Abstract
Objective
Changes of routine disease management associated with COVID‐19 lockdown might have potentially affected the clinical course of juvenile idiopathic arthritis (JIA). The aim of our study was to assess the rate of disease flare before and during COVID‐19 lockdown to investigate its impact on disease course in children with JIA.
Methods
A single‐center retrospective study was conducted, including patients presenting with inactive JIA between September 1, 2018 and March 9, 2019 (group A) and between September 1, 2019 and March 9, 2020 (group B). For each patient, demographic and clinical data were collected. The rate of JIA flare from March 10, 2019 to June 30, 2019 for group A and from March 10, 2020 to June 30, 2020 for group B was compared.
Results
Group A included 126 patients, and group B 124 patients. Statistical analysis did not show significant differences among the 2 cohorts with respect to age, sex, age at JIA onset, JIA subtype, co‐occurrence of uveitis, antinuclear antibody positivity, and past or ongoing medications. The rate of disease flare during lockdown at the time of the first COVID‐19 pandemic wave was significantly higher in comparison to the previous year (16.9% versus 6.3%; P = 0.009).
Conclusion
Our study showed that COVID‐19 lockdown was associated with a higher rate of joint inflammation in children with JIA. This finding has a considerable clinical implication, as restrictive measures may be necessary in order to contain pandemics. Our data highlight the need for rearrangement in the home and health care management of children with JIA during lockdowns.
INTRODUCTION
The first European country affected by the COVID‐19 pandemic was Italy, where the outbreak exploded in February 2020 having immediately far‐reaching health and social implications. Since the beginning of the COVID‐19 outbreak, restrictive measures were implemented to prevent the spreading of SARS–CoV‐2. During the so‐called “phase 1” of the COVID‐19 outbreak in Italy, starting on March 10, 2020, school closure was a major component of social distancing along with the shutdown of all nonessential activities, including leisure and sport. During “phase 2,” from May 4 to June 15, 2020, there was a progressive easing of the containment measures, although schools and gyms remained closed. While national and regional governments ordered the discontinuation of deferrable medical and surgical activities during phase 1, they were allowed in phase 2.
SIGNIFICANCE & INNOVATIONS.
In this population of children with juvenile idiopathic arthritis from Southern Italy, we observed that COVID‐19 lockdown was associated with a higher rate of disease flare.
Our data underlie the need for reconsidering home and health care management of children with chronic arthritis during lockdowns aimed to contain pandemics.
Children affected by juvenile idiopathic arthritis (JIA) might be considered a vulnerable population. In the first months of the COVID‐19 pandemic, JIA patients and their parents had to cope with major challenges in routine disease management, such as limiting nonessential health care visits and physical activity due to home confinement and the concerns raised by the use of immunosuppressive medications, like conventional disease‐modifying antirheumatic drugs (cDMARDs) and biologic DMARDs (bDMARDs) (1). These factors might potentially contribute to disease worsening during the pandemic. Current findings on the course of inflammatory rheumatic diseases during lockdown mainly regard adult patients (2, 3, 4), while physical effects of the pandemic on pediatric chronic arthritis (5) have not been widely reported. Therefore, we investigated the rate of JIA flare before and during COVID‐19 lockdown in order to explore its impact on disease course in children with JIA.
PATIENTS AND METHODS
A single‐center retrospective study was conducted by reviewing medical records of JIA patients admitted at the Pediatric Rheumatology Unit of the University of Naples Federico II with a minimum follow‐up duration of 6 months. All patients were diagnosed according to the International League of Associations for Rheumatology criteria (6) and were divided in 2 groups: group A (n = 126; patients with inactive disease between September 1, 2018 and March 9, 2019 [V1] and then reevaluated between March 10, 2019 and June 30, 2019 [V2]); and group B (n = 124; patients with inactive disease between September 1, 2019 and March 9, 2020 [V1] and then reevaluated between March 10, 2020 and June 30, 2020 [V2]).
