Abstract
The 1‐year mortality and health consequences of COVID‐19 in cancer patients are relatively underexplored. In this multicenter cohort study, 166 COVID‐19 patients with cancer were compared with 498 non‐cancer COVID‐19 patients and 498 non‐COVID cancer patients. The 1‐year all‐cause mortality and hospital mortality rates in Cancer COVID‐19 Cohort (30% and 20%) were significantly higher than those in COVID‐19 Cohort (9% and 8%, both P < .001) and Cancer Cohort (16% and 2%, both P < 0.001). The 12‐month all‐cause post‐discharge mortality rate in survival discharged Cancer COVID‐19 Cohort (8%) was higher than that in COVID‐19 Cohort (0.4%, P < .001) but similar to that in Cancer Cohort (15%, P = .084). The incidence of sequelae in Cancer COVID‐19 Cohort (23%, 26/114) is similar to that in COVID‐19 Cohort (30%, 130/432, P = .13). The 1‐year all‐cause mortality was high among patients with hematologic malignancies (59%), followed by those who have nasopharyngeal, brain, and skin tumors (45%), digestive system neoplasm (43%), and lung cancers (32%). The rate was moderate among patients with genitourinary (14%), female genital (13%), breast (11%), and thyroid tumors (0). COVID‐19 patients with cancer showed a high rate of in‐hospital mortality and 1‐year all‐cause mortality, but the 12‐month all‐cause post‐discharge mortality rate in survival discharged cancer COVID‐19 patients was similar to that in Cancer Cohort. Comparing to COVID‐19 Cohort, risk stratification showed that hematologic, nasopharyngeal, brain, digestive system, and lung tumors were high risk (44% vs 9%, P < 0.001), while genitourinary, female genital, breast, and thyroid tumors had moderate risk (10% vs 9%, P = .85) in COVID‐19 Cancer Cohort. Different tumor subtypes had different effects on COVID‐19. But if cancer patients with COVID‐19 manage to survive their COVID‐19 infections, then long‐term mortality appears to be similar to the cancer patients without COVID‐19, and their long‐term clinical sequelae were similar to the COVID‐19 patients without cancer.
Keywords: Cancer, consequences, coronavirus disease 2019, mortality, risk factors
1. INTRODUCTION
As of 30 March 2021, the number of COVID‐19 cases and deaths continued to rise with 127.3 million cumulative cases and 2.8 million deaths globally. 1 The global cancer burden is estimated to have risen to 19.3 million new cases and 10.0 million deaths in 2020. 2 Some initial reports suggested that patients with an active malignancy might be more likely to contract SARS‐CoV‐2 and have an increased risk of short‐term mortality. 3 , 4 , 5 , 6
However, these initial reports were restricted by the follow‐up time. 6 As some patients remained hospitalized for some time, longer‐term follow‐up is needed to better understand the effect of COVID‐19 on cancer patients. 3 , 6 , 7 Cancer encompasses a diverse array of primary tumor subtypes with different outcomes. 8 , 9 To the best of our knowledge, there are no reports to study the long‐term prognosis of cancer patients with COVID‐19, and few studies compare them with cancer patients without COVID‐19 as well as different tumor subtypes.
Herein, 166 cancer patients with COVID‐19, 498 COVID‐19 patients without cancer, and 498 cancer patients without COVID‐19 were compared. All of the patients were from the four hospitals in Wuhan, a COVID‐19 “hot spot” in China. All survival patients were followed up for at least 12 months after hospital admission.
2. METHODS
2.1. Study design and patients
In this multicenter ambidirectional comparative cohort study, we enrolled sequential cancer patients who were admitted with COVID‐19 (Cancer COVID‐19 Cohort) to the four hospitals in Wuhan, Hubei Province, China, the epicenter of the first COVID‐19 pandemic, between January 1, 2020, and March 18, 2020 (Table S1). Eligibility criteria for enrollment were laboratory confirmation of SARS‐CoV‐2 virus infection by RT‐PCR test and hospitalized patients with active cancer (Table S2). COVID‐19 disease severity was defined according to World Health Organization (WHO) guidelines. 10 Primary tumor subtypes were classified by the WHO Classification of Tumors series. 11 Each COVID‐19 patient with cancer was matched to patients without cancer from the same COVID‐19‐positive population (COVID‐19 Cohort) in a ratio of 1:3 based on age (±5 years) and sex. The patients served as uninfected controls were chosen at a ratio of 1:3 (based on age, gender, and cancer subtype) from cancer patients admitted to the same four hospitals between 1 January 2019 and 17 March 17 2020. All data were de‐identified. This study was done in accordance with the STROBE statement. 12
2.2. Study variables and outcomes
We define the acute phase as the time between symptom onset and hospital discharge. Data were collected on site by trained coordinators manually reviewing the electronic medical records and importing them into a secure online database using a standardized case report form (supplementary case report form, Data S1). Demographic characteristics, coexisting conditions, presenting symptoms, vital signs, biochemical findings, treatment practices, and a variety of hospital outcome data were collected. To ensure the enrollment of an unbiased population, the eligible patients were consecutively recruited from each site from 1 January 2020 to 17 March 2020.
All survivors who had been discharged from the hospital were selected into the follow‐up study to observe their health consequences in recovery from COVID‐19 (supplementary follow‐up studies, Data S1). Some patients were followed up in the outpatient clinic and asked to complete a series of questionnaires. We followed up with the patients until death or 18 February 2021, whichever came first.
The primary outcomes included the 1‐year all‐cause mortality rate and sequelae. Secondary outcomes included the hospital mortality rate, the length of hospital stay, and the 12‐month all‐cause post‐discharge mortality rate.
2.3. Statistical analysis
We aimed to generate a representative sample of cancer patients with COVID‐19 by starting with at least 5500 COVID‐19 patients and 40 900 cancer patients from the four hospitals (Figure S1). To describe baseline characteristics, treatment, and outcomes, we prespecified the following covariates for inclusion in the models. Descriptive statistics were used to describe the baseline data of the patients. Categorical variables were presented as percentages. Continuous variables were expressed as mean ± SD if they were normally distributed or as median (IQR) if they were not. Proportions for categorical variables were compared using the χ2 test.
