to the disease severity of the COVID‐19 patients included in our study was performed in Table 1. It was observed that lymphocyte, NLR, LDH, prothrombin time, CRP, PaO2/FiO2, d‐Dimer, ferritin, and fibrinogen levels, whose importance was pointed out in previous studies, were higher in the severe patient group (p = 0.001, 0.001, 0.001, 0.05, 0.001, 0.001, 0.005, 0.001, 0.001, respectively). The comparison of MIF levels of the patients according to the severity of the disease is shown in Table 2. Accordingly, it was observed that the MIF level, which was observed to be higher in patients compared to the control group, was statistically significantly higher in severe patients compared to moderate patients (p = 0.001).
Table 1.
Comparison of laboratory parameters in the hospitalization of patients with moderate to severe COVID‐19
|
Moderate illness (n: 65) Mean ± SD |
Severe illness (n: 45) Mean ± SD |
p | |
|---|---|---|---|
| Age (year) | 56.3 ± 15.5 | 55.0 ± 13.5 | 0.63 |
| WBC (/µl) | 9473.2 ± 4153.9 | 13,853.3 ± 5644.8 | 0.001 |
| Lymphocytes (/µl) | 934.3 ± 492.7 | 521.2 ± 331.5 | 0.001 |
| Neutrophils (/µl) | 7756.2 ± 4012.8 | 9188.3 ± 4311.2 | 0.77 |
| NLR | 10.3 ± 7.9 | 27.5 ± 31.4 | 0.001 |
| AST (U/L) | 58.5 ± 40.9 | 60.2 ± 39.9 | 0.825 |
| ALT (U/L) | 71.7 ± 68.5 | 78.3 ± 75.3 | 0.638 |
| LDH (U/L) | 433.6 ± 136.9 | 626.6 ± 193.9 | 0.001 |
| GGT (U/L) | 74.1 ± 39.9 | 127.3 ± 244.5 | 0.087 |
| ALP (U/L) | 97.1 ± 50.1 | 101.2 ± 56.4 | 0.691 |
| Creatine (mg/dl) | 1.1 ± 0.9 | 0.9 ± 1 | 0.543 |
| Prothrombin time (s) | 12.3 ± 2.3 | 14.4 ± 5.4 | 0.05 |
| CRP (mg/dl) | 58.4 ± 65.8 | 168.8 ± 88.2 | 0.001 |
| Troponin‐I (ng/dl) | 26.9 ± 61.9 | 40.2 ± 46.3 | 0.22 |
| PaO2/FiO2 | 274.4 ± 72.2 | 174.8 ± 26.3 | 0.001 |
| d‐Dimer (ng/ml) | 1250.2 ± 1768.1 | 3188.8 ± 4999.3 | 0.005 |
| Ferritin (ng/ml) | 626.1 ± 379.9 | 1512.5 ± 307.1 | 0.001 |
| Fibrinogen (ng/ml) | 411.6 ± 141.7 | 511.1 ± 157.1 | 0.001 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma‐glutamyl transferase; LDH, lactate dehydrogenase; NLR, neutrophil/lymphocyte ratio; p, Comparison of parameters between groups; SD, standard deviation; WBC, white blood cells.
Table 2.
Comparison of hospitalization MIF levels of COVID‐19 patients among themselves and with the control group according to the severity of the disease
| Severtiy of the illness |
Control (mean ± SD) (n:40) |
p*/p** | ||
|---|---|---|---|---|
|
Modarete (mean ± SD) (n: 65) |
Severe (mean ± SD) (n: 45) |
|||
| MIF (ng/ml) | 5.32 ± 3.34 | 9.1 ± 3.94 | 2.12 ± 2.1 | 0.001/0,001 |
Abbreviations: MIF, macrophage migration inhibitor factor; p*, statistical evaluation of the patients among themselves; p**, statistical evaluation of patient groups with the control group; SD, standard deviation.
In the correlation of MIF level with age and laboratory data, a positive correlation was observed with white blood cell, NLR, LDH, and CRP (r = 0.26, p = 0.01, r = 0.268, p = 0.01, r = 0.224, p = 0.05, r = 0.235, p = 0.05; Figure 1), it was observed to be inversely correlated with age and lymphocyte count (r = −0.195, p = 0.05, r = −0.2, p = 0.05; Figure 1).