Inactive disease was defined, according to the American College of Rheumatology (ACR) 2011 criteria (7), as no joint with active arthritis, no systemic manifestations due to JIA, no active uveitis, normal acute‐phase reactants, physician global assessment of disease activity (PhGA) indicating no disease activity (defined as score of 0 on a 0–10 visual analog scale), and duration of morning stiffness of <15 minutes. However, the full set of ACR 2011 criteria could not be applied before 2020 due to the limitations in the direct medical visits that precluded a PhGA. In those circumstances, when the other ACR 2011 criteria were met, the absence of disease activity was inferred through the review of the patient chart by consensus of 3 investigators (RN, RA, and MA). Also, patients evaluated with telemedicine tools during COVID‐19 lockdown and reporting no signs of active disease were included in group B (n = 31). In fact, during COVID‐19 lockdown, remote consultations (telephone or email interviews) were performed with patients' parents, investigating the occurrence of signs and symptoms consistent with JIA flare (morning stiffness, joint swelling and/or pain and/or limited range of motion). If any of those was present, in‐person consultation was ordered. Otherwise, the direct visit was deferred. For the purpose of the analysis and in agreement with Beukelman et al (8), patients were grouped in the functional phenotypes of oligoarthritis (≤4 affected joints), polyarthritis (≥5 affected joints), systemic JIA, and enthesitis‐related arthritis. Among patients with systemic JIA, only patients with a history of chronic arthritis that persisted in spite of inactive systemic features were included. In order to investigate lockdown effects only on articular symptoms in children with JIA, patients with active uveitis without active arthritis at V2 were excluded from the analysis. A subset of patients included in group A was also evaluated the following year in the same period and thus included also in group B (n = 71).
For each patient, data on demographic characteristics, JIA subtype, age at JIA onset, co‐occurrence of uveitis, antinuclear antibody (ANA) positivity, disease duration, and past therapeutic regimens were collected into a dedicated anonymized database. Date of disease onset was defined as the date when the first symptoms of arthritis were noted, as recorded in the clinical charts. For each consultation, data on the PhGA, presence of morning stiffness, presence of JIA flare, including the number and type of active joints (swelling or both tenderness and limited range of motion), erythrocyte sedimentation rate, routine out‐of‐school physical activity (defined as regular sport activity at least twice a week), and ongoing medications and therapeutic decisions at the visit were also collected. Type of consultation (in‐person or remote), missed days of school, and deferred medical visits were also recorded for patients undergoing V2 during the COVID‐19 pandemic. Medication adherence was assessed by parental report, including overall adherence (yes/no) and potential barriers. In patients experiencing flares in group B, information on contact history with COVID‐19 cases, suspected or confirmed COVID‐19 diagnosis before JIA relapse, and the results of SARS–CoV‐2 serology, if available, was also investigated and collected.
The JIA relapse rate at V2 was measured and compared between patients of group A and group B. Descriptive statistics were reported as the median and interquartile range (IQR) for continuous variables and as percentages for categorical variables. The rate of disease flare was expressed with 95% confidence intervals (95% CIs). Comparison of categorical variables between the 2 groups was performed by chi‐square test or Fisher's exact test in the case of expected frequencies of <5, whereas the Mann‐Whitney U test was used in order to compare continuous variables. All statistical tests were 2‐sided and considered significant with P values less than 0.05. The study protocol was approved by the Ethical Committee of the University of Naples Federico II (protocol number 440/20).
RESULTS
With regard to group A, 165 patients with JIA presented with inactive disease at V1; of those, 134 underwent V2. Eight subjects with systemic JIA without persistent arthritis were excluded, resulting in a cohort of 126 patients (Figure 1). With regard to group B, 178 patients presented inactive disease at V1, of those 137, underwent V2. One patient with active uveitis at V2 and 12 patients with systemic JIA without history of persistent arthritis were excluded, resulting in a cohort of 124 patients (Figure 1).
Figure 1.