The Kaplan–Meier method with log‐rank test was used for comparing survival curves between Cancer COVID‐19 Cohort and control groups. Cox proportional hazards regression was used to investigate the effect of several risk factors on survival. Univariable and multivariable analyses between 1‐year mortality and risk factors were performed. The risk factor variables were included in the stepwise Cox proportional hazards regression. A backward stepwise technique was used to evaluate all potential univariable correlates (P < .05) and create a multivariable model containing variables with P < .05.
Analyses were performed using SPSS Statistics 26.0 software (International Business Machines Corp, IBM) and Graph Pad Prism (Version 8.0.2, GraphPad Software, Inc.).
3. RESULTS
3.1. Patient characteristics at baseline
The median age of the 166 hospitalized cancer COVID‐19 patients was 65 (59–70) years old and 49% (82/166) of the patients were male (Table 1). The patients consist of different primary tumor subtypes following WHO classification criteria (Table 2). 13 Among the patients, most cancers were of the digestive system (25%, 42/166), followed by the lung (15%, 25/166), and genitourinary (13%, 22/166). In terms of primary organ tumor, lung cancer was the most common cancer type (15%, 25/166), followed by breast cancer (11%, 19/166), and colon cancer (11%, 18/166).
TABLE 1.
Characteristics of Cancer COVID‐19 Cohort, COVID‐19 Cohort, and Cancer Cohort
| Patients, no. (%) | |||||
|---|---|---|---|---|---|
| Cancer COVID‐19 Cohort (n = 166) | COVID‐19 Cohort (n = 498) | P value a | Cancer Cohort (n = 498) | P value b | |
| Demographics | |||||
| Age, median (IQR), y | 65 (59–70) | 65 (59–70) | 1.000 | 66 (58–73) | .49 |
| Sex | |||||
| Female | 84 (51) | 252 (51) | 1.000 | 252 (51) | 1.000 |
| Male | 82 (49) | 246 (49) | 246 (49) | ||
| Comorbidities | |||||
| Current smoking | 8 (5) | 14 (3) | .211 | 127 (26) | <.001 |
| Hypertension | 55 (33) | 171 (34) | .777 | 135 (27) | .137 |
| Diabetes | 24 (15) | 104 (21) | .069 | 47 (9) | .07 |
| Hyperlipidemia | 33 (20) | 76 (15) | .164 | 34 (7) | <.001 |
| Hyperuricemia | 16 (10) | 42 (8) | .634 | 14 (3) | <.001 |
| Coronary heart disease | 12 (7) | 40 (8) | .739 | 53 (11) | .2 |
| Cerebrovascular disease | 4 (2) | 18 (4) | .453 | 14 (3) | 1.000 |
| COPD | 11 (7) | 25 (5) | .429 | 7 (1) | .001 |
| Chronic kidney disease | 3 (2) | 11 (2) | 1.000 | 7 (1) | 1.000 |
| Chronic liver disease | 5 (3) | 7 (1) | .313 | 28 (6) | .18 |
| Arrhythmia | 8 (5) | 18 (4) | .488 | 27 (5) | .764 |
| Symptoms at admission | |||||
| Fever | 118 (71) | 363 (73) | .652 | NA | NA |
| Chills | 26 (16) | 88 (18) | .552 | NA | NA |
| Headache or dizzy | 26 (16) | 79 (16) | .951 | NA | NA |
| Myalgias | 26 (16) | 104 (21) | .142 | NA | NA |
| Fatigue | 88 (53) | 253 (51) | .622 | NA | NA |
| Rhinorrhea | 10 (6) | 20 (4) | .281 | NA | NA |
| Sore throat | 12 (7) | 69 (14) | .024 | NA | NA |
| Dry cough | 72 (43) | 264 (53) | .031 | NA | NA |
| Expectoration | 60 (36) | 105 (21) | <.001 | NA | NA |
| Hemoptysis | 10 (6) | 7 (1) | .003 | NA | NA |
| Chest congestion | 70 (42) | 203 (41) | .75 | NA | NA |
| Dyspnea | 40 (24) | 79 (16) | .017 | NA | NA |
| Nausea or vomiting | 21 (13) | 45 (9) | .178 | NA | NA |
| Abdominal pain | 8 (5) | 15 (3) | .27 | NA | NA |
| Diarrhea | 31 (19) | 101 (20) | .653 | NA | NA |
| Consciousness disorder | 18 (11) | 20 (4) | .001 | NA | NA |
| Complications | |||||
| Respiratory failure | 27 (16) | 52 (10) | .045 | NA | NA |
| Acute cardiac injury | 3 (2) | 2 (0) | .195 | NA | NA |
| Acute kidney injury | 29 (18) | 41 (8) | .001 | NA | NA |
| Acute liver injury | 53 (32) | 110 (22) | .011 | NA | NA |
| Prothrombotic coagulopathy | 2 (1) | 4 (1) | 1.000 | NA | NA |
| Electrolytic disturbance | 28 (17) | 63 (13) | .171 | NA | NA |
| In‐hospital infection | 6 (4) | 16 (3) | .802 | NA | NA |
| Treatment | |||||
| Antiviral drug | 108 (65) | 359 (72) | .086 | NA | NA |
| Intravenous antibiotics | 88 (53) | 224 (45) | .073 | NA | NA |
| Intravenous antifungal | 20 (12) | 36 (7) | .053 | NA | NA |
| Anticoagulation | 50 (30) | 37 (7) | <.001 | NA | NA |
| Intravenous corticosteroids | 44 (27) | 70 (14) | <.001 | NA | NA |
Cancer COVID‐19 Cohort vs COVID‐19 Cohort.
Cancer COVID‐19 Cohort vs Cancer Cohort.
TABLE 2.