Figure 1.
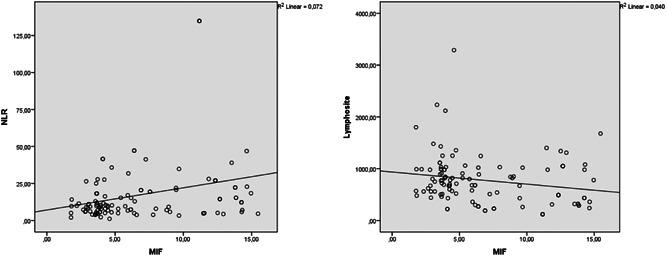
Correlation analysis of MIF level with NLR and lymphocyte count. MIF, migration inhibitory factor; NLR, neutrophil/lymphocyte ratio
In the ROC curve analysis performed to differentiate between severe and moderate COVID‐19 patients with MIF levels, the area under the curve was observed as 0.78. When the MIF level was taken as the cutoff value of 4.455 ng/ml, the sensitivity was 83% and the specificity was 62% (Figure 2).
Figure 2.
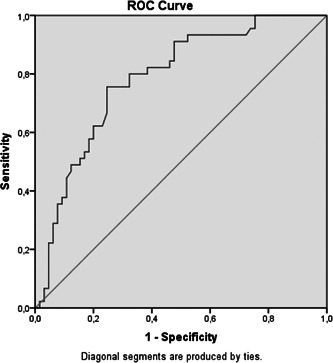
ROC curve analysis of MIF level in severe and moderate COVID‐19 patients. MIF, migration inhibitory factor; ROC, receiver operating characteristic
4. DISCUSSION
In line with the data of our study, it was observed that the levels of ferritin, LDH fibrinogen, CRP, and d‐Dimer, which was shown to have prognostic significance in COVID‐19, increased in correlation with the severity of the disease. In addition, it was observed that the MIF level, which forms the basis of our study, increased with the severity of the disease. It was observed that the MIF level was inversely correlated with age.
COVID‐19 mediated by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) emerged in late 2019 and the World Health Organization declared the SARS‐CoV‐2 epidemic as a pandemic due to serious public health threats. 10 , 11 Coronaviruses related to COVID‐19‐, SARS‐, and MERS are common in many genomic and structural features and are less pathogenic than SARS‐CoV‐2, SARS‐CoV, and MERS‐CoV. Cytokine storm has been found to play an important role in the pathogenesis of SARS‐CoV‐2, SARS‐CoV, and MERS‐CoV. Severe COVID‐19 is usually associated with hypercytokinemia that occurs in MAS, and increased cytokine levels can cause problems in many tissues and organs, especially the lungs. 10
Cytokine storm syndrome has a clinical picture similar to sepsis, as it is characterized by multiple organ failure, clinically persistent fever, hyperferritinemia, and potentially death. Induction of cytokine storm has different etiologies, such as iatrogenic, inflammatory, or infectious. 12 , 13
Cytokine storm syndrome, which is virally induced during the COVID‐19 process, occurs more severely in patients associated with a specific genetic predisposition. 14 , 15 According to the report of McGonagle et al. 16 the clinical phenotype of COVID‐19 was found to be similar to MAS. MAS occurs during autoimmune, tumor, and infectious diseases. Viral infections, especially in adults, are associated with MAS. Early diagnosis of MAS is difficult, as there is no uniform diagnostic criterion to distinguish MAS from underlying inflammatory conditions. 17 The clinical and laboratory findings of MAS include fever, hyperferritinemia, and pancytopenia. 18 However, hemophagocytosis and pancytopenia seen in MAS syndrome were not observed in COVID‐19. The presence of cytokine storm and overactivation of tissue macrophages is a common dominant trait observed in both MAS and severe COVID‐19 patients. 17
The pathophysiology of COVID‐19 disease has not been fully elucidated yet. SARS‐Cov‐2 causes fatal lung damage. The new coronavirus acts on T lymphocytes. MAS, which is characterized by an increase in the concentration of IL‐2, IL‐7, and IL‐10 released from T lymphocytes and a granulocyte colony‐stimulating factor, can be observed in the case of infection in most severe cases. The most prominent feature of MAS is pancytopenia, tissue hemophagocytosis, intravascular coagulation, and organ dysfunction. Acute lung injury has been found to result from inflammatory monocyte and macrophage activation in the pulmonary luminal epithelium, which causes the release of pro‐inflammatory cytokines such as IL‐6, IL‐1, and TNF‐α. 19 , 20
