Abstract
Understanding how β adrenergic agonists influence the physiology of heat stress could lead to mitigation options. We sought to investigate body surface temperatures in feedlot wethers supplemented with ractopamine or zilpaterol and exposed to heat stress for 18 d. Corneal and skin temperatures were assessed via infrared thermography at 1- and 2-m distances. Rectal temperatures and circulating leukocytes, metabolites, and electrolytes were also measured. Heat stress increased (P < 0.05) rectal temperatures in unsupplemented and zilpaterol-supplemented lambs but not in ractopamine-supplemented lambs. Heat stress also increased (P < 0.05) surface temperatures of the cornea, nose, ear, and back, regardless of supplement. Observations were comparable between thermography performed at 1 and 2 m, and higher emissivity settings generally produced less variation. Heat stress tended to increase (P = 0.08) blood monocytes in unsupplemented but not ractopamine- or zilpaterol-supplemented lambs. Granulocytes were increased (P < 0.05) by heat stress in ractopamine-supplemented lambs but decreased (P < 0.05) in zilpaterol-supplemented lambs. Blood glucose, triglycerides, and cholesterol did not differ among groups, and blood lactate was reduced (P < 0.05) by heat stress in zilpaterol-supplemented lambs only. Blood Na+ was reduced (P < 0.05) and Ca2+ increased (P < 0.05) by heat stress, regardless of supplement. These findings indicate that β1- and β2-adrenergic agonists differentially relieve some but not all heat stress-induced changes in stress indicators. Moreover, corneal and skin surface temperatures measured by infrared thermography reasonably identified body temperature changes at a distance of 2 m.
Keywords: animal health, β-adrenergic agonists, environmental stress, thermogenesis
Introduction
Chronic heat stress decreases growth and metabolic efficiency in livestock (Barnes et al., 2019; Swanson et al., 2020), which reduces profitability and capacity for food production. Conversely, growth promoters like β-adrenergic agonists improve growth performance and efficiency (Buntyn et al., 2016). Physiological responses to environmental heat stress include increases in circulating catecholamines (Swanson et al., 2020), which stimulate adrenergic pathways including those activated by β-agonist supplements (Navegantes et al., 2002). However, research regarding how the use of β agonists impacts the response to heat stress is sparse and inconsistent. For example, zilpaterol supplementation increased respiration but decreased body temperature in cattle housed without shade (Boyd et al., 2015), and ractopamine supplementation in sheep reduced the impact of heat stress on cardiovascular metrics and delayed the heat stress-induced increase in circulating TNFα (Swanson et al., 2020). In addition, recent studies have demonstrated that infrared thermography performed over short distances (i.e., less than 1 m) is an effective method for determining surface temperatures (Feng et al., 2019; Lowe et al., 2020). Moreover, one study in immune-challenged tropical sheep found that temperatures measured across the surface of the eye from a distance of 30 cm correlated with rectal temperatures at a remarkable coefficient of 0.87, and that the two identified febrile responses similarly and exhibited comparable variability (George et al., 2014). These studies provide a strong basis for infrared thermography as a no-touch option for assessing body temperature changes. However, they were performed at distances that would typically encroach upon an animal’s flight zone (Grandin, 1989), and thus, we were interested in performing thermography at a moderately greater distance. The present study is part of a larger project with the objective of understanding how β-agonist supplements influence the impact of heat stress on animal well-being and growth efficiency. Here, our objective was to focus specifically on temperature changes measured on the external surface of the face and body and how they relate to physiological responses after 18 d of heat stress (which would be considered chronic) and β-agonist supplementation.
Materials and Methods
Animals and experimental design