Primary tumor subtype among Cancer COVID‐19 Cohort
| Primary tumor subtype | Patients, no. (%) | ||||
|---|---|---|---|---|---|
| Total (n = 166) | Hospital mortality | 1‐year mortality | |||
| Dead (n = 34) | Alive (n = 132) | Dead (n = 49) | Alive (n = 117) | ||
| Total | 166 (100) | 34 (20) | 132 (80) | 49 (30) | 117 (70) |
| Solid tumors | 149 (90) | 26 (17) | 124 (83) | 38 (26) | 111 (74) |
| Thyroid and breast | 33 (20) | 2 (6) | 33 (84) | 2 (6) | 33 (84) |
| Thyroid | 14 (8) | 0 | 14 (100) | 0 | 14 (100) |
| Breast | 19 (11) | 2 (11) | 17 (89) | 2 (11) | 17 (89) |
| Female genital | 16 (9) | 1 (6) | 15 (94) | 2 (13) | 14 (87) |
| Cervical | 9 (5) | 1 (11) | 8 (89) | 1 (11) | 8 (89) |
| Ovary | 4 (2) | 0 | 4 (100) | 1 (25) | 3 (75) |
| Endometrial | 3 (2) | 0 | 3 (100) | 0 | 3 (100) |
| Genitourinary | 22 (13) | 3 (14) | 19 (86) | 3 (14) | 19 (86) |
| Prostatic | 7 (4) | 2 (29) | 5 (71) | 2 (29) | 5 (71) |
| Bladder | 7 (4) | 0 | 7 (100) | 0 | 7 (100) |
| Renal | 5 (3) | 1 (20) | 4 (80) | 1 (20) | 4 (80) |
| Penile | 2 (1) | 0 | 2 (100) | 0 | 2 (100) |
| Testicular | 1 (1) | 0 | 1 (100) | 0 | 1 (100) |
| Lung | 25 (15) | 4 (16) | 21 (84) | 8 (32) | 17 (68) |
| Digestive system | 42 (25) | 12 (29) | 30 (71) | 18 (43) | 24 (57) |
| Colon | 18 (11) | 3 (17) | 15 (83) | 7 (39) | 11 (61) |
| Gastric | 10 (6) | 5 (50) | 5 (50) | 5 (50) | 5 (50) |
| Liver | 6 (4) | 1 (17) | 5 (83) | 2 (33) | 4 (67) |
| Rectal | 5 (3) | 2 (40) | 3 (60) | 2 (40) | 3 (60) |
| Pancreas | 3 (2) | 1 (33) | 2 (67) | 2 (67) | 1 (33) |
| Other solid | 11 (7) | 4 (36) | 7 (64) | 5 (45) | 6 (55) |
| Bone | 4 (2) | 0 | 4 (100) | 1 (25) | 3 (75) |
| Nasopharyngeal | 4 (2) | 2 (50) | 2 (50) | 2 (50) | 2 (50) |
| Brain | 2 (1) | 1 (50) | 1 (50) | 1 (50) | 1 (50) |
| Skin | 1 (1) | 1 (100) | 0 | 1 (100) | 0 |
| Hematologic malignancies | 17 (10) | 8 (47) | 9 (53) | 11 (65) | 6 (35) |
| Lymphoid malignancy | 14 (8) | 7 (50) | 7 (50) | 9 (64) | 5 (36) |
| Multiple myeloma | 6 (4) | 4 (67) | 2 (33) | 5 (83) | 1 (17) |
| Non‐Hodgkin lymphoma | 4 (2) | 1 (25) | 3 (75) | 2 (50) | 2 (50) |
| Chronic lymphoblastic leukemia | 2 (1) | 0 | 2 (100) | 0 | 2 (100) |
| Acute lymphoblastic leukemia | 2 (1) | 2 (100) | 0 | 2 (100) | 0 |
| Myeloid malignancy | 3 (2) | 1 (33) | 2 (67) | 2 (67) | 1 (33) |
| Acute myelogenous leukemia | 2 (1) | 1 (50) | 1 (50) | 2 (100) | 0 |
| Myelodysplastic syndrome | 1 (1) | 0 | 1 (100) | 0 | 1 (100) |
At baseline, the demographics characteristics of the patients were well‐balanced in all three cohorts (Table 1). Comorbidities among Cancer COVID‐19 Cohort were generally similar to those among COVID‐19 Cohort. But comparing to Cancer Cohort, Cancer COVID‐19 Cohort was more likely to have a history of diabetes (9% vs 15%, P = .07), hyperlipidemia (7% vs 20%, P < .001), hyperuricemia (3% vs 10%, P < .001), and chronic obstructive pulmonary diseases (COPD; 1% vs 7%, P = .001), supporting the risks of these underlying conditions for contracting COVID‐19.
In terms of COVID‐19 symptoms, patients in Cancer COVID‐19 Cohort were more likely to have expectoration (36% vs 21%, P < .001), dyspnea (24% vs 16%, P = .017), and consciousness disorder (11% vs 4%, P = .001) than those in COVID‐19 Cohort, but less likely to have a sore throat (7% vs 14%, P = .024) or dry cough (43% vs 53%, P = .031; Table 1). Cancer COVID‐19 Cohort had more severe complications such as respiratory failure (16% vs 10%, P = .045), acute kidney injury (18% vs 8%, P = .001), and acute liver injury (32% vs 22%, P = .011) than COVID‐19 Cohort (Table 1).
3.2. One‐year all‐cause mortality
The median follow‐up time from the point of hospital admission was 12.2 (IQR 12.1–12.6) months (Table 3 and Figure 1A). In the Cox proportional hazards regression analysis, 30% (49/166) of the patients in Cancer COVID‐19 Cohort died within 12 months. The mortality was 9% (44/498) in COVID‐19 Cohort (relative risk/RR = 0.29; 95% CI 0.19 to 0.44, P < .001), for an absolute risk difference of −19 percentage points (95% CI −13 to −29, P < .001). And that was 16% (80/498) in Cancer Cohort (RR, 0.43; 95% CI 0.30 to 0.62, P < .001), for an absolute risk difference of −13 percentage points (95% CI −9 to −19, P < .001). Results were similar in the adjusted analysis. Figure 1A shows the main difference between Cancer COVID‐19 Cohort and Cancer Cohort was in the first 2 months, with mortality of 20% (34/166) and 4% (20/498, P < .001) in each cohort, respectively.
TABLE 3.
