Abstract
Objective
To provide the latest evidence for the efficacy and safety of arbidol (umifenovir) in COVID‐19 treatment.
Methods
A literature systematic search was carried out in PubMed, Cochrane Library, Embase, and medRxiv up to May 2021. The Cochrane risk of bias tool and Newcastle–Ottawa scale were used to assess the quality of included studies. Meta‐analysis was performed using RevMan 5.3.
Results
Sixteen studies were met the inclusion criteria. No significant difference was observed between arbidol and non‐antiviral treatment groups neither for primary outcomes, including the negative rate of PCR (NR‐PCR) on Day 7 (risk ratio [RR]: 0.94; 95% confidence interval (CI): 0.78–1.14) and Day 14 (RR: 1.10; 95% CI: 0.96–1.25), and PCR negative conversion time (PCR‐NCT; mean difference [MD]: 0.74; 95% CI: −0.87 to 2.34), nor secondary outcomes (p > .05). However, arbidol was associated with higher adverse events (RR: 2.24; 95% CI: 1.06–4.73). Compared with lopinavir/ritonavir, arbidol showed better efficacy for primary outcomes (p < .05). Adding arbidol to lopinavir/ritonavir also led to better efficacy in terms of NR‐PCR on Day 7 and PCR‐NCT (p < .05). There was no significant difference between arbidol and chloroquine in primary outcomes (p > .05). No remarkable therapeutic effect was observed between arbidol and other agents (p > .05).
Conclusion
The present meta‐analysis showed no significant benefit of using arbidol compared with non‐antiviral treatment or other therapeutic agents against COVID‐19 disease. High‐quality studies are needed to establish the efficacy and safety of arbidol for COVID‐19.
Keywords: 2019 novel coronavirus infection, 2019‐nCoV infection, arbidol, coronavirus, novel coronavirus, umifenovir
The evidence on the antivirus effect of Arbidol against COVID‐19 is controversial. The present meta‐analysis showed no significant benefit of using arbidol compared with non‐antiviral treatment or other therapeutic agents against COVID‐19 disease

1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the causative agent of coronavirus disease 2019 (COVID‐19), has rapidly spread throughout the world leading to a pandemic. 1 , 2 , 3 Up until now, some antiviral drugs have been proposed as promising therapeutic agents against SARS‐CoV‐2 infection including interferon, 4 lopinavir/ritonavir, 5 chloroquine, 6 remdesivir, 7 and arbidol. 8
Arbidol (umifenovir) is an oral antiviral drug 9 that was approved for prophylaxis in Russia and China several decades ago and used in the treatment of influenza A and B as well as other respiratory viral infections. 10 In addition to Arbidol's antiviral and anti‐inflammatory activities against various types of influenza viruses, 11 , 12 especially H1N1, 13 its broad‐spectrum antiviral activities against other viruses, such as Zika, 14 Ebola, 15 hepatitis B and C, 16 , 17 rhinovirus, 18 respiratory syncytial virus, 18 , 19 coxsackie, 18 , 20 chikungunya, 21 and adenovirus 18 are shown in vitro and in vivo.
Regarding the SARS‐CoV‐2 infection, the antivirus effect of arbidol against SARS‐CoV‐2 has yet been controversial. On the one hand, the efficacy of arbidol was shown in vitro 22 , 23 which seems to have inhibited the infection more efficiently among other WHO‐approved anti‐influenza drugs including baloxavir, laninamivir, oseltamivir, peramivir, zanamivir 23 by blocking the trimerization of the spike glycoprotein. 22 Also, some studies suggested its beneficial effects either in monotherapy or combination therapy with other agents against COVID‐19. 5 , 24 , 25 , 26 On the other hand, there exist other studies which have found no benefit of using arbidol in COVID‐19 patients 27 , 28 suggesting an urgent need to reach a conclusive decision on this matter. The present systematic review and meta‐analysis aim to provide the latest evidence on arbidol's efficacy and safety compared with other therapeutic agents in COVID‐19 treatment.
2. METHODS
We have registered the protocol of this systematic review and meta‐analysis with the registry number CRD42020207821 and used the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) checklist. 29
2.1. Literature search strategy
We conducted a systematic search in the leading bibliographic databases, including PubMed, the Cochrane Library, and Embase for the relevant records up to May 2021. We also searched in medRxiv, Google Scholar, and clinical registry databases, including ClinicalTrials.gov, the European Union Clinical Trials Register, and the Chinese Clinical Trial Registry for additional relevant documents. Finally, the reference lists of the included studies and review articles were screened and the search was limited to the articles the abstract or full text of which were in English. Search terms included 2019‐nCoV, SARS‐CoV‐2, COVID‐19, arbidol, and umifenovir. The following terms were used to explore PubMed: ((((((((Coronavirus[Title/Abstract]) OR (Coronavirus[MeSH Terms])) OR (COVID‐19[Title/Abstract])) OR (SARS‐CoV‐2[Title/Abstract])) OR (COVID‐19[MeSH Terms])) OR (SARS‐CoV‐2[MeSH Terms])) OR (2019 novel coronavirus infection[Title/Abstract])) OR (2019‐nCoV infection[Title/Abstract])) AND ((Umifenovir[Title/Abstract]) OR (Arbidol[Title/Abstract])).