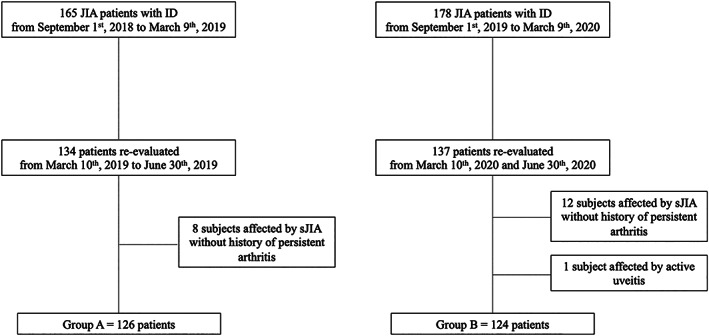
Diagram showing the composition of the patients' groups. Two groups of children with juvenile idiopathic arthritis (JIA) were enrolled, all presenting with clinically inactive disease (ID) at enrollment (V1) and then evaluated (V2) before (group A) and during (group B) the first COVID‐19 lockdown. sJIA = systemic JIA.
Looking at patients' demographic and clinical data (Table 1), in both groups, there was a predominance of female patients (77% in group A versus 75.8% in group B; P = 0.826), and oligoarticular was the most frequent functional JIA phenotype (65.8% versus 62.1%; P = 0.534). No significant difference was observed in regard to age at JIA onset, ANA positivity, and history of uveitis (Table 1). Median age at V1 was 10.9 years in both cohorts; median disease duration at V1 was 5.1 and 5.3 years in group A and B, respectively (P = 0.809). No difference was found in the ongoing JIA treatment at V1 (Table 1). Twenty of 126 patients (15.9%) presented with clinical inactive disease without medication in group A compared to 22.6% (28 of 124) subjects in group B (P = 0.178). The proportion of patients undergoing treatment with methotrexate was similar (46.8% in group A versus 37.1% in group B; P = 0.119), as well as the proportion of subjects treated with a bDMARD (43.7% versus 45.2%; P = 0.81). Among patients receiving medication, therapy was tapered or discontinued in 37.7% of patients in group A and 33.3% in group B at V1 (P = 0.514). The proportion of children participating in out‐of‐school physical activities at V1 was ~54% in both cohorts (Table 1). Altogether, these data suggest that clinical and demographic features at baseline did not differ between the 2 groups of patients.
Table 1.
Baseline characteristics of study patients*
| Characteristic | Group A | Group B | P † |
|---|---|---|---|
| (n = 126) | (n = 124) | ||
| Sex, female | 97 (77) | 94 (75.8) | 0.826 |
| Age at JIA onset, median (IQR) years | 4 (2.2–6.8) | 4.2 (2.0–6.9) | 0.71‡ |
| Age at V1, median (IQR) years§ | 10.9 (7.8–14.4) | 10.9 (8.0–14.4) | 0.933‡ |
| Disease duration at V1, median (IQR) years§ | 5.1 (3.2–8.6) | 5.3 (2.7–8.5) | 0.809‡ |
| JIA subtype | |||
| Oligoarticular | 83 (65.8) | 77 (62.1) | 0.534 |
| Polyarticular | 35 (27.8) | 41 (33.1) | 0.364 |
| Systemic | 7 (5.6) | 4 (3.2) | 0.369 |
| ERA | 1 (0.8) | 2 (1.6) | 0.62¶ |
| ANA positivity | 58 (46) | 52 (41.9) | 0.514 |
| History of uveitis | 28 (22.2) | 26 (21) | 0.81 |
| Past JIA treatment | |||
| Intraarticular glucocorticoid injections | 45 (35.7) | 40 (32.3) | 0.564 |
| Systemic glucocorticoids | 21 (16.7) | 18 (14.5) | 0.639 |
| Methotrexate | 54 (42.9) | 65 (52.4) | 0.13 |
| Other conventional DMARDs | 3 (2.4) | 2 (1.6) | 1.0¶ |
| Biologic DMARDs | 14 (11.1) | 16 (12.9) | 0.663 |
| Ongoing JIA treatment at V1§ | |||
| NSAIDs | 17 (13.5) | 8 (6.5) | 0.064 |
| Systemic glucocorticoids | 0 | 1 (0.8) | 0.496¶ |
| Methotrexate | 59 (46.8) | 46 (37.1) | 0.119 |
| Sulfasalazine | 1 (0.8) | 2 (1.6) | 0.62¶ |
| Biologic DMARDs | 55 (43.7) | 56 (45.2) | 0.81 |
| Etanercept | 28 (22.2) | 27 (21.8) | 0.932 |
| Adalimumab | 15 (11.9) | 14 (11.3) | 0.879 |
| Infliximab | 2 (1.6) | 3 (2.4) | 0.682¶ |
| Tocilizumab | 7 (5.6) | 9 (7.3) | 0.582 |
| Canakinumab | 0 | 1 (0.8) | 0.496¶ |
| Abatacept | 3 (2.4) | 2 (1.6) | 1.0¶ |
| Off‐therapy | 20 (15.9) | 28 (22.6) | 0.178 |
| Out‐of‐school physical activity in the last month, no./total no. (%)# | 50/91 (54.9) | 50/92 (54.3) | 0.9 |
Values are the number (%) unless indicated otherwise. ANA = antinuclear antibody; DMARDs = disease‐modifying antirheumatic drugs; ERA = enthesitis‐related arthritis; IQR = interquartile range; JIA = juvenile idiopathic arthritis; NSAIDs = nonsteroidal antiinflammatory drugs.