Studies have found that inflammatory cytokines and chemokines, including IL‐6 and IL‐1β, are significantly increased in COVID‐19 patients, and some severe COVID‐19 patients have significantly increased compared to other patients. Increased inflammatory cytokine levels cause postmortem pathology, tissue necrosis in the lung, heart, and gastrointestinal mucosa, and interstitial macrophage and monocyte infiltration in COVID‐19 patients. 19 A study found that cytokines, such as TNF‐α, IL‐6, and IL‐1β, play an important role in MAS. Macrophage migration inhibitory factor (MIF) isolated from T lymphocytes is a pro‐inflammatory cytokine. 4 , 5 The MIF level, which is low in the case of low inflammation, increases with the increase in inflammation. MIF increases the production of inflammatory cytokines such as TNF‐α, IL‐1β, IL‐6, and IFN. 6 , 7 It has been shown that the MIF level measured in patients hospitalized with sepsis and acute respiratory failure may be associated with poor prognosis in the early period, and it has also been suggested that the regulation of MIF level can be used in the treatment of these patients. It has also been stated that MIF suppresses glucocorticoid production, which plays an important role in the anti‐inflammatory effects. 21 , 22 , 23
In line with the data of our study, we observed that the levels of ferritin, fibrinogen, CRP, LDH, and d‐Dimer associated with disease prognosis and severity in COVID‐19 were higher in patients with severe COVID‐19. In addition, in accordance with previous MIF studies, it was observed that the MIF level increased with the increase in disease severity in COVID‐19. In COVID‐19, where the increase in pro‐inflammatory cytokine level cannot be controlled with anti‐inflammatory balance, the increase in MIF, which has an important place in pro‐inflammatory cytokine discharge, may indicate that it may have an important place in the clinical course. In addition, the high sensitivity observed in the case of taking the cutoff at 4.455 ng/ml in the differentiation of severe and moderate COVID‐19 patients in the ROC curve analysis can be considered to support this. The inverse correlation of MIF level with age can be considered as the reason why the immune response decreases with age in the foreground. In addition, although there is no statistical difference between the ages of middle and severe patients, the fact that the average age of moderate COVID‐19 patients is slightly higher than that of serious patients may have led to this situation.
The most important limitation observed in our study was the nonhomogeneous distribution of the patient population according to their gender. However, considering that COVID‐19 caused more hospitalizations in male patients, this difference developed secondary to this in the foreground.
As a result, MIF can be a guiding biomarker in determining the course of patients with COVID‐19 in the later stages. In addition, regulation of the synthesis of MIF, which has an important place in pro‐inflammatory cytokine discharge, shows that it can be used for therapeutic purposes. The relief of endogenous glucocorticoid synthesis blockade, which plays an important role in the anti‐inflammatory balance, by regulation of MIF level may also provide less systemic steroid treatment need in these patients.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Conceptualization, Methodology, Software, Validation, Formal analysis: Alperen Aksakal, Buğra Kerget, and Ferhan Kerget. Investigation, Resources, Data curation: Alperen Aksakal, Buğra Kerget, and Seda Aşkın. Writing – original draft, Writing – review & editing: Buğra Kerget and Ferhan Kerget. Visualization, Supervision, Project administration: Buğra Kerget and Ferhan Kerget.
Aksakal A, Kerget B, Kerget F, Aşkın S. Evaluation of the relationship between macrophage migration inhibitory factor level and clinical course in patients with COVID‐19 pneumonia. J Med Virol. 2021;93:6519‐6524. 10.1002/jmv.27189
All authors disclosure no Conflict of Interest.