This study was approved by the Institutional Animal Care and Use Committee at the University of Nebraska–Lincoln. Studies were performed at the university’s Animal Science Complex, which is accredited by AAALAC International. This study was performed on a subset of the Rambouillet x Columbia crossbred wether lambs during a project that was described in detail previously (Barnes et al., 2019). Briefly, lambs were individually penned in indoor climate-controlled rooms and randomly assigned to be fed an ad libitum corn-based feedlot ration for 21 d under thermoneutral (average THI = 62.5) or heat stress conditions (average THI = 84). Actual ambient temperatures at the time the present study was performed (i.e., when blood samples and thermal images were taken) were THI = 63.2, environmental temperature = 19.8 °C, and relative humidity = 16.2% for controls (n = 12) and were THI = 81.4, environmental temperature = 35.6 °C, and relative humidity = 31.0% for heat-stressed lambs (n = 12). In a 2 × 3 factorial design, lambs were also supplemented with no additive (i.e., unsupplemented), 39.96 mg/d ractopamine HCl, or 25 mg/d zilpaterol HCl, which was mixed into their daily ad libitum ration. These doses were chosen to reflect the approved doses for feedlot cattle, as no sheep-specific dosage has been established and lambs were used as a model for beef cattle. Blood samples (6 mL) were collected via jugular venipuncture and body temperatures were assessed at 1500 h on day 18, which was 7 h after feeding. Lambs were briefly restrained by hand for blood collection and temperature assessment.
Body temperature assessment
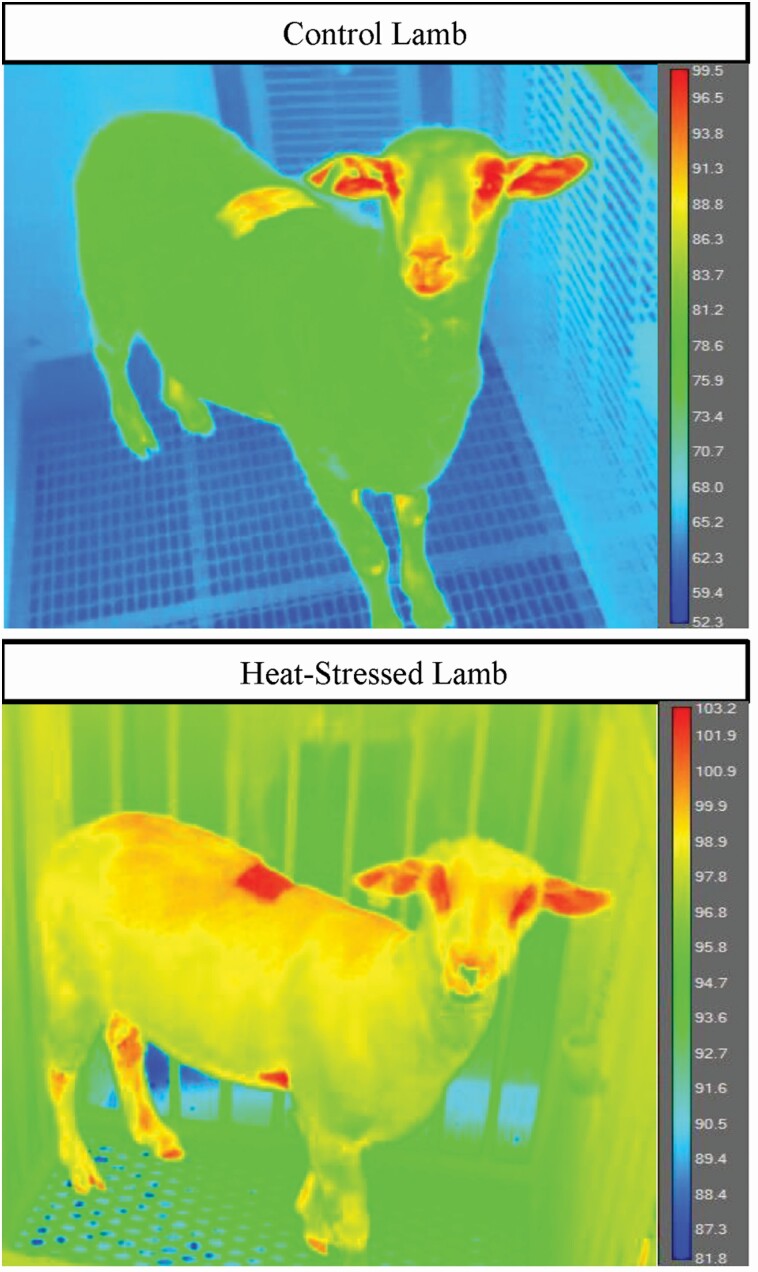
Rectal temperatures were determined by the average readings of two commercial digital thermometers. Three infrared images of each lamb were taken at or near a directly straight angle relative to the animal’s face (i.e., forward-facing) from distances of 1 and 2 m as described previously (Cadaret et al., 2021). Briefly, images were captured with an A655sc IR camera (FLIR Systems Inc., Wilsonville, OR) at an emissivity setting of 0.95 and were analyzed using FLIR ResearchIR Max (FLIR Systems Inc.). Maximal temperatures for the center of the eye surface (i.e., cornea), the central area between the nostrils (i.e., nose), and the center of the ear were recorded and averaged across the three images captured at each distance. Examples of infrared images are shown in Figure 1. Body surface temperatures were assessed with a pair of hand-held infrared thermometer guns (Model FB61354, Fisher Scientific, Pittsburgh, PA and Model TN418LD, Metris Instruments, Los Gatos, CA). Ten-second average temperatures were collected from a distance of 2 m for shorn and unshorn (i.e., ~3 cm wool length) sections of the loin area over the last rib. For each area, temperatures were determined across a range of emissivity values (i.e., the efficiency at which a surface emits thermal energy) from 0.40 to 1.00 and averaged between the two infrared guns. For all thermographic measurements, lambs were left unrestrained in their individual 2-m2 pens.
Figure 1.
Representative thermographic images for thermoneutral (controls) and heat-stressed wether lambs captured with a FLIR A655sc IR camera at an emissivity of 0.95 and at a distance of 2 m.
Blood analyses
Blood samples were collected into EDTA vacutainer tubes for hematology and blood counts and into heparinized syringes for blood gas analyses as described previously (Cadaret et al., 2019; Swanson et al., 2020). Total white blood cells, lymphocytes, monocytes, granulocytes, red blood cells, platelets, hematocrit, hemoglobin, mean corpuscular volumes, red blood cell width distributions, mean corpuscular hemoglobin concentrations, and mean packed cell volumes were determined from 125 µL of venous blood with a HemaTrue Veterinary Chemistry Analyzer (Heska, Loveland, CO). Blood glucose, lactate, HCO3, pH, O2 partial pressures (pO2), CO2 partial pressure (pCO2), Na+, K+, and Ca2+ were determined from 90 μL of venous blood with an ABL90 FLEX blood gas analyzer (Radiometer, Copenhagen, Denmark). Blood plasma triglycerides and cholesterol concentrations were determined with a Vitros-250 Chemistry Analyzer (Ortho Clinical Diagnostics, Linden, NJ) by the University of Nebraska Biomedical Obesity Research Core.