By chi‐square test unless otherwise specified.
By Mann‐Whitney U test.
V1 frame was from September 1, 2018 to March 9, 2019 in group A; and from September 1, 2019 to March 9, 2020 in group B.
By Fisher's exact test.
Data on sports activity outside school were available for 91 patients in group 1 and for 92 patients in group 2.
Due to discontinuation of deferrable medical activities, 31 of 124 (25%) patients in group B were evaluated only through a remote consultation at V2; 31 (25%) had their appointment postponed for over a month. At V2, no significant difference was found with respect to the ongoing JIA treatment between the 2 cohorts (Table 2). Temporary drug interruptions for >1 week were reported in 5 of 81 (6.2%) in group B, 4 of which were unrelated to COVID‐19. One patient delayed her monthly tocilizumab infusions without medical advice due to fear of being infected but did not develop a flare. The parents of another 10 children expressed worries about continuing drugs for JIA during the pandemic but did not report drug discontinuation. Data on physical activity were available for 77 patients in group A: 48 (62.3%) practiced regular sports activity at V2 in comparison to 4 of 110 (3.6%) in group 2 (P < 0.00001). Indeed, 53 of 57 patients (93%) practicing out‐of‐school physical activity prior to the lockdown had interrupted it for at least 1 month at V2 due to restrictive measures. In addition, patients of group B had not been attending school for a median time of 89.5 days (IQR 71.0–106.7).
Table 2.
Relapse rate and therapeutic regimens in group A and group B at V2*
| Group A | Group B | P † | |
|---|---|---|---|
| (n = 126) | (n = 124) | ||
| Patients with JIA relapse at V2 | 8 (6.3) | 21 (16.9) | 0.009‡ |
| Ongoing JIA treatment at V2 | |||
| NSAIDs | 6 (4.8) | 1 (0.8) | 0.120§ |
| Oral glucocorticoids | 0 | 0 | |
| Methotrexate | 45 (35.7) | 35 (28.2) | 0.204 |
| Sulfasalazine | 1 (0.8) | 2 (1.6) | 0.62§ |
| Biologic DMARDs | 53 (42.1) | 51 (41.1) | 0.881 |
| Etanercept | 28 (22.2) | 26 (21) | 0.810 |
| Adalimumab | 14 (11.1) | 10 (8.1) | 0.414 |
| Infliximab | 1 (0.8) | 3 (2.4) | 0.368§ |
| Tocilizumab | 7 (5.6) | 9 (7.3) | 0.582 |
| Canakinumab | 0 | 1 (0.8) | 0.496§ |
| Abatacept | 3 (2.4) | 2 (1.6) | 1.0§ |
| Off therapy | 36 (28.6) | 43 (34.7) | 0.299 |
| Therapeutic decision at V2 | |||
| Prescription of a new drug | 8 (6.3) | 19 (15.3) | 0.022‡ |
| Continuation of ongoing therapy, no./total no. (%) | 57/90 (63.3) | 50/81 (61.7) | 0.829 |
| Drug dosage increase, no./total no. (%) | 3/90 (3.3) | 4/81 (4.9) | 0.709§ |
| Drug tapering or 1 drug discontinuation, no./total no. (%)¶ | 25/90 (27.8) | 15/81 (18.5) | 0.153 |
| Therapy withdrawal, no./total no. (%) | 3/90 (3.3) | 4/81 (4.9) | 0.709§ |
| Out‐of‐school physical activity in the last month, no./total no. (%)# | 48/77 (62.3) | 4/110 (3.6) | <0.00001‡ |
Values are the number (%) unless indicated otherwise. V2 frame was from March 10, 2019 to June 30, 2019 in group A; and from March 10, 2020 to June 30, 2020 in group B. DMARDs = disease‐modifying antirheumatic drugs; JIA = juvenile idiopathic arthritis; NSAIDs = nonsteroidal antiinflammatory drugs.