2.2. Study selection
Two authors (Behnam Amani and Mahsa Zareei) independently screened the identified records based on inclusion and exclusion criteria. Disagreements between the authors were resolved by discussion among authors. The studies were included based on the following criteria: (1) patients with laboratory‐confirmed positive COVID‐19 test; (2) arbidol as monotherapy or in combination with other therapeutic agents; (3) any therapeutic intervention as a comparison (4); efficacy and safety outcomes of interest. The primary efficacy outcomes were the negative rate of PCR (polymerase chain reaction) and PCR negative conversion time and the secondary efficacy outcomes included the rate of improvement on chest CT, rate of cough alleviation, length of hospital stay, and disease progression. The safety outcome was the incidence of adverse events reported in patients; and (5) clinical trials or observational studies. The exclusion criteria were the studies conducted on animal models, case reports, case series, letters to editors, and editorials.
2.3. Data extraction and quality assessment
We used the Cochrane collaboration tool to assess the risk of bias of randomized clinical trials. 30 Quality assessment of observational studies was conducted using the Newcastle–Ottawa scale (NOS). 31 We extracted data using the same data extraction form. The extracted data included (1) study characteristics (author, year, setting, and design); (2) patient's characteristics (sample size, sex, and age); (3) intervention and comparison (sample size); and (4) efficacy and safety outcomes. All steps were performed independently by two authors (Behnam Amani and Mahsa Zareei).
2.4. Evidence synthesis
We performed a meta‐analysis using RevMan software, version 5.3. The mean difference (MD) with a 95% confidence interval (CI) was used for continuous variables and a risk ratio (RR) with 95% CI for dichotomous variables. The statistical heterogeneity was evaluated using the I 2 and Chi2 tests. The random‐effects model was used for studies with I 2 > 50% or p < .1. Otherwise, we used the fixed‐effect model.
3. RESULTS
3.1. The characteristics of studies
Figure 1 shows the literature search flow, removal of duplicates, and the screening based on title, abstract, and full text. As a result, 52 full‐text articles were reviewed and sixteen studies 24 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 entered the final analysis. The characteristics of the studies included in the systematic review are presented in Table 1. Assessment of the risk of bias using the Cochrane collaboration tool is presented in Figure 2.
Figure 1.
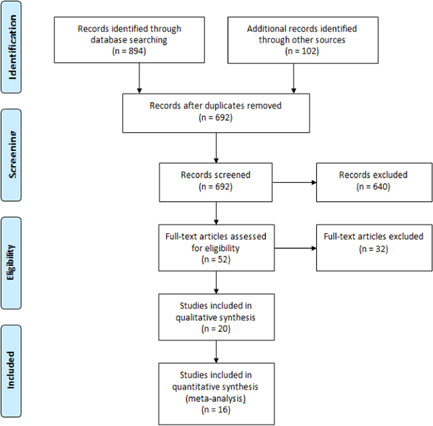
Study flow diagram
Table 1.
Characteristics of included studies
| Study, year | Country | Design | Age (mean) | N (M/F) | Intervention (n) | Comparison (n) | NOS |
|---|---|---|---|---|---|---|---|
| Chang Chen 2020, 47 | China | RCT | NA | 236 (110/126) | Arbidol (120) | Favipiravir (116) | RoB 2 |
| Huang 2020, 24 | China | R | NA | 27 (12/15) | Arbidol (11) | LPV/r (6), CQ (10) | 7 |
| Lisi Deng 2020, 33 | China | R | 44.5 | 33 (17/16) | Arbidol + LPV/r (16) | LPV/r (17) | 8 |
| Ping Xu 2020, 42 | China | R | 51.9 | 141 (74/67) | Arbidol + IFN (71) | IFN (70) | 6 |
| Qibin Liu 2020, 25 | China | R | 59.5 | 504 (259/245) | Arbidol (257) | Os (66), LPV/r (259) | 6 |
| Qiong Zhou 2020, 44 | China | R | NA | 77 (31/46) | Arbidol (24) | Arbidol + IFN (46), IFN (7) | 7 |