Outcomes of Cancer COVID‐19 Cohort, COVID‐19 Cohort, and Cancer Cohort
| Patients, no. (%) | |||||
|---|---|---|---|---|---|
| Cancer COVID‐19 cohort (n = 166) | COVID‐19 cohort (n = 498) | P value a | Cancer cohort (n = 498) | P value b | |
| COVID‐19 severity | |||||
| Non‐severe | 106 (64) | 399 (80) | <.001 | NA | NA |
| Severe | 60 (36) | 99 (20) | NA | NA | |
| Oxygenation and ventilation | |||||
| Not requiring supplement oxygen | 47 (29) | 170 (34) | <.001 | NA | NA |
| Requiring supplement oxygen | 93 (56) | 279 (56) | NA | NA | |
| HFNC | 2 (1) | 23 (5) | NA | NA | |
| NIV | 11 (7) | 8 (2) | NA | NA | |
| IMV or ECMO | 12 (7) | 18 (4) | NA | NA | |
| Length of hospital stay, median (IQR), d | 25 (15–33) | 21 (11–28) | .005 | NA | NA |
| Time from symptom onset to admission, median (IQR), d | 10 (7–16) | 10 (5–15) | .047 | NA | NA |
| Time from admission to follow‐up, median (IQR), m | 12.2 (12.1–12.6) | 12.2 (12.1–12.6) | .36 | 12.1 (11.7–12.4) | .063 |
| Time from discharge to follow‐up, median (IQR), m | 11.2 (10.8–11.6) | 11.4 (11.2–11.8) | .051 | 11.2 (10.7–11.7) | .82 |
| Mortality | |||||
| 1‐year all‐cause mortality | 49 (30) | 44 (9) | <.001 | 80 (16) | <.001 |
| 12‐month post‐discharge mortality | 15 (11) | 2 (0.4) | <.001 | 72 (15) | .084 |
| Hospital mortality | 34 (20) | 42 (8) | <.001 | 8 (2) c | <.001 |
| Consequences at 1‐year followed up | |||||
| Number of patients | 114 | 432 | NA | NA | |
| Any one of symptoms | 26 (23) | 130 (30) | 0.13 | NA | NA |
| Fatigue | 5 (4) | 53 (12) | 0.016 | NA | NA |
| Chest congestion | 3 (3) | 38 (9) | 0.027 | NA | NA |
| Cough | 10 (9) | 25 (6) | 0.24 | NA | NA |
| Expectoration | 4 (4) | 7 (2) | 0.20 | NA | NA |
| Dyspnea | 9 (8) | 27 (6) | 0.51 | NA | NA |
| Palpitations | 2 (2) | 9 (2) | 0.83 | NA | NA |
| Waist pain | 7 (6) | 20 (5) | 0.50 | NA | NA |
| Anxiety | 0 | 23 (5) | 0.021 | NA | NA |
| Sleep difficulties | 1 (1) | 5 (1) | 0.65 | NA | NA |
Abbreviations: ECMO, Extracorporeal membrane oxygenation; HFNC, high‐flow nasal canula for oxygen therapy; IMV, Invasive mechanical ventilation; NIV, Noninvasive mechanical ventilation.
Cancer COVID‐19 cohort vs COVID‐19 cohort.
Cancer COVID‐19 cohort vs cancer cohort.
As these patients were not necessarily hospitalized, their hospital mortality rate was calculated as when one deceased within the hospitalization time duration of their cancer COVID‐19 cohort match patient.
FIGURE 1.
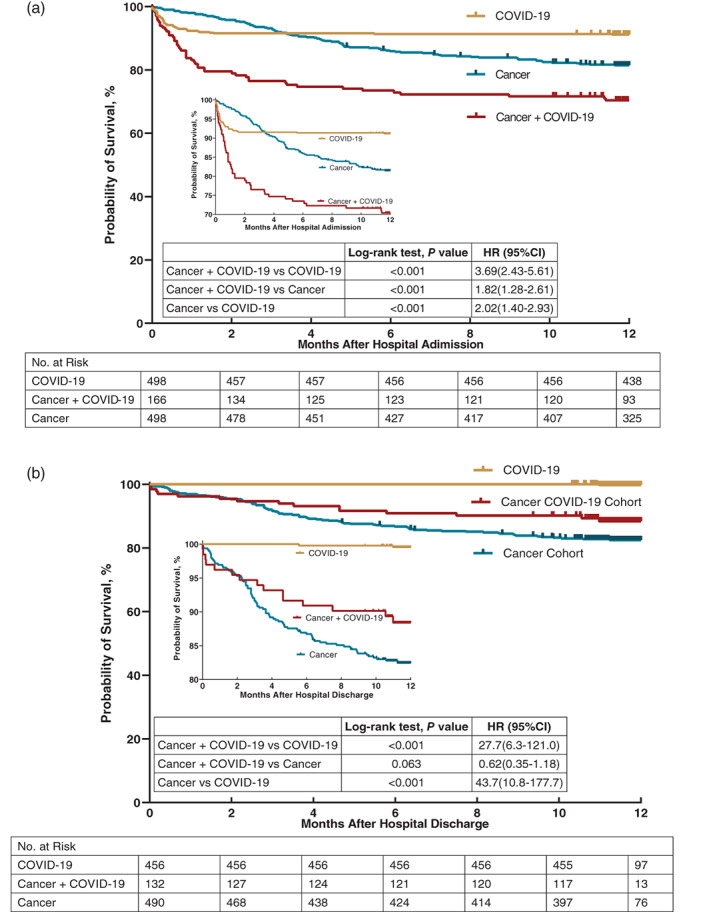
Kaplan‐Meier analysis mortality of Cancer COIVD‐19 Cohort, COVID‐19 Cohort, and Cancer Cohort. (a) Kaplan‐Meier analysis of Cancer COIVD‐19 Cohort, COVID‐19 Cohort and Cancer Cohort in 1‐year all‐cause post‐admission mortality; (b) Kaplan‐Meier analysis of Cancer COIVD‐19 Cohort, COVID‐19 Cohort and Cancer Cohort in 12‐month all‐cause post‐discharge mortality
3.3. One‐year health consequences
At the 1‐year follow‐up, 56 cancer COVID‐19 patients were excluded because 49 patients died and seven patients could not be reached, and 70 COVID‐19 patients were excluded because 44 patients died and 26 patients lost contact. As a result, 114 cancer COVID‐19 patients and 432 COVID‐19 participants were enrolled for the questionnaire interview. In this follow‐up investigation, the rate of having at least one symptom was 23% (26 /114) in Cancer COVID‐19 Cohort, generally similar to that in COVID‐19 Cohort (30%; 130/432, P = .13). Interestingly, patients in Cancer COVID‐19 Cohort were slightly likely to have fatigue (4% vs 12%, P = .016), chest congestion (3% vs 9%, P = .027), and anxiety (0 vs 5%, P = .021) than those in COVID‐19 Cohort (Table 3).