By chi‐square test unless otherwise specified.
Significant.
By Fisher's exact test.
In case of combined medications regimens.
Data on sports activity outside school were available for 77 patients in group 1 and for 110 patients in group 2.
The rate of relapse was statistically significantly higher in group B (21 of 124, 16.9% [95% CI 10.8–24.7%]) in comparison to group A (8 of 126, 6.3% [95% CI 2.8–12.1%]) (P = 0.009) (Table 2). In fact, a new drug was started in 15.3% of patients of group B compared to 6.3% of group A (P = 0.022), while the proportion of patients who underwent therapy tapering or discontinuation at V2 was only slightly lower in group B (15 of 81, 18.5% versus 25 of 90, 27.8%; P = 0.153). In more detail, with regard to patients experiencing flares in group B, 16 patients started an NSAID, 4 a new cDMARD or bDMARD, while 3 underwent glucocorticoid joint injection(s), and 3 of 10 required an increased dosage of the ongoing DMARD therapy (see Supplementary Table 1, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24768).
When considering medication adherence, 11 of 21 relapsing patients in group B were receiving medication at V2. None of these patients reported temporary therapeutic interruptions compared to 5 of 70 children with inactive disease (0% versus 7.1%; P > 0.05). The face‐to‐face visit had been postponed for >1 month in 33.3% of patients who had relapsed (7 of 21), which is the same as for patients presenting with inactive disease (24 of 72, 33.3%; P = 1). Data on out‐of‐school physical activity were available in 18 patients with JIA flare in group 2: 12 of them had interrupted physical activity due to COVID‐19 lockdown, and 6 did not practice sports before the COVID‐19 pandemic. Of note, none of the patients experiencing flares had either a suspected or confirmed COVID‐19 diagnosis or a COVID‐19 exposure, and 5 of them had a negative SARS–CoV‐2 serology finding in June 2020.
When comparing patients who had relapsed among the 2 groups, no differences in demographic and clinical features at V2 were found (see Supplementary Table 1, available at http://onlinelibrary.wiley.com/doi/10.1002/acr.24768). Notably, ankle arthritis was slightly more frequent in group B (38% versus 0%; P = 0.066).
DISCUSSION
To our knowledge, this study presented the largest pediatric JIA cohort in which the effects of COVID‐19 lockdown on disease course were investigated. Our data showed that more JIA patients experienced a disease flare during home confinement due to the SARS–CoV‐2 pandemic compared to the same period of the previous year, supporting our hypothesis that containment measures during COVID‐19 lockdown negatively impacted disease activity.
In contrast to the data published so far about the impact of the COVID‐19 pandemic on the course of inflammatory rheumatic diseases in adults (2, 3, 4), mostly based on patient‐reported data, in our study, disease flare assessment required physician evaluation, thus increasing the strength of our findings. While Ciurea and colleagues found no detrimental impact of containment measures on disease course in 666 patients with spondyloarthritis (SpA), rheumatoid arthritis, or psoriatic arthritis (3), Roux et al observed a significant difference in the rate of severe disease flare in 512 SpA patients before and during home confinement (20% versus 49%) (2). So far, only 1 study reported an increase of JIA flares in a small cohort of 58 children during March to July 2020 (5), in agreement with our findings. The higher relapse rate reported by these 2 latter studies was mainly attributed to changes of treatment regimens due to concerns about COVID‐19 (2, 5). Recently, a large survey did not reveal a decrease in therapy compliance during the first months of the pandemic in ~4,000 patients with rheumatic diseases (9). Accordingly, in our cohort, only 1 patient delayed the scheduled treatment due to apprehension of SARS–CoV‐2 infection, down‐sizing the possible impact of the pandemic outbreak on treatment adherence and thus on disease course. During lockdown, we remotely recommended patients to continue all therapies as usual, as suggested by the Paediatric Rheumatology European Association in March 2020 (10). This reassurance campaign might have limited the impact of COVID‐19–related fears on therapeutic compliance. Yet, a role of decreased drug adherence on disease activity during lockdown could not be entirely excluded, as it was not measured through a validated tool.