| Wenyu Chen 2020, 48 | China | RCT | NA | 62 (34/28) | Arbidol + control (42) | Control (20) | RoB 2 |
| Kaijin Xu 2020, 26 | China | R | NA | 111 (47/64) | Arbidol + ER (49) | ER (62) | 7 |
| Xiu Lan 2020, 35 | China | R | 55.8 | 73 (37/36) | Arbidol + LPV/r (39) | LPV/r (34) | 7 |
| Jun Chen 2020, 34 | China | R | 48 | 134 (69/65) | Arbidol (34) | LPV/r (52), non‐antiviral (48) | 5 |
| Xudan Chen 2020, 32 | China | R | 48 | 284 (131/153) | Arbidol (37) | Control (121), LPV/r (60), arbidol + LPV/r (16), CQ (17), Os (13), Other (16) | 9 |
| Yaya Zhou 2020, 45 | China | R | 55.5 | 238 (102/136) | Arbidol (82) | Arbidol + IFN (139) | 7 |
| Yueping Li 2020, 37 | China | RCT | 49.4 | 86 (40/46) | Arbidol (35) | LPV/r (34), control (17) | RoB 2 |
| Zhu 2020, 46 | China | R | 39.8 | 50 (26/24) | Arbidol (16) | LPV/r (34) | 7 |
| Wen 2020, 41 | China | R | 49.9 | 178 (81/97) | Arbidol (36) | LPV/r (59), control (58), arbidol + LPV/r (25) | 7 |
| Ming Li 2021, 36 | China | R | NA | 62 (24/38) | Arbidol (42) | CQ (20) | 7 |
| Jie 2021, 49 | China | R | 65 | 252 (106/146) | Arbidol (228) | No arbidol (24) | 8 |
| Ruan 2021, 50 | China | R | 64 | 331 (160/171) | Arbidol (273) | Non‐antiviral (58) | 8 |
| Ghaderkhani 2021, 51 | Iran | RCT | NA | 53 (32/21) | HCQ + arbidol (28) | HCQ (25) | RoB 2 |
| Nojomi 2020, 40 | Iran | RCT | 56.4 | 100 (60/40) | Arbidol (50) | LPV/r (50) | RoB 2 |
| Lian 2020, 38 | China | R | 60 | 81 (45/36) | Arbidol (45) | Control (36) | 8 |
| Liu 2021, 39 | China | R | 54.8 | 108 (47/61) | Arbidol (40) | Arbidol + LHQW (68) | 8 |
| Jing Chen 2020, 52 | China | R | NA | 200 (130/70) | Arbidol + SFJDC (100) | Arbidol (100) | 8 |
| Fang 2020, 53 | China | R | 61.5 | 162 (87/75) | Arbidol + LHQW (113) | LHQW (49) | 8 |
| Ping 2020, 43 | China | R | NA | 295 (171/124) | Arbidol (148) | LHQW + arbidol (147) | 8 |
| Xiang‐Kun 2020, 54 | China | R | NA | 70 (41/29) | Arbidol (30) | SFJD + arbidol (40) | 9 |
Abbreviations: CQ, chloroquine; ER, empirical regimens; F, female; HCQ, hydroxychloroquine; IFN, Interferon; LPV/r, lopinavir/ritonavir; LHQW: Lianhuaqingwen; M, male; N, number; NA, not acquired; Os, oseltamivir; R, retrospective; RCT, randomized clinical trial; RoB, risk of bias; SFJD, Shufeng Jiedu.
Figure 2.
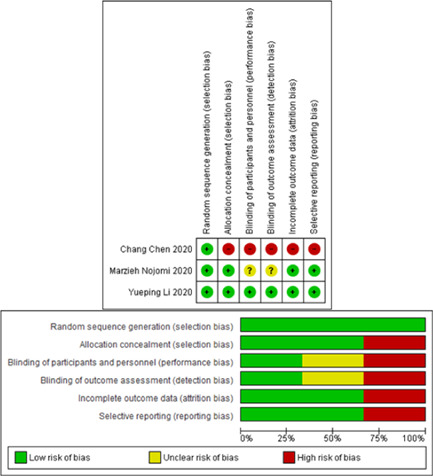
Risk of bias in the selected studies
3.2. Comparisons
3.2.1. Arbidol versus non‐antiviral treatment
The result of meta‐analysis showed that there was no significant difference between arbidol and non‐antiviral groups in terms of negative rate of PCR on Day 7 (RR: 0.94; 95% CI: 0.78–1.14; p = .55) and Day 14 (RR: 1.10; 95% CI: 0.96–1.25; p = .17), PCR negative conversion time (MD: 0.74; 95% CI: −0.87 to 2.34; p = .37) (Figure 3), rate of improvement on chest CT on Day 7 (RR: 1.53; 95% CI: 0.50–4.68; p = .46) and Day 14 (RR: 0.92; 95% CI: 0.56–1.54; p = .76), rate of cough alleviation on Day 7 (RR: 1.47; 95% CI: 0.64–3.39; p = .36) and Day 14 (RR: 1.19; 95% CI: 0.74–1.91; p = .47), hospital stay (MD: 3.97; 95% CI: 0.05–7.89; p = .05), and disease progression (RR: 1.88; 95% CI: 0.70–5.00; p = .21; Figure 4). Arbidol was associated with higher adverse events (RR: 2.24; 95% CI: 1.06–4.73; p = .04; Figure 4).
Figure 3.
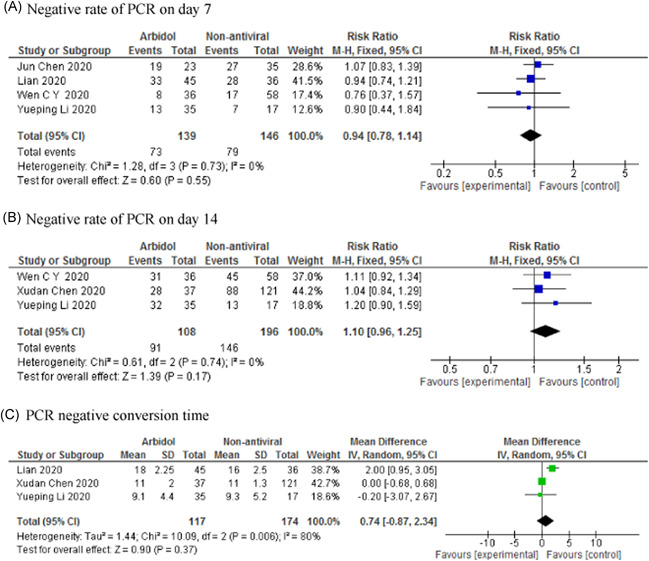
Forest plot of arbidol versus non‐antiviral for outcomes of negative rate of PCR on Day 7 (A), negative rate of PCR on Day 14 (B), and PCR negative conversion time (C)
Figure 4.