Statistical analysis
All data were analyzed by ANOVA using the mixed procedure of SAS 9.4 (SAS Institute, Cary, NC) to determine the effects of environmental condition, supplement, and their interactions in a 2 × 3 factorial. Appropriate covariance structures were selected based on best-fit statistics. Lamb was considered the experimental unit. Where necessary, means were separated with Fisher’s LSD test. Technical replicates for corneal and body temperatures were averaged for each lamb. The threshold for significance was P ≤ 0.05, and tendencies were noted when P ≤ 0.10. All data are presented as means ± standard error.
Results
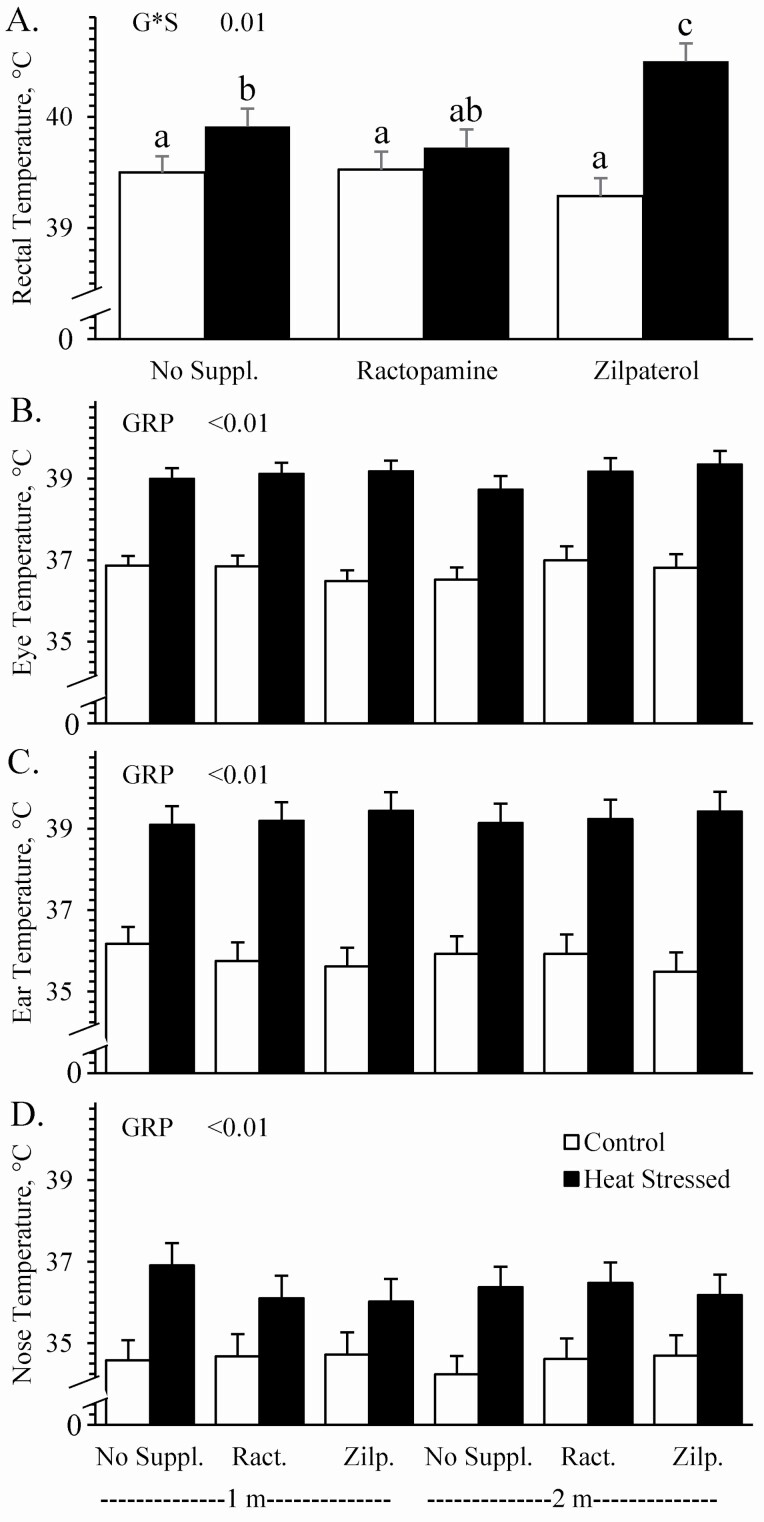
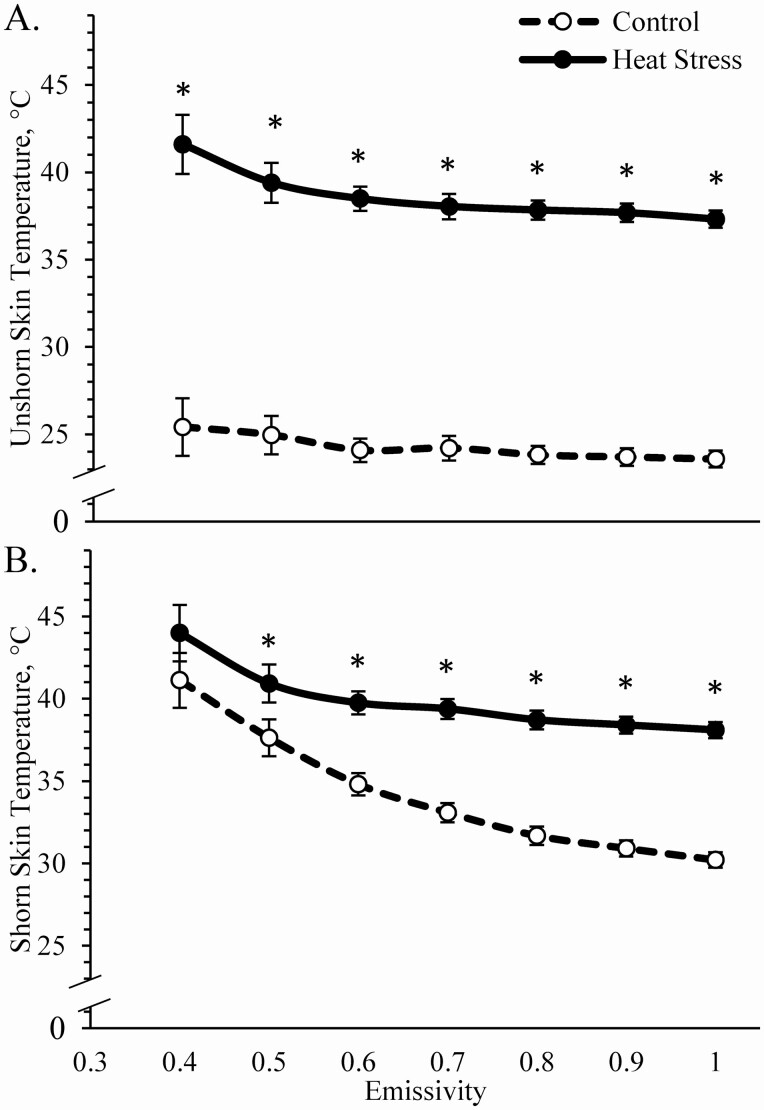
Environmental group x supplement interactions were observed (P < 0.05) for rectal temperatures, but not for any surface temperatures. Rectal temperatures did not differ among thermoneutral controls regardless of supplement (Figure 2A). Heat stress increased (P < 0.05) rectal temperatures for unsupplemented and zilpaterol-supplemented lambs but not for ractopamine-supplemented lambs. Whether measured at 1 or 2 m, corneal temperatures (Figure 2B), ear temperatures (Figure 2C), and nose temperatures (Figure 2C) assessed by infrared camera were greater (P < 0.05) for heat-stressed lambs than for controls, regardless of supplement. Surface temperatures across the unshorn loin area were greater (P < 0.05) for heat-stressed lambs than for controls when measured at an emissivity higher than 0.40, regardless of supplement (Figure 3A). Temperatures across the shorn loin were greater (P < 0.05) for heat-stressed lambs than for controls when measured at an emissivity higher than 0.50, regardless of supplement (Figure 3B). Spearman correlation coefficients for body temperature assessments are provided in Table 1. Corneal and ear temperatures measured by an IR camera were closely correlated with ambient temperature, ambient relative humidity, and ambient temperature–humidity index but were less strongly correlated with rectal temperatures. Nose temperatures measured by the IR camera were less strongly correlated with ambient and rectal temperatures than were corneal and ear temperatures. In general, there were little or no differences in correlation coefficients when IR images were captured at 2 m rather than 1 m. Shorn and unshorn skin temperatures were closely correlated with ambient temperature, humidity, and temperature–humidity index, but less strongly correlated with rectal temperatures.
Figure 2.
Rectal and facial temperatures in heat-stressed wether lambs supplemented with ractopamine or zilpaterol. Data are shown for rectal temperatures measured with digital thermometers (A) and for corneal (B), ear (C), and nose (D) temperatures measured with an infrared camera at distances of 1 and 2 m. Effects of experimental group (GRP), supplement, and the interaction (G*S) were evaluated and are noted where significant (P < 0.05). a,b,cMeans with different superscripts differ (P < 0.05).
Figure 3.
Skin temperatures in heat-stressed wether lambs supplemented with ractopamine or zilpaterol. Data are shown for unshorn skin (A) and shorn skin (B) temperatures measured by hand-held infrared guns at emissivity of 0.4 to 1.0. No effects of supplement or interaction (G*S) were observed, and thus only main effects of environmental group are shown. *Means differ (P < 0.05) within the emissivity level.
Table 1.
Spearman correlation coefficients1 for body temperatures in heat-stressed feedlot lambs supplemented with ractopamine or zilpaterol for 18 d
| Temperature | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Corneal (IR camera2) | Ear (IR camera2) | Nose (IR camera2) | Shorn skin (IR guns3) | Unshorn skin (IR guns3) | |||||
| Measurement | Rectal (Digital) | 1 m | 2 m | 1 m | 2 m | 1 m | 2 m | ||
| Ambient temperature | 0.61 | 0.83 | 0.89 | 0.90 | 0.91 | 0.77 | 0.75 | 0.89 | 0.90 |
| RH | 0.51 | 0.73 | 0.70 | 0.77 | 0.76 | 0.65 | 0.62 | 0.72 | 0.71 |
| THI | 0.63 | 0.81 | 0.85 | 0.91 | 0.92 | 0.76 | 0.72 | 0.92 | 0.91 |
| Rectal temperature | 0.55 | 0.59 | 0.66 | 0.66 | 0.36 | NS | 0.71 | 0.65 |
1Spearman correlation coefficients were calculated across all 24 lambs.
