Highlights
It is best to store COVID‐19 swab samples in VTM to get the most reliable PCR results.
Positive COVID‐19 swab samples can be stored for at least 5 days at both room temperature and 4°C without loss of positivity.
Samples with high viral loads with CTs below 25 can be stored at both room temperature and 4°C without loss of positivity for up to 12 days.
Keywords: coronavirus, COVID‐19, polymerase chain reaction, RT‐PCR, SARS‐CoV‐2, viral transport medium
Abstract
Reliable and rapid detection of severe acute respiratory syndrome coronavirus 2 in laboratory setting is critical to control the pandemic. We aimed to an evaluated polymerase chain reaction (PCR) efficiency of nasopharyngeal swabs stored in viral transport medium (VTM) in different temperatures. Ninety swabs taken into VTM were analyzed at the first hour, then divided into two groups with similar numbers of positive and negative samples. Positive samples of each group were also subgrouped according to Fam CT values as low CT (<25), medium CT (25–32), and high CT (32–38) groups. One group was stored at 4°C, while the other was stored at room temperature, PCR analyses were repeated every 24 h for 5 days and on Day 12. There was a total of 30 positive samples (12 low CT, 11 medium CT, and 7 high CT). The CT values of both groups remained unchanged in first 3 days while the CT values of the room temperature group increased after the third day. All of the positive samples remained positive in both groups for the first 5 days. On the 12th day, the total number of positives decreased to 8 in the room temperature group and 11 in the 4°C groups. All the low CT samples remained positive in both groups. In conclusion, it is safe to store positive samples in room temperature for up to 5 days. Only samples with high viral loads remain positive for 12 days, regardless of whether stored at room temperature or 4°C. Negative samples don't turn to invalid if stored in VTM.
1. INTRODUCTION
Since the early 21st century, three types of coronaviruses have affected mankind through deadly pneumonia. The severe acute respiratory syndrome coronavirus (SARS‐CoV), which emerged in Guangdong, China, infected 8098 people and 29 countries in 2003, while the Middle East respiratory syndrome coronavirus emerged in Saudi Arabia in 2012 and spread to 27 different countries. However, nowadays, a new member of the coronavirus family, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) first reported in the Wuhan city of China in December 2019 has caused a global pandemic all over the world and infected more than 184 million people, killed more than 3.9 million according to the last update in July 2021. 1 , 2
In COVID‐19 (coronavirus disease 2019), people get infected usually through the airway with respiratory droplets, although infections via contaminated surfaces and close contacts were also shown. Following infection, an incubation period of SARS‐CoV‐2 is on average 5–6 days up to 14 days. 3 Viral load and the contagiousness of the disease are very high during incubation and the first days of the disease. The symptoms show up mostly with fever, fatigue, myalgia, back pain, cough, loss of taste and smell, and diarrhea. In severe cases, it deteriorates to pneumonia and respiratory failure. 4 On the other hand, asymptomatic cases have been reported with positive polymerase chain reaction (PCR) results depending on their viral loads. Estimates of the proportion of asymptomatic cases range from 8% to 80%. 5
In the clinical practice of COVID‐19 fast and reliable diagnosis of the infected people gain importance to limit the spread of the infection. The gold standard of the diagnosis is a positive real‐time reverse transcriptase‐polymerase chain reaction test (RT‐PCR). 6 Various techniques and materials have been developed such as various sampling methods, transport media, test kits, PCR conditions, and storage conditions to avoid false test results. 7 Although the number of molecular virology laboratories increases day by day, the number of infected people is very high and increases in different periods that this number may exceed the capacity of the laboratories. Thus, the need for storing the samples for long periods before RT‐PCR could arise. The recommended storage condition for nasopharyngeal swabs is +4°C during transport and it is recommended that RT‐PCR be performed as soon as possible. It is stated that the sample can be stored at 4°C or between 2°C and 8°C for up to 4 or 5 days if the swab is in viral transport medium (VTM). 6 , 8 Here we aimed to compare RT‐PCR results of nasopharyngeal swabs taken into VTM and stored in different conditions to see in which situations the test results are affected.
2. MATERIAL AND METHODS
2.1. Sample collection, transportation, and storage
For the diagnosis of COVID‐19, nasopharyngeal swabs were collected by trained personnel and transferred to Kanuni Sultan Suleyman Training and Research Hospital in VTM. All samples were transferred to our COVID‐19 Diagnostic Center within 1 h. Ninety randomly selected samples were tested with Bio‐Speedy (SARS CoV‐2 Double Gene RT‐qPCR Kit [version 1]) RT‐PCR kit and analyzed on Biorad CFX96 platform and all results and quantification cycle (CT) values were evaluated. A second experiment was done in the 0–3 h. period for comparison of the CT values of the samples on Roche LightCycler480. Then the samples were divided into two groups possessing an equal number of negative and positive samples. One group was stored at +4°C, while the other group was stored at room temperature (20–25°C). All swab samples were stored in VTM solution in test tubes.