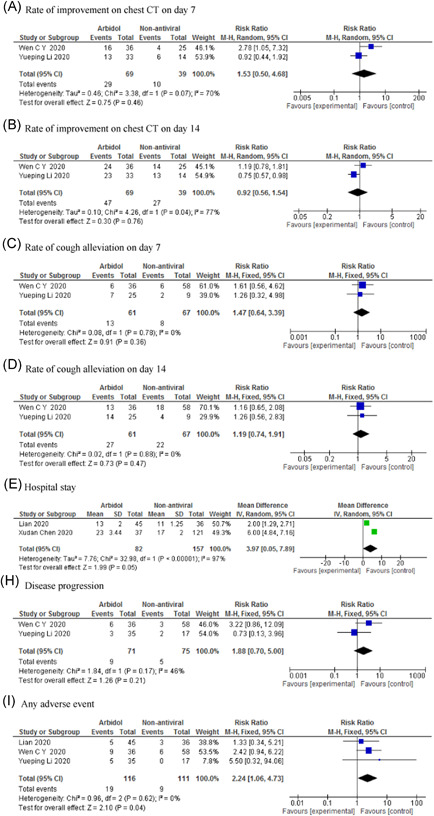
Forest plot of arbidol versus non‐antiviral for outcomes of rate of improvement on chest CT on Day 7 (A), rate of improvement on chest CT on Day 14 (B), rate of cough alleviation on Day 7 (C), rate of cough alleviation on Day 14 (D), hospital stay (E), disease progression (H), and any adverse event (I)
3.2.2. Arbidol versus favipiravir
Only one study 47 compared arbidol with favipiravir. The result showed no significant difference between arbidol and favipiravir groups in the clinical recovery rate. However, favipiravir was associated with better efficacy in relieving pyrexia and cough. The frequencies of drug‐related adverse events for arbidol and favipiravir were 23.33% and 31.9%, respectively.
3.2.3. Arbidol versus chloroquine
There was no significant difference between arbidol and chloroquine in terms of negative rate of PCR on Day 14 (RR: 1.27; 95% CI: 0.64–2.51; p = .50) and PCR negative conversion time (MD: 0.69; 95% CI: −3.72 to 5.10; p = .76; Table 2). However, the length of hospital stay in patients taking chloroquine was significantly shorter than patients taking arbidol (MD: 4.59; 95% CI: 0.58–8.60; p = .02; Table 2).
Table 2.
The pooled estimate of arbidol versus other therapeutic agents and sensitivity analysis
| Analysis | No. of studies | Participants | Pooled estimate (95% CI) | p | Heterogeneity | ||
|---|---|---|---|---|---|---|---|
| Ch2 | p | I 2 | |||||
| Sensitivity analysis | |||||||
| Arbidol versus non‐antiviral | |||||||
| Negative rate of PCR | 4 | 405 | 1.21 (1.06–1.38) | .005 | 5.79 | .12 | 48% |
| Arbidol versus chloroquine | |||||||
| Negative rate of PCR on Day 14 | 3 | 137 | 1.27 (0.64–2.51) | .50 | 12.54 | .002 | 84% |
| PCR negative conversion time | 2 | 75 | 0.69 (−3.72 to 5.10) | .76 | 14.71 | .0001 | 93% |
| Hospital stay | 2 | 75 | 4.59 (0.58–8.60) | .02 | 8.44 | .004 | 88% |
| Arbidol versus LPV/r | |||||||
| Negative rate of PCR on Day 7 | 4 | 276 | 1.35 (1.03–1.76) | .03 | 4.18 | .24 | 28% |
| Negative rate of PCR on Day 14 | 5 | 328 | 1.47 (1.06–2.04) | .02 | 24.07 | <.0001 | 83% |
| PCR negative conversion time | 5 | 328 | −2.28 (−3.83 to −0.72) | .004 | 21.91 | .0002 | 82% |
| Hospital stay | 3 | 214 | −1.87 (−8.01 to 4.27) | .55 | 50.39 | <.00001 | 96% |
| Rate of improvement on chest CT on Day 7 | 2 | 156 | 1.14 (0.77–1.69) | .50 | 0.29 | 0.59 | 0% |
| Rate of improvement on chest CT on Day 14 | 2 | 156 | 0.99 (0.80–1.23) | .92 | 0.24 | 0.62 | 0% |
| Disease progress | 2 | 164 | 1.08 (0.13–9.29) | .94 | 5.64 | 0.02 | 82% |
| Rate of cough alleviation on Day 7 | 2 | 141 | 1.61 (0.21–12.22) | .64 | 5.48 | 0.02 | 82% |
| Rate of cough alleviation on Day 14 | 2 | 141 | 0.81 (0.58–1.15) | .24 | 0.32 | 0.57 | 0% |
| Adverse events | 5 | 367 | 0.44 (0.28–0.68) | .0002 | 2.70 | 0.61 | 0% |
| Arbidol + LPV/r versus LPV/r | |||||||
| Negative rate of PCR on Day 7 | 2 | 117 | 2.06 (1.13–3.76) | .02 | 0.01 | 0.91 | 0% |
| Negative rate of PCR on Day 14 | 3 | 193 | 0.99 (0.55–1.80) | .99 | 9.44 | 0.009 | 79% |
| PCR negative conversion time | 3 | 229 | 2.21 (−0.13 to 4.54) | .06 | 6.61 | 0.04 | 70% |
| Hospital stay | 2 | 145 | 1.51 (−3.94 to 6.97) | .59 | 6.46 | 0.01 | 85% |
| Rate of imrovement on chest CT on Day 7 | 2 | 117 | 1.05 (0.20–5.50) | .96 | 6.99 | 0.008 | 86% |
| Arbidol versus arbidol + IFN | |||||||
| PCR negative conversion time | 2 | 291 | −0.99 (−16.67 to 14.69) | .90 | 715.70 | <.00001 | 100% |
| Arbidol + IFN versus IFN | |||||||
| PCR negative conversion time | 2 | 194 | 2.31 (−7.78 to 12.40) | .65 | 28.11 | <.00001 | 96% |
| Arbidol + LHQW versus arbidol | |||||||
| Rate of improvement on chest CT | 2 | 403 | 1.27 (0.88–1.85) | .20 | 2.91 | 0.09 | 66% |
Abbreviations: CI, confidence interval; IFN, interferon; LHQW, Lianhuqingwen; LPV/r, lopinavir/ritonavir; P, p‐value; PCR, polymerase chain reaction.