2A655sc IR camera (FLIR Systems Inc.).
3Average of two hand-held IR guns: Model FB61354 (Fisher Scientific) and Model TN418LD (Metris Instruments).
IR, infrared; RH, relative humidity; THI, temperature–humidity index.
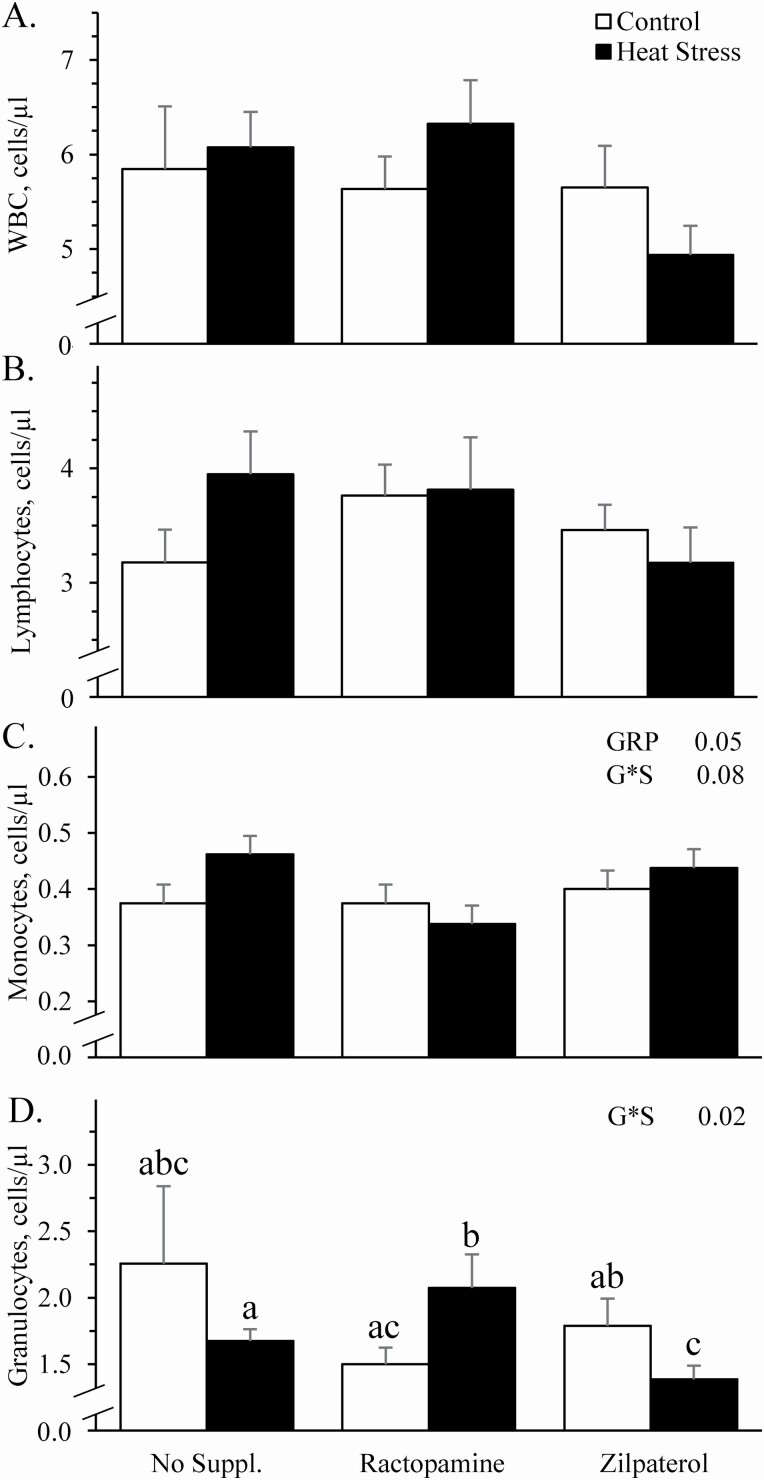
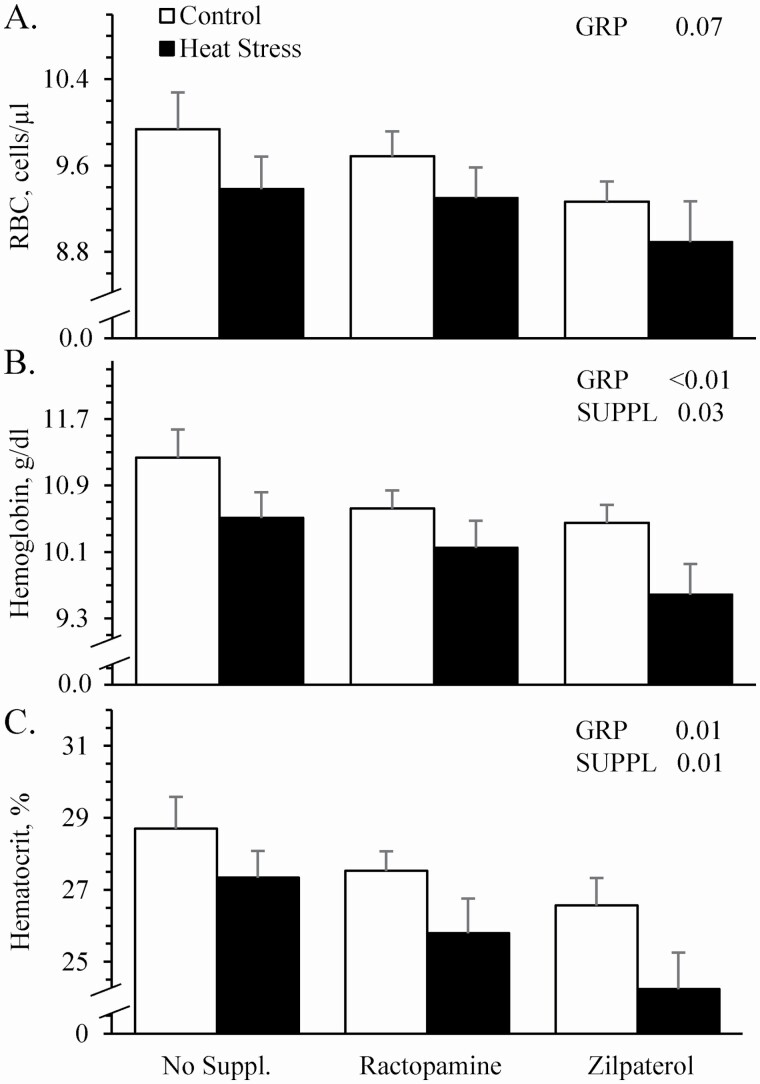
Environmental group x supplement interactions were observed (P < 0.05) for granulocyte concentrations and tended to be observed (P = 0.08) for monocyte concentrations but were not observed for total white blood cells, lymphocytes, or any hematology component. Circulating concentrations of total white blood cells (Figure 4A) and lymphocytes (Figure 4B) did not differ between controls and heat-stressed lambs or among unsupplemented, ractopamine-supplemented, or zilpaterol-supplemented groups. Circulating concentrations of monocytes were greater (P ≤ 0.05) for heat-stressed lambs than for controls across all lambs (Figure 4C). When evaluated by supplemental group based on the tendency for an environment x supplement interaction, monocytes tended to be greater (P = 0.08) for heat-stressed lambs compared to controls when unsupplemented but did not differ between environmental groups when supplemented with ractopamine or zilpaterol. Circulating concentrations of granulocytes did not differ among controls regardless of supplement but were greater (P < 0.05) for ractopamine-supplemented heat-stressed lambs and were less (P < 0.05) for zilpaterol-supplemented heat-stressed lambs compared to unsupplemented heat-stressed lambs (Figure 4D). Circulating red blood cell concentrations (Figure 5A) tended to be less (P = 0.07) and hemoglobin concentrations and hematocrit (Figure 5B and C, respectively) were less (P < 0.05) for heat-stressed lambs than for controls, regardless of supplement. Hemoglobin concentrations and hematocrit were also less (P < 0.05) for ractopamine-supplemented and zilpaterol-supplemented lambs compared to unsupplemented lambs, regardless of environment. Mean corpuscular volume (28.7 ± 0.3 vs. 28.1 ± 0.4 fl, respectively), mean corpuscular hemoglobin concentrations (39.1 ± 0.2 vs 39.2 ± 0.2 g/dL, respectively), platelet concentrations (225 ± 8 vs. 222 ± 10 no./µL, respectively), and mean packed cell volumes (5.18 ± 0.04 vs. 5.18 ± 0.05 fl, respectively) did not differ between controls and heat-stressed lambs. They also did not differ due to supplement.