2.2. RT‐PCR tests
All swab samples were tested every 24 h for 5 days and on the 12th day by RT‐PCR SARS‐CoV‐2 detection kit according to the kit protocols. The preferred kit did not require any extra RNA extraction step due to the use of VTM with nucleic acid extraction property. Vigorous vortexing of VTM solution was enough for RNA extraction. Utilized primers of the kit were designed for the conserved regions of ORF1ab and RNaseP genes of SARS‐CoV‐2. Analysis in Fam and Hex channels was recommended in the kit protocol for ORFlab and RNaseP gene, respectively. All PCRs were performed by the same two operators and analyzed with both Biorad CFX96 and Roche LightCycler480 platforms.
Bio‐Speedy SARS CoV‐2 Double Gene RT‐qPCR Kit (version 1) kit was used for PCR; according to kit protocol, 5 µl patient samples with VTM were added to 15 µl ready kit mixture to achieve 20 µl PCR mixture in total. Thermal cycle parameters of RT‐PCR amplification were as follows: 52°C for 5 min for reverse transcription, 95°C for 10 s for holding, then 40 cycles of 95°C for 1 s, and 55°C for 30 s for denaturation, annealing, and extension, respectively.
2.3. Test interpretation
On Biorad CFX96 platform the threshold value was set as 200 according to kit protocol and on Roche Light Cycler 480 platform it was set automatically. The positive values were interpreted as sigmoids with CT values below 38 for Fam channel irrespective of Hex values. Nonsigmoidal signals or sigmoidal signals with CT values above 38 in the Fam channel and sigmoidal signals with CT values below 38 in the Hex channel were interpreted as negative. Nonsigmoidal signals and sigmoids below 38 CT on both Fam and Hex channels were interpreted as invalid results according to the kit protocol.
2.4. Statistical analysis
Statistical analysis was performed using Microsoft Excel version 2019. The correlation coefficient was calculated by t‐test; p value was chosen as 0.05. When p value is made meaningful by using descriptive statistics, no significant difference was detected between CT values according to the p < 0.05 results of CT values.
2.5. Statement of ethics
All experiments were performed in compliance with relevant laws and institutional guidelines and in accordance with the ethical standards of the Declaration of Helsinki. This study protocol was reviewed and approved by Ethics Committee of Kanuni Sultan Suleyman Training and Research Hospital (KAEK: 2021.03.84).
3. RESULTS
In this study, nasopharyngeal swab samples taken into and stored in VTM with RNA extraction before storage were evaluated. The total number of the samples were 90, a total of 30 samples were positive. In the first study on Biorad CFX96 there were 27 positives while in the repeat of the same study on Roche Light Cycler 480 there were 29 positive samples. These conflicting numbers were because the samples with low viral load remained under the threshold in some of the studies and were evaluated as negative (Table 1). We grouped the positives according to CT values as low (CTs < 25, nr: 12), medium (CTs between 25 and 32, nr: 11), and high (CTs between 32 and 38, nr: 7) (Table 1). CT values did not change significantly in the first 3 days in any of the groups, CT values started to increase in samples stored at room temperature on the fourth day (Table 1). The number of samples with low CT decreased while the number of ones with medium CT increased after the third day at the room temperature group. In the first 5 days, the total number of positives remained nearly the same in both groups. There were 29 positives on Biorad CFX96 platform, and 28 positives on Roche Light Cycler 480 platform on the fifth day. Sample 14 in the room temperature group turned to negative after 24 h. and sample 15 in 4°C group turned negative in the fourth day on Roche Light Cycler 480. Also, sample 14 turned to negative on Biorad CFX96 after 48 h. On the 12th day, 7 of the positive samples stored at room temperature were evaluated as negative on both analyzers; 4 and 5 of the positive samples stored at 4°C were evaluated as negative on Biorad CFX96 and Roche LightCycler480, respectively. On the 12th day, there were no low Fam CT value on the room temperature group, while there were 2 and 3 low CT values on the Biorad CFX96 and Roche LightCycler480 analyzer results, respectively. The mean CT values of all positives increased in both groups as shown in Figure 1A,B. All of the low CT samples remained positive on the 12th day study (one was negative only on Biorad CFX96 run). Hex CT values of negative samples were above 38 in all experiments thus there was no invalid sample in any of the experiments.