3.2.4. Arbidol versus oseltamivir
Chen et al. 27 found that the clearance rate of arbidol and oseltamivir during 14 days were 75.7% and 61.5%, respectively. The median length of hospital stay in both groups was similar. The result of another study 25 showed that arbidol was more effective than oseltamivir in reducing mortality. Also, arbidol was more effective in the reduction of lesion size (46.43% vs. 41.18%).
3.2.5. Arbidol versus lopinavir/ritonavir
Arbidol showed better efficacy compared to lopinavir/ritonavir in terms of negative rate of PCR on Day 7 (RR: 1.35; 95% CI: 1.03–1.76; p = .03) and Day 14 (RR: 1.47; 95% CI: 1.06–2.04; p = .02), as well as PCR negative conversion time (MD: −2.28; 95% CI: −3.83 to − 0.72; p = .004; Table 2). However, there was no significant difference between two drugs in terms of rate of improvement on chest CT on Day 7 (RR: 1.14; 95% CI: 0.77–1.69; p = .50) and Day 14 (RR: 0.99; 95% CI: 0.80–1.23; p = .92), rate of cough alleviation on Day 7 (RR: 1.61; 95% CI: 0.21–12.22; p = .64) and Day 14 (RR: 0.81; 95% CI: 0.58–1.15; p = .24), hospital stay (MD: −1.87; 95% CI: −8.01 to 4.27; p = .55), and disease progression (RR: 1.08; 95% CI: 0.13–9.29; p = .94; Table 2). Compared with lopinavir/ritonavir, arbidol had fewer adverse events (RR: 0.44; 95% CI: 0.28–0.68; p = .0002; Table 2).
3.2.6. Arbidol plus lopinavir/ritonavir versus lopinavir/ritonavir
Arbidol combined with lopinavir/ritonavir versus exclusive administration of lopinavir/ritonavir was associated with higher negative rate of PCR on Day 7 (RR: 2.06; 95% CI: 1.13–3.76; p = .02; Table 2). However, no significant effect was observed between two administrations in terms of negative rate of PCR on Day 14 (RR: 0.99; 95% CI: 0.55–1.80; p = .99), PCR negative conversion time (MD: 2.21; 95% CI: −0.13 to 4.54; p = .06), rate of improvement on chest CT on Day 7 (RR: 1.05; 95% CI: 0.20–5.50; p = .96), and hospital stay (MD: 1.51; 95% CI: −3.94 to 6.97; p = .59; Table 2).
3.2.7. Arbidol and interferon
The meta‐analysis result showed no significant difference between exclusive arbidol and interferon/arbidol combination regarding the PCR negative conversion time (MD: −0.99; 95% CI: −16.67 to 14.69; p = .90; Table 2). Also, interferon/arbidol combination showed no beneficial effect compared with interferon alone regarding PCR negative conversion time (MD: 2.31; 95% CI: −7.78 to 12.40; p = .65; Table 2).
3.2.8. Arbidol combined with traditional Chinese medicines
Several studies 52 , 53 , 54 compared the efficacy of arbidol as a combination therapy with traditional Chinese medicines. The meta‐analysis of improvement rate of chest CT found no greater benefit of arbidol combined with Lianhuaqingwen compared to arbidol alone in the treatment of COVID‐19 patients (RR: 1.27; 95% CI: 0.88–1.85; p = .20; Table 2). Fang et al. 53 found that the simultaneous treatment of arbidol and Lianhuaqingwen was associated with higher improvement in patients with moderate COVID‐19 compared with Lianhuaqingwen alone. There are other studies 52 , 54 that reported the efficacy and safety of Shufeng Jiedu capsule combined with arbidol versus arbidol alone in patients with COVID‐19.
3.2.9. Sensitivity analysis
We conducted a sensitivity analysis by including the case‐series study 55 (Table 2).
4. DISCUSSION
This study aimed to provide the latest available evidence on the efficacy and safety of arbidol in the treatment of COVID‐19 disease. The meta‐analysis results showed that arbidol had no clinical efficacy for all primary and secondary outcomes, including the negative rate of PCR, PCR negative conversion time, rate of improvement on chest CT, cough alleviation, hospital stay, and disease progression.
Similar to our finding, a meta‐analysis by Huang et al. 56 indicated that arbidol was not associated with significant improvement in terms of efficacy outcomes but for the negative rate of PCR on Day 14 compared to the control group. However, they performed a subgroup analysis only on primary outcomes based on without or with antiviral drugs.