Figure 4.
Circulating leukocyte concentrations in heat-stressed wether lambs supplemented with ractopamine or zilpaterol. Data are shown for total white blood cells (WBC; A), lymphocytes (B), monocytes (C), and granulocytes (D). Effects of experimental group (GRP), supplement, and the interaction (G*S) were evaluated and are noted where significant (P < 0.05) or tending toward significant (P < 0.10). a,b,cMeans with different superscripts differ (P < 0.05).
Figure 5.
Hematology indicators in heat-stressed wether lambs supplemented with ractopamine or zilpaterol. Data are shown for red blood cell (RBC) concentrations (A), hemoglobin concentrations (B), and hematocrit (C). Effects of experimental group (GRP), supplement (SUPPL), and the interaction were evaluated and are noted where significant (P < 0.05).
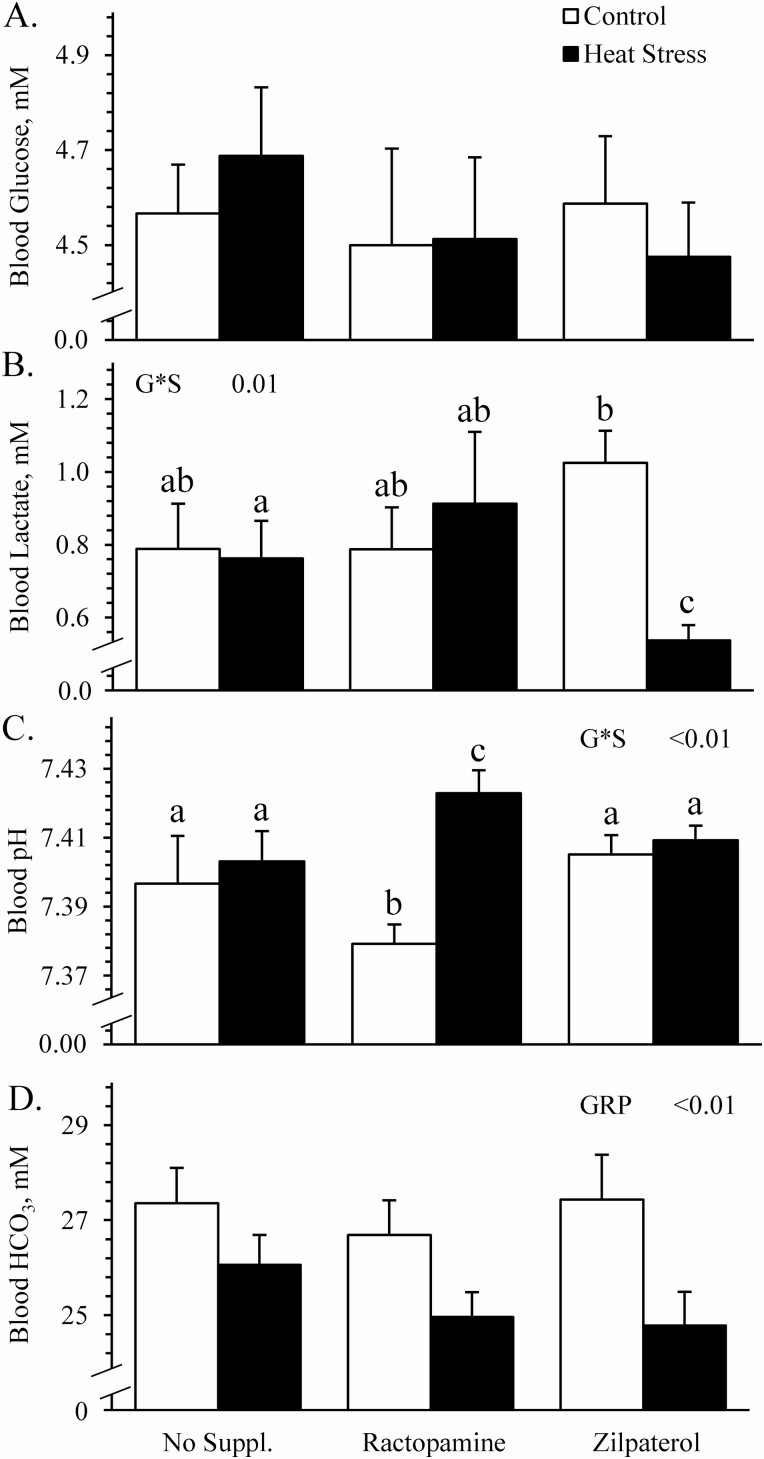
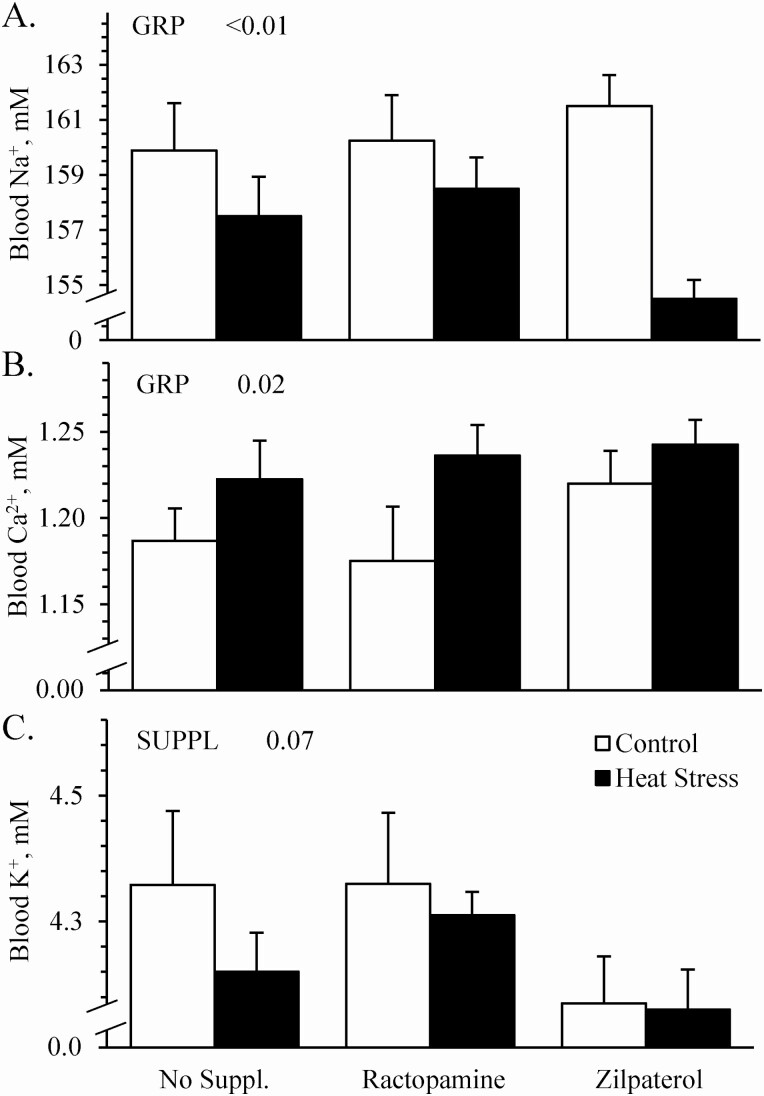
Environmental group x supplement interactions were observed (P < 0.05) for blood lactate and pH but not for triglycerides, cholesterol, glucose, pO2, pCO2, HCO3, Na+, K+, or Ca2+. Blood glucose concentrations (Figure 6A), blood plasma triglyceride concentrations (17.8 ± 1.1 vs. 15.8 ± 1.7 mg/dL, respectively), and blood plasma cholesterol concentrations (64.8 ± 3.4 vs. 58.1 ± 3.3 mg/dL, respectively) did not differ between controls and heat-stressed lambs. They also did not differ due to supplement. Blood lactate concentrations did not differ among controls regardless of supplement but were less (P < 0.05) for zilpaterol-supplemented heat-stressed lambs than for unsupplemented or ractopamine-supplemented heat-stressed lambs (Figure 6B). Thus, heat stress reduced (P < 0.05) lactate concentrations for zilpaterol-supplemented lambs only. Blood pH was less (P < 0.05) for ractopamine-supplemented controls compared to unsupplemented or zilpaterol-supplemented controls and was greater (P < 0.05) for ractopamine-supplemented heat-stressed lambs than unsupplemented or zilpaterol-supplemented heat-stressed lambs (Figure 6C). Blood HCO3 concentrations were less (P < 0.05) for heat-stressed lambs than for controls, regardless of supplement (Figure 6D). Blood pO2 did not differ between controls and heat-stressed lambs (41.3 ± 1.9 vs. 42.5 ± 0.8 mmHg, respectively), or among supplemental groups, but pCO2 was less (P < 0.05) for heat-stressed lambs than for controls (40.6 ± 0.6 vs. 45.4 ± 0.7 mmHg, respectively), regardless of supplement. Blood Na+ concentrations (Figure 7A) were less (P < 0.05) and blood Ca2+ concentrations (Figure 7B) were greater (P < 0.05) for heat-stressed lambs than for controls, regardless of supplement. Blood K+ concentrations did not differ between environmental groups but tended to be less (P = 0.07) for zilpaterol-supplemented lambs than for unsupplemented or ractopamine-supplemented lambs, regardless of environment (Figure 7C).
Figure 6.
Blood metabolic indicators in heat-stressed wether lambs supplemented with ractopamine or zilpaterol. Data are shown for blood glucose (A), lactate (B), pH (C), and HCO3 (D). Effects of experimental group (GRP), supplement, and the interaction (G*S) were evaluated and are noted where significant (P < 0.05). a,b,cMeans with different superscripts differ (P < 0.05).