REFERENCES
- 1. Hussain A, Bhowmik B, do Vale Moreira NC. COVID‐19 and diabetes: knowledge in progress. Diabetes Res Clin Pract. 2020;162:108142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Otsuka R, Seino K‐i. Macrophage activation syndrome and COVID‐19. Inflamm Regen. 2020;40(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Behrens EM, Koretzky GA. Cytokine storm syndrome: looking toward the precision medicine era. Arthritis Rheum. 2017;69(6):1135‐1143. [DOI] [PubMed] [Google Scholar]
- 4. Avau A, Matthys P. Therapeutic potential of interferon‐γ and its antagonists in autoinflammation: lessons from murine models of systemic juvenile idiopathic arthritis and macrophage activation syndrome. Pharmaceuticals. 2015;8(4):793‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schulert GS, Grom AA. Macrophage activation syndrome and cytokine‐directed therapies. Best Pract Res Clin Rheumatol. 2014;28(2):277‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nobre CC, de Araújo JM, Fernandes TA, et al. Macrophage migration inhibitory factor (MIF): biological activities and relation with cancer. Pathol Oncol Res. 2017;23(2):235‐244. [DOI] [PubMed] [Google Scholar]
- 7. de Souza GF, Muraro SP, Santos LD, et al. Macrophage migration inhibitory factor (MIF) controls cytokine release during respiratory syncytial virus infection in macrophages. Inflamm Res. 2019;68(6):481‐491. [DOI] [PubMed] [Google Scholar]
- 8. Kerget B, Kerget F, Koçak AO, et al. Are serum interleukin 6 and surfactant protein D levels associated with the clinical course of COVID‐19? Lung. 2020;198(5):777‐784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sjoding MW, Hofer TP, Co I, Courey A, Cooke CR, Iwashyna TJ. Interobserver reliability of the Berlin ARDS definition and strategies to improve the reliability of ARDS diagnosis. Chest. 2018;153(2):361‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jafarzadeh A, Chauhan P, Saha B, Jafarzadeh S, Nemati M. Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in COVID‐19: lessons from SARS and MERS, and potential therapeutic interventions. Life Sci. 2020;257:118102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kermali M, Khalsa RK, Pillai K, Ismail Z, Harky A. The role of biomarkers in diagnosis of COVID‐19—a systematic review. Life Sci. 2020;254:117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruscitti P, Berardicurti O, Iagnocco A, Giacomelli R. Cytokine storm syndrome in severe COVID‐19. Autoimmun Rev. 2020;19(7):102562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Canna SW, Behrens EM. Making sense of the cytokine storm: a conceptual framework for understanding, diagnosing, and treating hemophagocytic syndromes. Pediatr Clin. 2012;59(2):329‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pedersen SF, Ho Y‐C. SARS‐CoV‐2: a storm is raging. J Clin Invest. 2020;130:5‐2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caso F, Costa L, Ruscitti P, et al. Could Sars‐coronavirus‐2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev. 2020;19(5):102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McGonagle D, Sharif K, O'Regan A, Bridgewood C. The role of cytokines including interleukin‐6 in COVID‐19 induced pneumonia and macrophage activation syndrome‐like disease. Autoimmun Rev. 2020;19(6):102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang C, Xie J, Zhao L, et al. Aveolar macrophage activation and cytokine storm in the pathogenesis of severe COVID‐19. EBioMedicine. 2020;57:102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Crayne CB, Albeituni S, Nichols KE, Cron RQ. The immunology of macrophage activation syndrome. Front Immunol. 2019;10:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Addeo A, Obeid M, Friedlaender A. COVID‐19 and lung cancer: risks, mechanisms and treatment interactions. J Immunother Cancer. 2020;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu L, Wei Q, Lin Q, et al. Anti–spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS‐CoV infection. JCI Insight. 2019;4(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Calandra T, Echtenacher B, Roy DL, et al. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nature Med. 2000;6(2):164‐170. [DOI] [PubMed] [Google Scholar]
- 22. Guo Y, Xie C. The pathogenic role of macrophage migration inhibitory factor in acute respiratory distress syndrome. Zhonghua Jie He He Hu Xi za Zhi = Zhonghua Jiehe He Huxi Zazhi = Chinese J Tuberc Respir Dis. 2002;25(6):337‐340. [PubMed] [Google Scholar]
- 23. Calandra T, Bucala R. Macrophage migration inhibitory factor: a counter‐regulator of glucocorticoid action and critical mediator of septic shock. J Inflamm. 1995;47(1–2):39‐51. [PubMed] [Google Scholar]