In another similar meta‐analysis done by Li et al., 27 arbidol was associated with a higher negative rate of PCR compared with control in patients with COVID‐19. Nevertheless, this study found no efficacy for PCR negative conversion time and improvement rate on chest CT and progression disease. The finding of these meta‐analyses for the negative rate of PCR contrast with our findings due to the differences in control groups. In fact, the present study boasts specified control subgroups in which each non‐arabidol treatment was considered as a separate control group, but other studies take more general categories into accounts such as all non‐arabidol treatments in Huang et al.'s study and all other antiviral/no antiviral drugs in the one done by Li et al. It should be noted that the inclusion of different interventions in a control group in the meta‐analysis may cause problems including the risk of bias, heterogeneity, and imprecision, which finally affect the interpretation of findings. 57
Although the present study found no significant treatment benefit for arbidol compared with non‐antiviral interventions, recent findings from two studies 5 , 58 have suggested its efficacy and safety for prophylaxis in patients with COVID‐19. The result of a clinical and laboratory data analysis also 58 showed that arbidol improved SARS‐CoV‐2 infection though without any effect on the hospitalization rate. Zhang et al. 5 found that arbidol was associated with the improvement in SARS‐CoV‐2 infection. It seems that more evidence is needed to approve the potential of arbidol for prophylaxis of COVID‐19.
Based on the meta‐analysis results, arabidol showed different efficacies in various outcomes in comparison to other treatments. Arbidol was not more effective than chloroquine in the negative rate of PCR and PCR negative conversion time. Also, chloroquine led to a shorter length of hospital stay than arabidol. However, arbidol showed better efficacy than oseltamivir in terms of the negative rate of PCR, the length of hospital stay, and the mortality rate. Compared with lopinavir/ritonavir, arbidol had better efficacy in the negative rate of PCR and PCR negative conversion time, and also was associated with fewer adverse events, with no significant difference between them for other efficacy outcomes.
Our meta‐analysis showed that adding arbidol to lopinavir/ritonavir increased the negative rate of PCR on Day 7 and decreased PCR negative conversion time compared to lopinavir/ritonavir alone. Furthermore, simultaneous prescription of arbidol with interferon has no effect on the PCR negative conversion time in patients. Similar results were also found for interferon as a combination therapy with arbidol. The present meta‐analysis found no benefit for arbidol in combination with traditional Chines medicine. However, more studies are needed to approve this therapeutic alternative. The meta‐analysis of Huang et al. 56 found no significant adverse events for arbidol. However, in our study arbidol was associated with higher adverse events in patients.
5. LIMITATIONS
Despite the efforts to minimize limitations, there were still several limitations to the current study. One of the challenging limitations of our study was the study design. Studies conducted were mostly retrospective and associated with a higher risk of bias. To reduce bias, we applied some strategies recommended by Almeida et al. 59 Another important limitation was the location of the studies. The majority of studies were conducted in China, which makes our findings prone to a selection bias. Finally, we could not perform subgroup analyses on variables such as the severity of illness, dosage, sample size, and other variables due to an insufficient number of available studies.
6. CONCLUSION
The finding of this meta‐analysis revealed that arbidol was not superior to non‐antiviral treatment in patients with COVID‐19. Compared with lopinavir/ritonavir, arbidol showed better efficacy for primary outcomes. No remarkable treatment effect was observed compared with other therapeutic agents. A well‐designed randomized controlled trial with a large sample size is necessary to conclude the efficacy and safety of arbidol against COVID‐19.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Study concept and design: Bahman Amani and Behnam Amani. Literature searching: Bahman Amani and Behnam Amani. Study selection and appraisal: Behnam Amani and Mahsa Zareei. Data extraction: Sara Zareei and Mahsa Zareei. Data analysis and interpretation: Bahman Amani and Behnam Amani. Critical revision of the manuscript: Sara Zareei. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Supporting information
Supplementary information.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Ahmad Khanijahani for his contribution during the preparation of this study.
Amani B, Amani B, Zareei S, Zareei M. Efficacy and safety of arbidol (umifenovir) in patients with COVID‐19: A systematic review and meta‐analysis. Immun Inflamm Dis. 2021;9:1197‐1208. 10.1002/iid3.502
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are included within the Supporting Information file.
REFERENCES
- 1. Lai C‐C, Shih T‐P, Ko W‐C, Tang H‐J, Hsueh P‐R. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and corona virus disease‐2019 (COVID‐19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paudel S, Dangal G, Chalise A, Bhandari TR, Dangal O. The coronavirus pandemic: what does the evidence show? J Nepal Health Res Counc. 2020;18(2):1‐9. [DOI] [PubMed] [Google Scholar]
- 3. Vellingiri B, Jayaramayya K, Iyer M, et al. COVID‐19: a promising cure for the global panic. Sci Total Environ. 2020;725:138277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Payandemehr P, Azhdarzadeh M, Bahrami‐Motlagh H, et al. Interferon beta‐1a as a candidate for COVID‐19 treatment; an open‐label single‐arm clinical trial. Front Emerg Med. 2020;4(2s):e51. [Google Scholar]
- 5. Deng L, Li C, Zeng Q, et al. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: a retrospective cohort study. J Infect. 2020;81:e1‐e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou L, Huang W, Huang H, et al. Chloroquine, arbidol (umifenovir) or lopinavir/ritonavir as the antiviral monotherapy for COVID‐19 patients: a retrospective cohort study; 2020.