Figure 7.
Blood electrolytes in heat-stressed wether lambs supplemented with ractopamine or zilpaterol. Data are shown for blood concentration of Na+ (A), Ca2+ (B), and K+ (C). Effects of experimental group (GRP), supplement (SUPPL), and the interaction were evaluated and are noted where significant (P < 0.05) or trending toward significant (P < 0.10).
Discussion
In this study, we found that dietary supplementation of ractopamine but not zilpaterol for 18 d partially moderated the hyperthermic response to heat stress in wether lambs. Moreover, supplementation of ractopamine or zilpaterol tended to moderate the heat stress-induced increases in circulating monocytes observed in unsupplemented lambs, and zilpaterol also reduced circulating granulocytes. This corroborates our previous observations in which long-term β-agonist supplementation improved indicators of systemic inflammation in heat-stressed animals (Swanson et al., 2020). The mild reductions in circulating concentrations of red blood cells, hemoglobin, and hematocrit due to heat stress and β-adrenergic agonist supplementation would perhaps be consistent with greater water intake, which we previously observed in these and other heat-stressed lambs (Barnes et al., 2019; Swanson et al., 2020). Metabolic indicators including circulating glucose, triglycerides, and cholesterol concentrations were not affected by heat stress or by β agonists, which was unexpected. Blood lactate was also not affected by heat stress or either β agonist alone but was substantially reduced by the combination of heat stress and zilpaterol. This was surprising based on seemingly normal lactate concentrations observed at earlier and later time points (Barnes et al., 2019; Swanson et al., 2020). Nevertheless, this could have been the result of reduced skeletal muscle glucose utilization due to heat stress-associated inflammation and β2-adrenergic stimulation (Cadaret et al., 2017), which would indirectly reduce lactate production. Although liver function was not assessed in this study, stress hormones and β1-adrenergic stimulation have also been shown to increase hepatic clearance of lactate from circulation (Brockman, 1991; Taurà et al., 2010). Heat stress-induced elevations in blood Ca2+ and reductions in blood K+ were not compounded by the supplementation of either β-adrenergic agonist, despite early work linking adrenergic stimulation to hypercalcemia and hyponatremia (Hsu and Cooper, 1975; Johansson et al., 1988). The differences in the collective effects between the two supplements in heat-stressed lambs are perhaps not surprising, as they have separate primary modes of action. Despite some affinity for β2-adrenergic receptors, ractopamine acts primarily via β1-adrenergic receptors, whereas zilpaterol acts primarily via β2-adrenergic receptors (Mersmann, 1998; Johnson et al., 2014). Corneal and skin temperatures measured by infrared thermography indicated that body surface temperatures were consistently elevated by exposure to heat stress, indicating that infrared thermography performed at up to 2 m is a reasonable no-touch option for assessing hyperthermy in feedlot lambs.