3.4. Hospital mortality
The median length of hospital stay in Cancer COVID‐19 Cohort (25 days; IQR 15–33 days) was longer than that in COVID‐19 Cohort (21 days; IQR 11–28 days; P = .005; Table S4). Patients in Cancer COVID‐19 Cohort were more likely to develop severe COVID‐19 than those in COVID‐19 Cohort (36% vs 20%, P < .001; Table 3). Cancer COVID‐19 Cohort also required significantly more mechanical ventilation than COVID‐19 Cohort (14% vs 6%, P < .001). Regarding the hospital mortality, 20% (34/166) patients in Cancer COVID‐19 Cohort died in hospital, 8% (42/498) were found in COVID‐19 Cohort (RR 0.36; 95% CI 0.22 to 0.59, P < .001), and 2% (8/498) were observed in Cancer Cohort (RR 0.10; 95% CI 0.05 to 0.19, P < .001).
3.5. Twelve‐month post‐discharge mortality
The median follow‐up time of cancer COVID‐19 patients after discharge was 11.2 (IQR 10.8–11.6) months among those who were alive (Table 3 and Figure 1B). The 12‐month all‐cause post‐discharge mortality rate was 11% (15/132) in Cancer COVID‐19 Cohort, significantly higher than that in COVID‐19 Cohort (0.4%, 2/456, P < .001) and showing no statistical difference from Cancer Cohort (15%, 72/490, P = .084).
3.6. Outcomes of primary tumor subtype
For 1‐year all‐cause post‐admission mortality, comparing to COVID‐19 Cohort (9%, 44/498), COVID‐19 patients with hematologic malignancies (65%, 11/17, P < .001) had the highest rate, followed by some solid tumors such as brain/nasopharyngeal/bone and skin tumors (45%, 5/11, P < .001), digestive system tumors (43%, 18/42, P < .001), and lung cancers (32%, 8/25, P < .001). COVID‐19 patients with breast and thyroid tumors were associated with relatively low mortality (6%, 2/33, P = .57), while patients with female genital tumors (13%, 2/16, P = .43) and genitourinary (14%, 3/22, P = .61) had moderate high mortality rate (Table 2 and Figure 2). Compared with1‐year all‐cause post‐admission mortality in the COVID‐19 Cohort (9%, 44/498), patients with hematologic, brain, nasopharyngeal, digestive system, and lung malignancies, combined as the high‐risk group (43%, 42/95, P < .001), showed a significantly higher risk of 1‐year post‐admission mortality [5.4 (3.5–8.3), P < .001] and 12‐month post‐discharge mortality [80.9 (10.5–627.0), P < .001]; patients with breast and endocrine, genitourinary, and female genital tumors, combined as the moderate‐risk group (10%, 7/71, P = .71), showed a moderate risk of 1‐year post‐admission mortality [1.6 (0.5–2.5), P = .79] and 12‐month post‐discharge mortality [6.9 (0.4–110.5), P = .17] (Figure 2).
FIGURE 2.
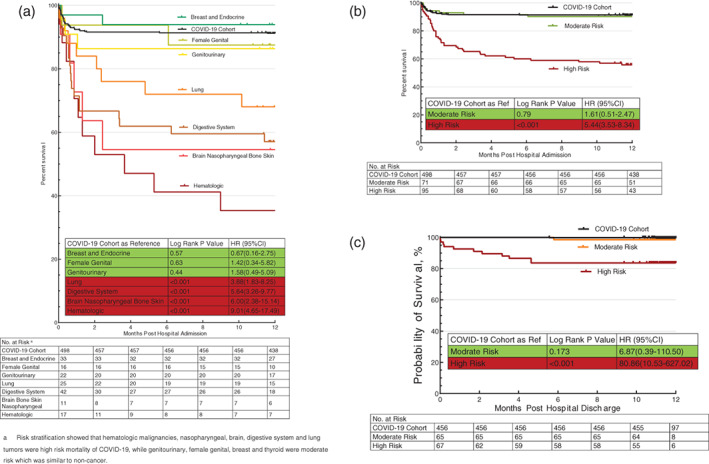
Kaplan‐Meier analysis of mortality in primary tumor subtype among Cancer COIVD‐19 Cohort. (a) Kaplan‐Meier analysis of primary tumor subtype among Cancer COIVD‐19 Cohort. Compared with the COVID‐19 Cohort, COVID‐19 patients with hematologic, brain, nasopharyngeal, digestive system, and lung malignancies showed a significantly high risk of 1‐year all‐cause post‐admission mortality, defined as the high‐risk group; while patients with breast and endocrine, genitourinary, and female genital tumors showed a moderate risk of 1‐year post‐admission mortality with no statistical difference from the COVID‐19 Cohort, defined as the moderate‐risk group. (b) Kaplan–Meier analysis of high and moderate risk stratification of primary tumor subtype among cancer COIVD‐19 Cohort in 1‐year all‐cause post‐admission mortality; (c) Kaplan‐Meier analysis of high and moderate risk stratification of primary tumor subtype among cancer COIVD‐19 Cohort in 12‐month all‐cause post‐discharge mortality
3.7. Risk factors for outcomes of Cancer COVID‐19 cohort
In the multivariate cox regression model (Figure 3), we observed that male [HR 2.0, 95% CI 1.1–3.6], severe COVID‐19 disease [non‐severe; HR 7.5, 95%CI 3.9–14.6], hyperuricemia [HR 3.9, 95% CI 1.8–8.2], stroke [HR 3.7, 95% CI, 1.1–12.9], dyspnea [HR 3.1, 95% CI 1.6–5.8], consciousness disorder [HR 9.2, 95% CI, 4.5–18.2], respiratory failure [HR 11.4, 95% CI 5.3–24.6], and acute kidney injury [HR 2.2, 95% CI 1.1–4.5] were significantly associated with increased mortality. Characteristic analysis of Cancer COVID‐19 Cohort on the 1‐year all‐cause mortality did not show any age bias.