- 7. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid‐19—preliminary report. N Engl J Med. 2020;383:1813‐1826. [DOI] [PubMed] [Google Scholar]
- 8. Zhu Z, Lu Z, Xu T, et al. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID‐19. J Infect. 2020;81:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blaising J, Polyak SJ, Pécheur E‐I. Arbidol as a broad‐spectrum antiviral: an update. Antiviral Res. 2014;107:84‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang Y, Xu Q, Sun Z, Zhou L. Current targeted therapeutics against COVID‐19: based on first‐line experience in china. Pharmacol Res. 2020;157:104854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leneva IA, Falynskova IN, Makhmudova NR, Poromov AA, Yatsyshina SB, Maleev VV. Umifenovir susceptibility monitoring and characterization of influenza viruses isolated during ARBITR clinical study. J Med Virol. 2019;91(4):588‐597. [DOI] [PubMed] [Google Scholar]
- 12. Pshenichnaya NY, Bulgakova VA, Lvov NI, et al. Clinical efficacy of umifenovir in influenza and ARVI (study ARBITR). Ter Arkh. 2019;91(3):56‐63. [DOI] [PubMed] [Google Scholar]
- 13. Liu Q, Xiong H‐r, Lu L, et al. Antiviral and anti‐inflammatory activity of arbidol hydrochloride in influenza A (H1N1) virus infection. Acta Pharmacol Sin. 2013;34(8):1075‐1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fink SL, Vojtech L, Wagoner J, et al. The antiviral drug arbidol inhibits Zika virus. Sci Rep. 2018;8(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pécheur E‐I, Borisevich V, Halfmann P, et al. The synthetic antiviral drug arbidol inhibits globally prevalent pathogenic viruses. J Virol. 2016;90(6):3086‐3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pécheur E‐I, Lavillette D, Alcaras F, et al. Biochemical mechanism of hepatitis C virus inhibition by the broad‐spectrum antiviral arbidol. Biochemistry. 2007;46(20):6050‐6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wei X‐F, Gan C‐Y, Cui J, et al. Identification of compounds targeting hepatitis B virus core protein dimerization through a split luciferase complementation assay. Antimicrob Agents Chemother. 2018;62(12):e01302‐e01318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shi L, Xiong H, He J, et al. Antiviral activity of arbidol against influenza A virus, respiratory syncytial virus, rhinovirus, coxsackie virus and adenovirus in vitro and in vivo . Arch Virol. 2007;152(8):1447‐1455. [DOI] [PubMed] [Google Scholar]
- 19. Brooks MJ, Burtseva EI, Ellery PJ, et al. Antiviral activity of arbidol, a broad‐spectrum drug for use against respiratory viruses, varies according to test conditions. J Med Virol. 2012;84(1):170‐181. [DOI] [PubMed] [Google Scholar]
- 20. Zhong Q, Yang Z, Liu Y, et al. Antiviral activity of arbidol against Coxsackie virus B5 in vitro and in vivo . Arch Virol. 2009;154(4):601‐607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Delogu I, Pastorino B, Baronti C, Nougairède A, Bonnet E, de Lamballerie X. In vitro antiviral activity of arbidol against Chikungunya virus and characteristics of a selected resistant mutant. Antiviral Res. 2011;90(3):99‐107. [DOI] [PubMed] [Google Scholar]
- 22. Vankadari N. Arbidol: a potential antiviral drug for the treatment of SARS‐CoV‐2 by blocking the trimerization of viral spike glycoprotein? Int J Antimicrob Agents. 2020;56:105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang X, Cao R, Zhang H, et al. The anti‐influenza virus drug, arbidol is an efficient inhibitor of SARS‐CoV‐2 in vitro . Cell Discov. 2020;6(1):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang H, Guan L, Yang Y, et al. Chloroquine, arbidol (umifenovir) or lopinavir/ritonavir as the antiviral monotherapy for COVID‐19 patients: a retrospective cohort study; 2020.
- 25. Zhang K, Liu F, Zhang Y, et al. The effect of Arbidol Hydrochloride on reducing mortality of Covid‐19 patients: a retrospective study of real world date from three hospitals in Wuhan. medRxiv. 2020;146:1275‐1284. [Google Scholar]
- 26. Xu K, Chen Y, Yuan J, et al. Clinical efficacy of arbidol in patients with 2019 novel coronavirus‐infected pneumonia: a retrospective cohort study; 2020.