The heat stress-induced rise in core body temperature was mitigated by the β1-adrenergic agonist ractopamine after 18 d of supplementation, which was comparable to findings from our previous study in which mitigation occurred after 21 d (Swanson et al., 2020). In that study, the mitigated hyperthermic response coincided with diminished inflammation, as the heat stress-induced elevation of circulating TNFα was delayed and diminished by ractopamine. TNFα was not assessed in the present study, but ractopamine supplementation prevented heat stress-induced increases in monocytes, which are a primary source of circulating inflammatory cytokines (Rossol et al., 2011). Zilpaterol supplementation also prevented the heat stress-induced rise in macrophages but failed to prevent hyperthermia. This was rather unexpected, as zilpaterol supplementation in feedlot steers and heifers reduced body temperatures under heat stress and thermoneutral conditions (Boyd et al., 2015; Buntyn et al., 2016). Zilpaterol increases skeletal muscle oxidative metabolism (Cadaret et al., 2017; Barnes et al., 2019), a thermogenic process (Tappy et al., 1986), and it is perhaps possible that the higher rectal temperatures were the product of greater metabolic heat production. However, it is not clear why this might have occurred in our heat-stressed lambs but not in our thermoneutral controls or in the previous studies with cattle. Moreover, β-adrenergic antagonists increased body temperature in humans when metabolic rates were elevated by exercise (Pescatello et al., 1987).
Elevated surface temperatures were detected for the cornea, ears, nose, and back in heat-stressed lambs, which is important for situations where safety concerns or logistics prevent measurement of rectal or vaginal temperatures. Previous studies have demonstrated the accuracy of infrared thermography performed over short distances in livestock (George et al., 2014; Feng et al., 2019; Lowe et al., 2020). In the present study, thermographic images obtained at 2 m from the animal produced comparable results and interpretation to images obtained at 1 m. This indicates that thermography can be effectively performed from outside an animal’s flight zone, which in reasonably acclimated sheep and other herd animals is typically within a 2-m radius of the animal (Grandin, 1989; Markowitz et al., 1998). Our findings also illustrate that core and surface temperatures can be affected differently, as ractopamine moderated heat stress-induced increases in rectal temperatures but not those in corneal, eye, ear, or nose temperatures. Previous studies in rats have shown that β-adrenergic stimulation can have differing effects on core body temperatures and surface temperatures (Wright and Katovich, 1996) due to differential changes in blood flow through the skin (Yamazaki and Yuge, 2011; Hodges et al., 2015). These studies indicated that the effects of thermo-stimulatory factors on skin temperatures were more variable than on core body temperatures, and that they differed among different areas of the body surface. In our study, variability for corneal (pooled SE: ± 0.16°C), nose (pooled SE: ± 0.29°C), and ear (pooled SE: ± 0.26°C) temperatures was only marginally greater than for rectal temperatures (pooled SE: ± 0.09°C) and was similar between experimental groups and distances from the animal. Conversely, variability in surface temperatures measured with the hand-held infrared guns was notably higher than for those measured with the IR camera, although it improved with increasing emissivity levels. Our findings also indicated that the presence of wool over the loin reduced the accuracy of the hand-held infrared guns. This may further limit their commercial application in sheep, since most are closely shorn for only a short period each year. Temperatures measured at the nose were perhaps the least meaningful despite the area’s absence of wool, which we postulate was a product of greater air movement across the surface due to breathing. Measurements on the inner surface of the ear produced good results but were often difficult to capture based on the posture of the animal. Moreover, ear tags used for identification in commercial animals may reduce the practicality of this site. Corneal temperatures, however, were easily obtainable from the forward-facing angle and produced the least amount of variability. Thus, they provided the best option for assessing body surface temperatures, which corroborates the earlier observations at closer distances (George et al., 2014). As with any technology, potential limitations of infrared thermography should be considered along with the potential benefits. For example, a comprehensive review of the existing literature led Wijffels et al. (2021) to conclude that although mean surface temperatures were generally accurate, there were more frequent outlier readings relative to rectal or vaginal temperatures. Although not applicable to the present (indoor) study, they also noted that surface temperatures measured outdoors could be affected by uncontrolled or unaccounted differences of solar radiation, wind speed, humidity, and surface moisture or by differences among equipment or operators.
The findings of this study allow us to conclude that the supplementation of β1- and β2-adrenergic agonists to heat-stressed sheep can each relieve certain indicators of stress. In addition, this study demonstrates that temperatures of the corneal and other surface areas measured by infrared thermography are reasonable options for assessing hyperthermia when rectal or vaginal temperatures are not possible. Additionally, our results indicate that thermography can be effective even when performed from outside the flight zone of acclimated livestock, which widens the implications of its commercial use.
Acknowledgments
This manuscript is based on the research that was supported in part by the USDA National Institute of Food and Agriculture Foundational Grants 2019-67015-29448 and 2020-67015-30825, the National Institute of General Medical Sciences Grant 1P20GM104320 (J. Zempleni, Director), the Nebraska Agricultural Experiment Station with funding from the Hatch Act (accession number 1009410), and Hatch Multistate Research capacity funding program (accession numbers 1011055 and 1009410) through the USDA National Institute of Food and Agriculture. The Biomedical and Obesity Research Core (BORC) in the Nebraska Center for Prevention of Obesity Diseases (NPOD) receives partial support from NIH (NIGMS) COBRE IDeA award NIH 1P20GM104320. The contents of this publication are the sole responsibility of the authors and do not necessarily represent the official views of the NIH or NIGMS.