Table 1.
FAM channel CT values of positive samples in both temperatures stored and, in both analyzers
| 0 | 0–3 h | D1 | D2 | D3 | D4 | D5 | D12 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CT | Sample | B | R | B | R | B | R | B | R | B | R | B | R | B | R | |
| <25 | 1 | 21.98 | 21.1 | 21.76 | 22.56 | 23.09 | 21.98 | 22.59 | 22.8 | 24.31 | 22.84 | 22.07 | 23.8 | 26.01 | 25.4 | |
| 2 | 22.1 | 17.94 | 21.36 | 21.36 | 23.02 | 23.52 | 23.94 | 22.66 | 26.21 | 25.67 | 24.27 | 26.79 | 27.89 | 28.7 | ||
| 3 | 22.25 | 19.94 | 23.45 | 21.8 | 24.73 | 20.75 | 24.04 | 22.06 | 26.07 | 24.14 | 24.9 | 25.56 | 30.44 | 28.55 | ||
| 4 | 22.59 | 21.73 | 22.37 | 22.72 | 23.11 | 23.21 | 33.01 | 24.22 | 25.44 | 25.23 | 25.03 | 26.05 | 29.13 | 28.84 | ||
| 5 | 24.38 | 23.23 | 24.58 | 25 | 25.08 | 24.73 | 26.12 | 25.06 | 26.52 | 26.93 | 25.76 | 27.29 | 30.25 | 29.97 | ||
| 25–32 | 6 | 25.58 | 20.09 | 21.25 | 21.72 | 22.06 | 22.41 | 23.63 | 23.67 | 25.95 | 26.44 | 26.37 | 28.86 | 30.43 | 30.4 | |
| Room | 7 | 28.76 | 26.71 | 28.51 | 28.46 | 28.31 | 28.16 | 28.79 | 27.78 | 29.88 | 29.37 | 27.92 | 30.98 | – | – | |
| Temperature | 8 | 29.3 | 26.81 | 29.92 | 27.74 | 30.72 | 28.66 | 28.04 | 28 | 31.31 | 31.04 | 28.52 | 31.71 | – | – | |
| 9 | 29.05 | 27.67 | 27.83 | 28.35 | 28.01 | 27.56 | 28.39 | 28.66 | 28.16 | 28.7 | 27.33 | 28.86 | 34 | 34.62 | ||
| 10 | 29.38 | 27.62 | 26.99 | 28.56 | 27.39 | 27.94 | 27.94 | 29 | 29.32 | 29.88 | 29.23 | 29.57 | – | – | ||
| 11 | 30.76 | 30.63 | 29.38 | 28.62 | 34.73 | 30.37 | 34.16 | 29.11 | 33.53 | 32.61 | 31.87 | 33.29 | – | – | ||
| 12 | 30.9 | 27.67 | 26.95 | 27.7 | 28.07 | 28.04 | 28.37 | 27.82 | 28.33 | 28.96 | 27.37 | 29.25 | – | – | ||
| 32–38 | 13 | 32.18 | 28.83 | 29.01 | 29.51 | 29.63 | 30.66 | 29.67 | 28.47 | 30.06 | 30.54 | 28.34 | 30.02 | 33.68 | 36.14 | |
| 14 | 35.83 | 33.01 | 34.62 | – | – | – | – | – | – | – | – | – | – | – | ||
| 15 | – | 36.81 | 34.62 | – | – | 34.77 | 32.66 | 36.95 | 34.51 | 36.78 | 32.89 | 35.96 | – | – | ||
| 0–3 h | D1 | D2 | D3 | D4 | D5 | D12 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CT | B | R | B | R | B | R | B | R | B | R | B | R | B | R | ||