During COVID‐19 lockdown, children spent less time engaged in physical activity, with a parallel increase in sleeping and TV or video watching/playing time (11, 12). These lifestyle modifications may impact on daily life of patients with chronic diseases (13) and possibly contribute to a higher flare rate in children with JIA. As expected, in our population, the proportion of patients performing regular physical activity was significantly lower during the COVID‐19 pandemic compared to the previous year. In addition, the children with JIA in our study had not been attending school for ~3 months at the time of consultation. It is well‐known that arthritis symptoms worsen in the morning or after prolonged rest (14) and that physical therapy may lead to pain reduction and increased range of motion in JIA patients (15). Indeed, along with medications, exercise is recommended as a therapeutic tool to children and adolescents with JIA in order to counteract the disease‐related inflammation and improve clinical symptoms (16). Besides, it has been shown that peripheral blood lymphocytes of less active children present a proinflammatory profile, suggesting that physical activity may decrease systemic inflammatory responses (17). Therefore, the physical inactivity associated with home confinement could be a possible explanation for clinical worsening in our patients. On this basis, we believe that prescription of home‐based exercise programs conducted by a physical therapist should be promoted to implement JIA management in case of public lockdowns.
The temporary interruption of nonessential health care in‐person consultations during the “phase 1” of the COVID‐19 pandemic might have led to delays in patients' management; however, the proportion of delayed face‐to‐face visits was the same in patients with or without arthritis relapse, suggesting that limitations in outpatient rheumatology medical service were not a main contributor to the worsening of JIA in our cohort. As a matter of fact, outpatient in‐person visits were postponed only if parents reported no signs or symptoms consistent with JIA relapse at the telemedicine call. Even though recent data suggest that telemedicine alone may be insufficient to guide a treat‐to‐target strategy (18), the use of telehealth tools might have limited the impact of the partial closure of ambulatory services on disease management according to other reports (19). From this point of view, the development of validated telemedicine models for JIA may be critical to guarantee effective management of JIA in case of confinement measures and to monitor disease activity at home.
Our findings should be interpreted within the limitations of the study, which are mainly inherent to its observational and retrospective nature. Besides, our results reflect a single tertiary care center experience, so they may not be extended to other clinical settings. Since our study was not randomized and observational, we cannot exclude that patients in group B presented a more aggressive disease than those in group A. Likewise, the slightly higher number of patients off‐therapy in group B may represent a possible confounding factor in our analysis. Nevertheless, the comparison of the 2 cohorts showed homogeneity in regard to demographic and clinical features. Finally, since subtle signs of active arthritis might have been underrecognized and not reported during telemedicine, the relapse rate during lockdown could be even potentially higher than observed.
In conclusion, this study provides new evidence that COVID‐19 lockdown was associated with a higher rate of relapse in children with JIA, even in the absence of reduced drug adherence. This finding has considerable clinical implications because restrictive measures are still occurring in several countries as the pandemic evolves. Our data highlight the need for implementing health care management of patients with JIA, including personalized at‐home exercise programs in case of new lockdowns.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Alessio had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Naddei, Alessio.
Acquisition of data
Naddei, Alfani, Bove, Mozzillo.
Analysis and interpretation of data
Naddei, Alfani, Discepolo, Guarino, Alessio.
Supporting information
Disclosure Form
Supplementary Table 1 Clinical features of flaring patients at V2.
ACKNOWLEDGMENTS
Open Access Funding provided by Universita degli Studi di Napoli Federico II within the CRUI‐CARE Agreement.