- 27. Li Y, Jin Y, Ge Q, et al. Efficacy and safety of arbidol in the treatment of novel coronavirus pneumonia: a systematic review based on current and previous antiviral therapy. Adverse Drug React J. 2020;22(6). [Google Scholar]
- 28. Lian N, Xie H, Lin S, Huang J, Zhao J, Lin Q. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin Microbiol Infect. 2020;26:917‐921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264‐269. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 30. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25(9):603‐605. [DOI] [PubMed] [Google Scholar]
- 32. Yang Y, Pang M, Du C, et al. Associations of clinical characteristics and antiviral drugs with viral RNA clearance in patients with COVID‐19 in Guangzhou, China: a retrospective cohort study. medRxiv. 2020;23:57‐64. 10.1101/2020.04.09.20058941 [DOI] [Google Scholar]
- 33. Deng L, Li C, Zeng Q, et al. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: a retrospective cohort study. J Infect. 2020;81(1):e1‐e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jun C, Yun L, Xiuhong X, et al. Efficacies of lopinavir/ritonavir and abidol in the treatment of novel coronavirus pneumonia. Chin J Infect Dis. 2020:E008. [Google Scholar]
- 35. Lan X, Shao C, Zeng X, Wu Z, Xu Y. Lopinavir‐ritonavir alone or combined with arbidol in the treatment of 73 hospitalized patients with COVID‐19: a pilot retrospective study. medRxiv. 2020;59:378‐385. [DOI] [PubMed] [Google Scholar]
- 36. Li M, Yu T, Zhu J, et al. Comparison of the antiviral effect of Arbidol and Chloroquine in treating COVID‐19. Annals Palliative Med. 2021;10(3):3307‐3312. [DOI] [PubMed] [Google Scholar]
- 37. Li Y, Xie Z, Lin W, et al. Efficacy and safety of lopinavir/ritonavir or arbidol in adult patients with mild/moderate COVID‐19: an exploratory randomized controlled trial. Med. 2020;1(1):105‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lian N, Xie H, Lin S, Huang J, Zhao J, Lin Q. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin Microbiol Infect. 2020;26(7):917‐921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu L, Shi F, Tu P, et al. Arbidol combined with the Chinese medicine Lianhuaqingwen capsule versus arbidol alone in the treatment of COVID‐19. Medicine. 2021;100(4):24475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nojomi M, Yassin Z, Keyvani H, et al. Effect of arbidol (umifenovir) on COVID‐19: a randomized controlled trial. BMC Infect Dis. 2020;20(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wen CY, Xie ZW, Li YP, et al. Real‐world efficacy and safety of lopinavir/ritonavir and arbidol in treating with COVID‐19: an observational cohort study. Zhonghua Nei Ke Za Zhi. 2020;59:E012. [DOI] [PubMed] [Google Scholar]
- 42. Xu P, Huang J, Fan Z, et al. Arbidol/IFN‐α2b therapy for patients with corona virus disease 2019: a retrospective multicenter cohort study. Microb Infect. 2020;22(4–5):200‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yu P, Li Y‐Z, Wan S‐B, Wang Y. Effects of Lianhua Qingwen Granules plus Arbidol on treatment of mild corona virus disease‐19. Chin Pharm J. 2020:1042‐1045. [Google Scholar]
- 44. Zhou Q, Chen V, Shannon CP, et al. Interferon‐α2b treatment for COVID‐19. Front Immunol. 2020;11:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xia P, Wen Y, Duan Y, et al. Prolonged SARS‐CoV‐2 viral shedding in patients with COVID‐19 was associated with delayed initiation of Arbidol treatment: a retrospective cohort study. medRxiv. 2020;31:2205‐2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhu Z, Lu Z, Xu T, et al. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID‐19. J Infect. 2020;81(1):e21‐e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen C, Zhang Y, Huang J, et al. Favipiravir versus arbidol for COVID‐19: a randomized clinical trial. medRxiv. 2020. 10.1101/2020.03.17.20037432 [DOI] [Google Scholar]
- 48. Chen W, Yao M, Fang Z, Lv X, Deng M, Wu Z. A study on clinical effect of Arbidol combined with adjuvant therapy on COVID‐19. J Med Virol. 2020;92(11):2702‐2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jie X, Hongmei Y, Ping F, Kuikui Z, Bohan Y, Rui M. Beneficial effect of Arbidol in the management of COVID‐19 infection. Aging. 2021;13(7):9253‐9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ruan X, Lu X, Wang K, et al. Liver injury after antiviral treatment of critically ill patients with COVID‐19: a single‐centered retrospective cohort study. Ann Palliat Med. 2021;10:2429‐2438. [DOI] [PubMed] [Google Scholar]
- 51. Ghaderkhani S, Khaneshan AS, Salami A, et al. Efficacy and safety of Arbidol in treatment of patients with COVID‐19 infection: a randomized clinical trial. Res Sq. 2021. 10.21203/rs.3.rs-91430/v1 [DOI] [Google Scholar]
- 52. Chen J, Lin S, Niu C, Xiao Q. Clinical evaluation of Shufeng Jiedu Capsules combined with umifenovir (Arbidol) in the treatment of common‐type COVID‐19: a retrospective study. Expert Rev Respir Med. 2020;15:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fang J, Li H, Du W, et al. Efficacy of early combination therapy with Lianhuaqingwen and Arbidol in moderate and severe COVID‐19 patients: a retrospective cohort study. Front Pharmacol. 2020;11:1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Qu X‐K, Hao S‐L, Ma J‐H, et al. Observation on clinical effect of Shufeng Jiedu Capsule combined with Arbidol Hydrochloride Capsule in treatment of COVID‐19. Chin Trad Herbal Drugs. 2020;5:1167‐1170. [Google Scholar]
- 55. Gao W, Chen S, Wang K, et al. Clinical features and efficacy of antiviral drug, Arbidol in 220 nonemergency COVID‐19 patients from East‐West‐Lake Shelter Hospital in Wuhan: a retrospective case series. Virol J. 2020;17(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Huang D, Yu H, Wang T, Yang H, Yao R, Liang Z. Efficacy and safety of umifenovir for coronavirus disease 2019 (COVID‐19): a systematic review and meta‐analysis. J Med Virol. 2020;93:481‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Levack WM, Martin RA, Graham FP, Hay‐Smith EJ. Compared to what? An analysis of the management of control groups in Cochrane reviews in neurorehabilitation. Eur J Phys Rehabil Med. 2019;55:353‐363. [DOI] [PubMed] [Google Scholar]
- 58. Yang C, Ke C, Yue D, et al. Effectiveness of Arbidol for COVID‐19 prevention in health professionals. Front Public Health. 2020;8:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. de Almeida CPB, de Goulart BNG. How to avoid bias in systematic reviews of observational studies. Rev CEFAC. 2017;19(4):551‐555. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Data Availability Statement
The data used to support the findings of this study are included within the Supporting Information file.