| <25 | 1 | 19.13 | 17.68 | 20.42 | 20.58 | 22.01 | 18.53 | 20.6 | 17.38 | 21.58 | 19.49 | 19.37 | 20.48 | 19.9 | 17.84 | |
| 2 | 20.09 | 19.68 | 20.12 | 19.81 | 19.52 | 19.16 | 20.11 | 19.15 | 19.38 | 19.66 | 18.78 | 17.76 | 19.51 | 18.38 | ||
| 3 | 22.99 | 20.82 | 22.64 | 21.95 | 22.09 | 21.25 | 21.47 | 20.67 | 22.81 | 22.63 | 30.26 | 19.5 | 27.29 | 26.88 | ||
| 4 | 22.05 | 20.92 | 23.71 | 24.57 | 23.54 | 22.48 | 22.67 | 21.81 | 22.28 | 22.56 | 21.56 | 20.44 | 31.05 | 29.74 | ||
| 5 | 23.89 | 22.75 | 24.03 | 23.5 | 23.4 | 24.6 | 22.63 | 21.81 | 23.44 | 25.35 | 21.66 | 22.61 | – | 34.51 | ||
| 6 | 24.12 | 21.69 | 23.6 | 24.88 | 23.97 | 23.81 | 26.09 | 23.42 | 24.87 | 24.42 | 23.74 | 22.15 | 27.18 | 25.88 | ||
| 7 | 24.42 | 22.63 | 24.03 | 23.6 | 23.1 | 22.48 | 23.57 | 20.56 | 23.47 | 24.47 | 21.8 | 22.88 | 29.84 | 30.91 | ||
| 25–32 | 8 | 25.42 | 23.33 | 25.07 | 24.36 | 23.68 | 23.57 | 23.1 | 24.6 | 24.73 | 23.56 | 21.74 | 23.22 | 25.64 | 24.48 | |
| 4°C | 9 | 26.36 | 24.61 | 25.05 | 25.39 | 25.39 | 24.42 | 24.26 | 24.35 | 25.01 | 24.42 | 23.49 | 22.77 | 31.78 | – | |
| 10 | 29.94 | 26.34 | 27.22 | 27.57 | 27.12 | 26.22 | 26.7 | 26.13 | 27.18 | 26.94 | 25.85 | 26.21 | 25.06 | 25.85 | ||
| 11 | 30.26 | 27.53 | 29.19 | 28.94 | 28.48 | 28.71 | 27.62 | 27.29 | 28.1 | 28.94 | 26.44 | 28.43 | – | – | ||
| 32–38 | 12 | 34.24 | 26.17 | 28.06 | 30.57 | 28.76 | 27.88 | 33.35 | 29.21 | 31.9 | 38.1 | 29.04 | 30.56 | 29.51 | 29.47 | |
| 13 | 35.97 | 33.01 | v | – | 34.46 | 33.45 | 33.47 | 33.83 | 33.09 | 32.91 | 32.1 | 33.9 | 31.99 | – | ||
| 14 | v | 31.83 | 32.9 | 32.45 | 32.28 | 31.36 | 35.1 | 30.34 | 33.4 | 32.28 | 30.01 | 31.92 | – | – | ||
| 15 | – | – | 34.82 | 34.26 | ‐ | 34.68 | –‐ | 34.96 | – | – | 33.8 | – | – | – | ||
Abbreviations: B, Biorad CFX96; R, Roche Light Cycler 480.
Figure 1.
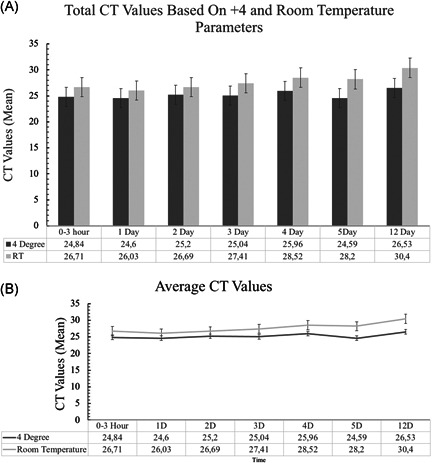
(A) and (B) Means and 95% confidence interval of CT values for both temperature conditions through all the studies. Mean values are also presented at the bottom of the diagrams
Although in some studies the CT values of some samples were lower on Roche Light Cycler 480 comparing to Biorad CFX96, there was no significant difference in general for most of the samples as shown in Figure 2.
Figure 2.