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Facr.24768&file=acr24768‐sup‐0001‐Disclosureform.pdf.
REFERENCES
- 1. Michaud K, Wipfler K, Shaw Y, et al. Experiences of patients with rheumatic diseases in the United States during early days of the COVID‐19 pandemic. ACR Open Rheumatol 2020;2:335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roux CH, Brocq O, Gerald F, et al. Impact of home confinement during the COVID‐19 pandemic on medication use and disease activity in spondyloarthritis patients [letter]. Arthritis Rheumatol 2020;72:1771–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ciurea A, Papagiannoulis E, Bürki K, et al. Impact of the COVID‐19 pandemic on the disease course of patients with inflammatory rheumatic diseases: results from the Swiss Clinical Quality Management cohort. Ann Rheum Dis 2021;80:238–41. [DOI] [PubMed] [Google Scholar]
- 4. Maldonado D, Tu E, Mahmood SN, et al. Association of medication access difficulty and COVID‐19–related distress with disease flares in rheumatology patients during the COVID‐19 pandemic. Arthritis Care Res (Hoboken) 2021;73:1162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Conti G, Galleta F, Carucci NS, et al. Negative effect of lockdown on juvenile idiopathic arthritis patients. Clin Rheumatol 2021;20:3723–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Petty RE, Southwood TR, Manners P, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004;31:390–2. [PubMed] [Google Scholar]
- 7. Wallace CA, Giannini EH, Huang B, et al. American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2011;63:929–36. [DOI] [PubMed] [Google Scholar]
- 8. Beukelman T, Patkar NM, Saag KG, et al. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res (Hoboken) 2011;63:465–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hasseli R, Müller‐Ladner U, Keil F, et al. The influence of the SARS‐CoV‐2 lockdown on patients with inflammatory rheumatic diseases on their adherence to immunomodulatory medication: a cross sectional study over 3 months in Germany. Rheumatology (Oxford) 2021;60:S151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paediatric Rheumatology European Association . Updated PRES recommendations for coronavirus outbreak. 2020. URL: https://www.pres.eu/news/newsstory.html?id=29.
- 11. Pietrobelli A, Pecoraro L, Ferruzzi A, et al. Effects of COVID‐19 lockdown on lifestyle behaviors in children with obesity living in Verona, Italy: a longitudinal study. Obesity (Silver Spring) 2020;28:1382–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Orgilés M, Morales A, Delvecchio E, et al. Immediate psychological effects of the COVID‐19 quarantine in youth from Italy and Spain. Front Psychol 2020;11:579038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saqib MA, Siddiqui S, Qasim M, et al. Effect of COVID‐19 lockdown on patients with chronic diseases. Diabetes Metab Syndr 2020;14:1621–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crayne CB, Beukelman T. Juvenile idiopathic arthritis: oligoarthritis and polyarthritis. Pediatr Clin North Am 2018;65:657–74. [DOI] [PubMed] [Google Scholar]
- 15. Long AR, Rouster‐Stevens KA. The role of exercise therapy in the management of juvenile idiopathic arthritis. Curr Opin Rheumatol 2010;22:213–7. [DOI] [PubMed] [Google Scholar]
- 16. Catania H, Fortini V, Cimaz R. Physical exercise and physical activity for children and adolescents with juvenile idiopathic arthritis: a literature review. Pediatr Phys Ther 2017;29:256–60. [DOI] [PubMed] [Google Scholar]
- 17. Merlin M, de Oliveira HH, Passos ME, et al. Relationship between children physical activity, inflammatory mediators and lymphocyte activation: possible impact of social isolation (COVID‐19). Sport Sci Health 2021;17:431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boone NW, Sepriano A, van der Kuy PH, et al. Routine Assessment of Patient Index Data 3 (RAPID3) alone is insufficient to monitor disease activity in rheumatoid arthritis in clinical practice. RMD Open 2019;5:e001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. López‐Medina C, Escudero A, Collantes‐Estevez E. COVID‐19 pandemic: an opportunity to assess the utility of telemedicine in patients with rheumatic diseases. Ann Rheum Dis 2021;80:e50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form
Supplementary Table 1 Clinical features of flaring patients at V2.