FIGURE 3.
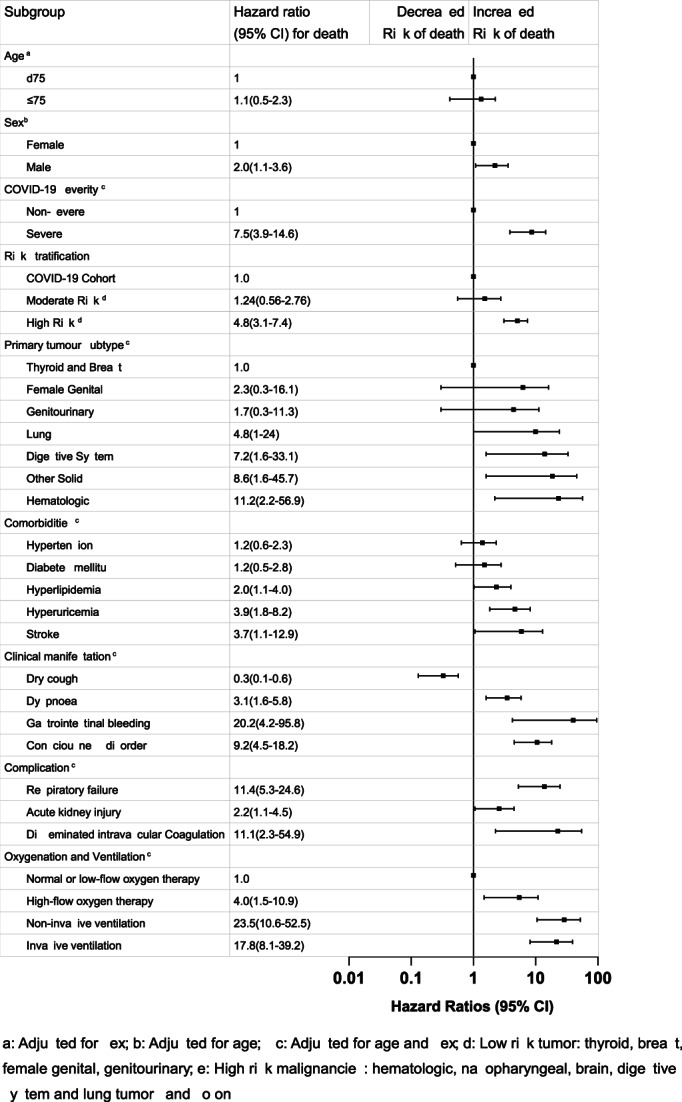
Multivariable cox regression model among Cancer COIVD‐19 Cohort for 1‐year all‐cause post‐admission mortality
4. DISCUSSION
The current COVID‐19 pandemic, a novel coronavirus first detected in Wuhan, China, has created a global crisis. The cancer center of Union Hospital of Huazhong University of Science and Technology was enlisted as a designated hospital to fight against COVID‐19. The Asian National Cancer Centers have also implemented pragmatic strategies, distancing strategies, and telemedicine in challenges and countermeasures against COVID‐19. 14 Judging from recent studies, patients with active malignancies might be more likely to contract SARS‐CoV‐2 and have an increased risk of short‐term mortality. However, the 1‐year mortality and health consequences of COVID‐19 in cancer patients have remained elusive.
To our knowledge, this study represents the longest follow‐up on the mortality and health consequences of hospitalized cancer patients with COVID‐19. One‐year mortality (30%) of Cancer COVID‐19 Cohort was nearly two times higher than that of Cancer Cohort (16%, P < .001) and more than three times higher than that of COVID‐19 Cohort (9%, P < .001). As it was difficult to determine whether COVID‐19 was the direct cause of death or an inevitably terminal event of cancer caused the death, we are reporting the all‐cause case fatality rate. All COVID‐19 patients in our cohort had finished their clinical treatment. The hospital mortality of the Cancer COVID‐19 Cohort was 20%, 2.5 times higher than that of the COVID‐19 Cohort (8%, P < .001) and 10 times higher than that of the Cancer Cohort (2%, P < .001). The difference in the hospital mortality among the three cohorts shows the strong adverse effect of COVID‐19 on both cancer and non‐cancer patients. A cohort study reported a death rate of 20% (40/205) in cancer patients with COVID‐19 in Hubei, China. 3 A series of 218 cancer patients with COVID‐19 from a New York Hospital System reported a case fatality rate of 28% because of a bias towards more severe cases. 5 The 12‐month all‐cause post‐discharge mortality rate in survival discharged Cancer COVID‐19 Cohort (8%) was higher than that in COVID‐19 Cohort (0.4%, P < .001) but similar to that in Cancer Cohort (15%, P = .084). Therefore, if cancer patients manage to survive their COVID‐19 infections, then long‐term sequelae appear to be similar to other cancer patients. The patients served as uninfected controls were based on age, gender, and cancer subtype from cancer patients admitted to the same four hospitals. The risk factors of age, gender, and cancer subtype had been excluded.