Glossary
Abbreviations
- EDTA
ethylenediaminetetraacetic acid
- HCO3
bicarbonate
- IL-6
interleukin 6
- IR
infrared
- pCO2
partial pressure of CO2
- pO2
partial pressure of O2
- RBC
red blood cells
- RH
relative humidity
- SE
standard error of the mean
- THI
temperature-humidity index
- TNFα
tumor necrosis factor α
- WBC
total white blood cells
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Barnes, T. L., Cadaret C. N., Beede K. A., Schmidt T. B., Petersen J. L., and Yates D. T.. . 2019. Hypertrophic muscle growth and metabolic efficiency were impaired by chronic heat stress, improved by zilpaterol supplementation, and not affected by ractopamine supplementation in feedlot lambs1. j. Anim. Sci. 97:4101–4113. doi: 10.1093/jas/skz271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd, B. M., Shackelford S. D., Hales K. E., Brown-Brandl T. M., Bremer M. L., Spangler M. L., Wheeler T. L., King D. A., and Erickson G. E.. . 2015. Effects of shade and feeding zilpaterol hydrochloride to finishing steers on performance, carcass quality, heat stress, mobility, and body temperature. j. Anim. Sci. 93:5801–5811. doi: 10.2527/jas.2015-9613. [DOI] [PubMed] [Google Scholar]
- Brockman, R. P. 1991. Effects of epinephrine on the net hepatic uptake of lactate, pyruvate, and glycerol in sheep. Can. j. Physiol. Pharmacol. 69:475–479. doi: 10.1139/y91-071. [DOI] [PubMed] [Google Scholar]
- Buntyn, J. O., Burdick Sanchez N. C., Schmidt T. B., Erickson G. E., Sieren S. E., Jones S. J., and Carroll J. A.. . 2016. The metabolic, stress axis, and hematology response of zilpaterol hydrochloride supplemented beef heifers when exposed to a dual corticotropin-releasing hormone and vasopressin challenge. j. Anim. Sci. 94:2798–2810. doi: 10.2527/jas.2015-0192. [DOI] [PubMed] [Google Scholar]
- Cadaret, C. N., Abebe M. D., Barnes T. L., Posont R. J., and Yates D. T.. . 2021. Lipopolysaccharide endotoxin injections elevated salivary TNFα and corneal temperatures and induced dynamic changes in circulating leukocytes, inflammatory cytokines, and metabolic indicators in wether lambs. J Anim Sci 99. doi: 10.1093/jas/skab120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadaret, C. N., Beede K. A., Riley H. E., and Yates D. T.. . 2017. Acute exposure of primary rat soleus muscle to zilpaterol HCl (β2 adrenergic agonist), TNFα, or IL-6 in culture increases glucose oxidation rates independent of the impact on insulin signaling or glucose uptake. Cytokine 96:107–113. doi: 10.1016/j.cyto.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadaret, C. N., Merrick E. M., Barnes T. L., Beede K. A., Posont R. J., Petersen J. L., and Yates D. T.. . 2019. Sustained maternal inflammation during the early third-trimester yields intrauterine growth restriction, impaired skeletal muscle glucose metabolism, and diminished β-cell function in fetal sheep1,2. j. Anim. Sci. 97:4822–4833. doi: 10.1093/jas/skz321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, Y. Z., Zhao H. T., Jia G. F., Ojukwu C., and Tan H. Q.. . 2019. Establishment of validated models for non-invasive prediction of rectal temperature of sows using infrared thermography and chemometrics. Int. j. Biometeorol. 63:1405–1415. doi: 10.1007/s00484-019-01758-2. [DOI] [PubMed] [Google Scholar]
- George, W. D., Godfrey R. W., Ketring R. C., Vinson M. C., and Willard S. T.. . 2014. Relationship among eye and muzzle temperatures measured using digital infrared thermal imaging and vaginal and rectal temperatures in hair sheep and cattle. j. Anim. Sci. 92:4949–4955. doi: 10.2527/jas.2014-8087. [DOI] [PubMed] [Google Scholar]
- Grandin, T. 1989. Behavioral principles of livestock handling. The Professional Animal Scientist 5:1–11. doi: 10.15232/S1080-7446(15)32304-4 [DOI] [Google Scholar]
- Hodges, G. J., Kellogg D. L., and Johnson J. M.. . 2015. Effect of skin temperature on cutaneous vasodilator response to the β-adrenergic agonist isoproterenol. j. Appl. Physiol. (1985). 118:898–903. doi: 10.1152/japplphysiol.01071.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, W. H., and Cooper C. W.. . 1975. Hypercalcemic effect of catecholamines and its prevention by thyrocalcitonin. Calcif. Tissue Res. 19:125–137. doi: 10.1007/BF02563997. [DOI] [PubMed] [Google Scholar]
- Johansson, B. W., Hansen O., Juul-Möller S., and Svensson O.. . 1988. Adrenaline-induced changes in serum electrolytes, ECG, and blood pressure, with Ca-blockade pretreatment. Angiology 39:345–354. doi: 10.1177/000331978803900403. [DOI] [PubMed] [Google Scholar]
- Johnson, B. J., Smith S. B., and Chung K. Y.. . 2014. Historical Overview of the Effect of β-Adrenergic Agonists on Beef Cattle Production. Asian-Australas. j. Anim. Sci. 27:757–766. doi: 10.5713/ajas.2012.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe, G., McCane B., Sutherland M., Waas J., Schaefer A., Cox N., and Stewart M.. . 2020. Automated collection and analysis of infrared thermograms for measuring eye and cheek temperatures in calves. Animals (Basel) 10:292. doi: 10.3390/ani10020292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz, T. M., Dally M. R., Gursky K., and Price E. O.. . 1998. Early handling increases lamb affinity for humans. Anim. Behav. 55:573–587. doi: 10.1006/anbe.1997.0640. [DOI] [PubMed] [Google Scholar]
- Mersmann, H. J. 1998. Overview of the effects of beta-adrenergic receptor agonists on animal growth including mechanisms of action. j. Anim. Sci. 76:160–172. doi: 10.2527/1998.761160x. [DOI] [PubMed] [Google Scholar]
- Navegantes, L. C., Migliorini R. H., and do Carmo Kettelhut I.. . 2002. Adrenergic control of protein metabolism in skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care 5:281–286. doi: 10.1097/00075197-200205000-00007. [DOI] [PubMed] [Google Scholar]
- Pescatello, L. S., Mack G. W., C. N.Leach, Jr, and Nadel E. R.. . 1987. Effect of beta-adrenergic blockade on thermoregulation during exercise. j. Appl. Physiol. (1985). 62:1448–1452. doi: 10.1152/jappl.1987.62.4.1448. [DOI] [PubMed] [Google Scholar]
- Rossol, M., Heine H., Meusch U., Quandt D., Klein C., Sweet M. J., and Hauschildt S.. . 2011. LPS-induced cytokine production in human monocytes and macrophages. Crit. Rev. Immunol. 31:379–446. doi: 10.1615/critrevimmunol.v31.i5.20. [DOI] [PubMed] [Google Scholar]
- Swanson, R. M., Tait R. G., Galles B. M., Duffy E. M., Schmidt T. B., Petersen J. L., and Yates D. T.. . 2020. Heat stress-induced deficits in growth, metabolic efficiency, and cardiovascular function coincided with chronic systemic inflammation and hypercatecholaminemia in ractopamine-supplemented feedlot lambs. J Anim Sci 98. doi: 10.1093/jas/skaa168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappy, L., Randin J. P., Felber J. P., Chiolero R., Simonson D. C., Jequier E., and DeFronzo R. A.. . 1986. Comparison of thermogenic effect of fructose and glucose in normal humans. Am. j. Physiol. 250(6 Pt 1):E718–E724. doi: 10.1152/ajpendo.1986.250.6.E718. [DOI] [PubMed] [Google Scholar]
- Taurà, P., Fuster J., Mercadal J., Martinez-Palli G., Fondevila C., Blasi A., Balust J., and Garcia-Valdecasas J. C.. . 2010. The use of beta-adrenergic drugs improves hepatic oxygen metabolism in cirrhotic patients undergoing liver resection. j. Hepatol. 52:340–347. doi: 10.1016/j.jhep.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Wijffels, G., Sullivan M., and Gaughan J.. . 2021. Methods to quantify heat stress in ruminants: Current status and future prospects. Methods 186:3–13. doi: 10.1016/j.ymeth.2020.09.004. [DOI] [PubMed] [Google Scholar]
- Wright, B. E., and Katovich M. J.. . 1996. Effect of restraint on drug-induced changes in skin and core temperature in biotelemetered rats. Pharmacol. Biochem. Behav. 55:219–225. doi: 10.1016/s0091-3057(96)00071-8. [DOI] [PubMed] [Google Scholar]
- Yamazaki, F., and Yuge N.. . 2011. Limb-specific differences in the skin vascular responsiveness to adrenergic agonists. j. Appl. Physiol. (1985). 111:170–176. doi: 10.1152/japplphysiol.00068.2011. [DOI] [PubMed] [Google Scholar]