We found that at 1 year after hospital admission, 23% of cancer COVID‐19 patients endorsed at least one symptom, a similar risk to that of COVID‐19 Cohort (30%, P = .13). Cough (9%) or dyspnea (8%) were common among Cancer COVID‐19 Cohort, while fatigue (12%) or chest congestion (9%) were common in COVID‐19 Cohort. Huang and colleagues found that fatigue or muscle weakness (63%) were the most common symptoms in a 6‐month follow‐up survey of 1733 COVID‐19 patients. 15 A 3‐month follow‐up survey of 538 COVID‐19 patients reported that fatigue (28%) was the most common symptom. 16
Our results showed that different tumor subtypes among Cancer COVID‐19 Cohort had different outcomes. Compared with the COVID‐19 Cohort, COVID‐19 patients with hematologic, brain, nasopharyngeal, digestive system, and lung malignancies showed a significantly high risk of mortality (44% vs 9%, P < .001), while patients with breast and endocrine, genitourinary, and female genital tumors showed a moderate mortality risk just similar to the COVID‐19 Cohort (10% vs 9%, P = .85). This is consistent with a few other studies. Studies showed that COVID‐19 patients with hematologic malignancies were reported to have high mortality rate in China (41%‐62%), 3 , 17 USA (37%, 20/54), 5 UK (36%, 81/224), 8 and Spain (33%, 230/697). 18 Thoracic cancer patients with COVID were reported with high mortality rate in The Thoracic Cancers International COVID‐19 Collaboration (TERAVOLT) registry (33%, 66/200), 19 China (25%, 6/24), 3 USA (55%,6/11), 5 UK (39%, 43/111), 8 and Turkey [11%, 18/157 (vs total cancers 4%, 50/1122)]. 15 Digestive system tumors were associated with high mortality rate in China (23%, 9/40), 3 USA (38%,15/39), 5 UK (30%, 63/219). 8 COVID‐19 patients with breast, thyroid or endocrine tumors had lower mortality rate in China (7%, 4/56), 3 USA (13%,4/31), 5 UK (18%, 26/143), 8 and Turkey (1%, 4/442). 15 However, COVID‐19 patients with genitourinary or gynecologic tumors in our study, showing a generally low mortality rate with small sample size, had different outcomes in other studies (genitourinary: low in the USA, 15%, 7/46; high in the UK, 38%, 72/191; gynecologic, high in USA 38%, 5/13; low in UK 13%, 7/56). 5 , 8 The UK study might be more representative as it had a bigger sample size.
Many of the predictive risk factors for mortality in the Cancer COVID‐19 Cohort were similar to data reported in COVID‐19 patients. 20 In the adjusted Cox proportional hazards model, we observed significant associations of death with chronic diseases including hypertension, diabetes, hyperuricemia, COPD, cardiovascular disease, and cerebrovascular disease in COVID‐19 patients. 21 , 22 Serologic predictors for mortality in our dataset included anemia, and elevated LDH, D‐dimer, and lactic acid, which correlated with available data from all COVID‐19 patients. 23
5. LIMITATION
This study has several limitations. First of all, this study population only included hospitalized COVID‐19 patients within Hubei Province, China. Therefore, the cohort might not exactly represent all cancer patient populations, such as the patients who were on end‐of‐life care and/or residing in nursing homes, which may potentially bias our study to more severe cases of COVID‐19. Secondly, our Cancer Cohort was admitted into hospital for active cancer during a different time frame (1 January 2019 to 17 March 2020) from the COVID‐19 Cohorts (1 January 2020 to 17 March 2020), the hospitals might be under different stress levels and operation modes during these two time frames, although it should not affect the patient care significantly. Nonetheless, it would be desired to use contemporaneous control groups when these data become available. Lastly, it would be ideal to have cancer stages identified and matched between cohorts in addition to the cancer subtypes. However, the cancer staging information required extensive manual review and verifications, eventually becoming prohibitively time‐consuming and technically difficult for the involved COVID‐19 clinicians to collect and confirm on, especially on the deceased patients. Also, the sample size of stratified populations would be too limited to be meaningful. Hopefully, our larger sample size of controls compensates for some of the potential skews.
6. CONCLUSION
If cancer patients with COVID‐19 manage to survive their COVID‐19 infections and immediate sequelae, then long‐term mortality appears to be similar to the cancer patients without COVID‐19, and their long‐term clinical sequelae were similar to the COVID‐19 patients without cancer. Different tumor subtypes had different effects on COVID‐19. Comparing to the COVID‐19 Cohort, risk stratification showed that hematologic, nasopharyngeal, brain, digestive system, and lung tumors were high risk (44% vs 9%, P < .001), while genitourinary, female genital, breast, and thyroid tumors had moderate risk (10% vs 9%, P = .85) in the COVID‐19 Cancer Cohort.
Abbreviations
- COVID‐19
Coronavirus disease 2019
- RT‐PCR
Reverse Transcriptase Polymerase Chain Reaction
- SARS‐CoV‐2
Severe Acute Respiratory Syndrome Coronavirus 2
- WHO
World Health Organization
- IQR
Interquartile range
- CI
Confidence interval
- RR
Relative Risk
- HR
Hazard Ratio
- USA
The United States of America
- UK
United Kingdom
- LDH
Lactic dehydrogenase
- HFNC
High low nasal cannula
- NIV
Non‐invasive ventilation
- IMV
Invasive mechanical ventilation
- ECMO
Extracorporeal membrane oxygenation
- ALT
Alanine transaminase
- AST
Aspartate transaminase
- CRRT
Continuous renal replacement therapy
- RR
Respiratory rate
- FiO2
Fraction of inspired oxygen
- PaO2
Partial pressure of oxygen
- PEEP
Positive end‐expiratory pressure
- CPAP
Continuous positive airway pressure
- ICU
Intensive care unit
- DVT
Deep vein thrombosis
- eGFR
Estimated glomerular filtration rate
- PE
Pulmonary embolism
- COPD
Chronic obstructive pulmonary disease
- CHD
Coronary heart disease
- APTT
Activated partial thromboplastin time
CONFLICT OF INTEREST
The authors declare no conflict of interests.
AUTHOR CONTRIBUTIONS
Zehai Tang, Yuxiong Weng, Jinnong Zhang, Chen Chai conceived the project; Zehai Tang, Xiaojun Feng, Meixia Lu, Shoupeng Li, Kui Chen, Hongxiang Wang, Wendan Wang, Zhaoming Tang, Gang Cheng, Xiaoxiong Wu, Yunfeng Li, Yuying Wen, Banghong Da, Hong Fan, Lei Wang, Fen Ai, Wei Li, Cao Peng, Hongrong Zhang, and Shuang Wen analyzed the data; Zehai Tang, Chen Chai, Xiaojun Feng, Meixia Lu, Shoupeng Li, Kui Chen, Hongxiang Wang, and Wendan Wang extracted data and generated figures; Zehai Tang, Chen Chai, Xiaojun Feng, Meixia Lu, Shoupeng Li, Kui Chen, Hongxiang Wang, and Wendan Wang wrote the manuscript which was reviewed and edited by the other co‐authors.
ETHICS STATEMENT
The study was approved by the Institutional Ethics Committee of Union Hospital (2021‐0005‐01) and Institutional Ethics Committee of the Central Hospital of Wuhan (2020‐7), Tongji Medical College, Huazhong University of Science and Technology. The data used were de‐identified. No reference has been made at any point to individually identifiable data. All of the data used in this study come from Wuhan Union Hospital and its affiliated hospitals or the Central Hospital of Wuhan.
Supporting information
APPENDIX S1. Supporting Information.
ACKNOWLEDGMENTS
We thank all patients who participated in this study and their families. We also thank all the staff of follow‐up study team.
Chai C, Feng X, Lu M, Li S, Chen K, Wang H, et al. One‐year mortality and consequences of COVID‐19 in cancer patients: A cohort study. IUBMB Life. 2021;73:1244–1256. 10.1002/iub.2536
Chen Chai, Xiaojun Feng, Meixia Lu, Shoupeng Li, Kui Chen, and Hongxiang Wang have contributed equally to this study.
Funding information The Natural Science Foundation of Hubei Province, Grant/Award Number: 2020CFB804
Contributor Information
Shoupeng Li, Email: 604911775@qq.com.
Zehai Tang, Email: tangzehai@hust.edu.cn.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. World Health Organization . WHO Coronavirus Disease (COVID‐19) Dashboard. WHO (COVID‐19) Homepage: WHO Health Emergency Dashboard; 2021. https://covid19.who.int/
- 2. The International Agency for Research on Cancer of World Health Organization . Latest global cancer data: Cancer burden rises to 19.3 million new cases and 10.0 million cancer deaths in 2020. Lyon, France: The International Agency for Research on Cancer, 2020. https://www.iarc.fr/faq/latest-global-cancer-data-2020-qa/. [Google Scholar]
- 3. Yang KY, Sheng YH, Huang CL, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID‐19 in Hubei, China: A multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):904–913. 10.1016/s1470-2045(20)30310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dai M, Liu D, Liu M, et al. Patients with Cancer appear more vulnerable to SARS‐CoV‐2: A multicenter study during the COVID‐19 outbreak. Cancer Discov. 2020;10(6):783–791. 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID‐19 in a New York hospital system. Cancer Discov. 2020;10(7):935–941. 10.1158/2159-8290.Cd-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID‐19 on patients with cancer (CCC19): A cohort study. Lancet. 2020;395(10241):1907–1918. 10.1016/s0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yelin D, Wirtheim E, Vetter P, et al. Long‐term consequences of COVID‐19: Research needs. Lancet Infect Dis. 2020;20(10):1115–1117. 10.1016/s1473-3099(20)30701-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee LYW, Cozier J‐B, Starkey T, et al. COVID‐19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: A prospective cohort study. Lancet Oncol. 2020;21(10):1309–1316. 10.1016/s1470-2045(20)30442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Plass C. A new series of invited reviews on WHO tumor classification. Int J Cancer. 2020;146(12):3243–3243. 10.1002/ijc.32983. [DOI] [PubMed] [Google Scholar]
- 10. Huang C, Huang L, Wang Y, et al. 6‐month consequences of COVID‐19 in patients discharged from hospital: A cohort study. Lancet. 2021;397(10270):220–232. 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization . Clinical management of severe acute respiratory infection when Novel coronavirus (nCoV) infection is suspected: interim guidance. 2020. https://www.who.int/docs/default‐source/coronaviruse/clinical‐management‐of‐novel‐cov.pdf
- 12. Sharp MK, Glonti K, Hren D. Online survey about the STROBE statement highlighted diverging views about its content, purpose, and value. J Clin Epidemiol. 2020;123:100–106. 10.1016/j.jclinepi.2020.03.025. [DOI] [PubMed] [Google Scholar]
- 13. Uttley L, Indave BI, Hyde C, et al. Invited commentary‐WHO classification of Tumours: How should tumors be classified? Expert consensus, systematic reviews or both? Int J Cancer. 2020;146(12):3516–3521. 10.1002/ijc.32975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luh KMD, Laureline G, Kanaga S, et al. An Asian perspective of the management of COVID‐19: The Asian National Cancer Centers Alliance led regional comparison. Asian Pac J Cancer Care. 2020;5(suppl 1):27–42. 10.31557/APJCC.2020.5.S1.2. [DOI] [Google Scholar]
- 15. Özdemir N, Dizdar Ö, Yazıcı O, et al. Clinical features and outcomes of COVID‐19 in patients with solid tumors: Turkish National Registry Data. Int J Cancer. 2021;148(10):2407–2415. 10.1002/ijc.33426. [DOI] [PubMed] [Google Scholar]
- 16. Xiong Q, Xu M, Li J, et al. Clinical sequelae of COVID‐19 survivors in Wuhan, China: A single‐Centre longitudinal study. Clin Microbiol Infect. 2021;27(1):89–95. 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. He W, Chen L, Chen L, et al. COVID‐19 in persons with haematological cancers. Leukemia. 2020;34(6):1637–1645. 10.1038/s41375-020-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garcia‐Suarez J, de la Cruz J, Cedillo A, et al. Impact of hematologic malignancy and type of cancer therapy on COVID‐19 severity and mortality: Lessons from a large population‐based registry study. J Hematol Oncol. 2020;13(1):133–146. 10.1186/s13045-020-00970-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garassino MC, Whisenant JG, Huang LC, et al. COVID‐19 in patients with thoracic malignancies (TERAVOLT): First results of an international, registry‐based, cohort study. Lancet Oncol. 2020;21(7):914–922. 10.1016/s1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID‐19): A review. JAMA. 2020;324(8):782–793. 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 21. Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1–12. 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. Jama‐Journal of the American Medical Association. 2020;323(20):2052–2059. 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harrison SL, Fazio‐Eynullayeva E, Lane DA, et al. Comorbidities associated with mortality in 31,461 adults with COVID‐19 in the United States: A federated electronic medical record analysis. PLoS Med. 2020;17(9):e1003321. 10.1371/journal.pmed.1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1. Supporting Information.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