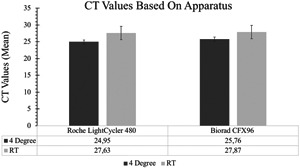
Comparison of polymerase chain reaction analyzers according to means and 95% confidence interval of CT values. Mean values are also presented at the bottom of the diagrams
4. DISCUSSION
Since December 2019, the world has been battling a highly contagious and deadly viral pandemic. It is vital to isolate infected individuals and diagnose them quickly and reliably to control the spread of the disease. RT‐PCR is still the most accurate and fast tool for the diagnosis of SARS‐CoV‐2 infection. The efficiency of the PCR and interpretation of the results can be affected by various preanalytical and postanalytical factors. These factors were evaluated in detail by Lippi et al. 6 As it is stated in the report the preanalytical phase is the major source of errors in laboratory tests. These factors include: Inadequate specimen collection, handling, transport, and storage; collection of inappropriate or inadequate material for quality or volume; manual‐operator (such as pipetting) errors and sample contamination. Although the major critical preanalytical factor is the viral load in the patient and in the specimen collected from the patient, the transportation and storage conditions of the samples are also important factors to consider in the interpretation of the test results. If samples are enclosed in VTM, the CDC (disease control and prevention centers) recommends that samples be stored at 2–8°C for up to 4 days, while WHO recommends that they can be stored at 4°C for a maximum of 5 days. 6 , 8
VTMs are used to preserve viruses during transport and storage before PCR or virus cultures. 9 , 10 They are designed to preserve the virus being dealt with, while preventing contamination with other agents such as bacteria or fungi. In different articles, it was recommended to use dry swabs taken into saline solutions like phosphate‐buffered saline or Tris EDTA buffer in the absence or depletion of VTM in pandemics like nowadays. 11 , 12 While VTMs are used for the preservation of the viruses they can also be used for virus inactivation and extraction of the viral nucleic acid for PCR. Our COVID‐19 diagnostic center is one of the most high‐capacity centers in Turkey. As around the world, at the beginning of the pandemic, the supply of standard VTMs was not sufficient for high demand, and swab samples were transferred to our center as a dry swab or saline solution. In those days, we observed that samples degraded and molded‐in as little as 24 h, and also observed that the number of samples with invalid results without the internal control signal or with abnormal signals was quite high, possibly due to these contaminated and degraded samples. With the use of VTMs, the number of invalid results significantly decreased. In our study we observed the effect of VTM, so none of our negative samples lost the internal control (Hex channel) signal in any run.
In different studies, PCR results of samples of SARS‐CoV‐2 stored at different temperatures for different periods were compared. 13 , 14 , 15 Rogers et al. 13 compared the PCR results of different sample types (oropharyngeal swab, nasopharyngeal swab, sputum, and bronchoalveolar lavage fluid) stored for up to 14 days at temperatures ranging from 26 to −30°C. They observed that the differences between the CT values of positive samples, even when stored in saline, were not more than two amplification cycles and did not turn negative even on the 14th day. 13 In the study of Basso et al. the effect of storage in room temperature and 4°C for 5 days were compared. In this study, samples were taken into VTMs without nucleic acid extraction feature. For only one group of samples, the authors performed additional nucleic acid extraction before storage. They observed that the CT values of samples kept at room temperature without prior nucleic acid extraction increased after 48 h. And the most stable CT values observed belonged to samples stored at 4°C with prior nucleic acid extraction. They have concluded that prior nucleic acid extraction before storage maximizes the RNA preservation of the sample. Another observation by the authors was that samples with low viral load and CT values above 33 may yield unreliable results in repeated tests during storage. 15 In the study of Agaoglu et al., 14 30 positive samples with initial CT values below 29 and stored at 4°C and room temperature were tested for 9 days. Since most of the sample volumes were not sufficient to complete the study, they did not observe any sample that turned negative in the run from samples remaining on Day 9. Unlike other studies, they observed that samples stored at room temperature had more stable CT values during tests. 14 In our study, the VTMs used had the feature of RNA extraction, thus providing additional preservation of the viral load in the samples. During our study, similar to Basso's study, samples kept at room temperature began to lose their viral load, but unlike their study, this decrease was observed after 72 h rather than 48 h, which may be due to the use of the RNA extraction step before storage, unlike theirs. Most of the samples stored at 4°C had CT values almost the same over 5 days. Most of the samples stored at room temperature had CT values increased by 1–4 amplification cycles at the end of Day 5.
In the study of Rogers et al., 13 they observed a maximum increase of two amplification cycles in samples stored at room temperature even on Day 14. In a study with respiratory viruses, the authors detected and isolated viral nucleic acid from a dry nasopharyngeal swab even after 2 weeks. 16 The maximum duration of research for SARS‐CoV‐2 in the literature was 14 days. 13 However, the study was done with very few samples. In our study, we used a complete batch of 90 samples containing 30 positives to observe different probabilities for samples with different values, and we observed that the stability of the positives was not as in the literature, while the negatives did not lose the internal control signals. On the 12th day, the difference between the room temperature group and the 4°C group became more pronounced as almost all medium and high CT samples at room temperature turned negative. Unlike the reported studies, although most of the samples in the 4°C group remained positive, five samples in this group also turned negative. On Day 12, all positive samples in the room temperature group appeared to have significantly increased CT values compared to their initial CT values, while half of the samples in the 4°C group (5 out of 9) retained their initial CT values.
Basso et al. 15 reported that samples with CT values above 33 tend to give variable results. Although the variability observed in their study had increased over time, our observation in our study was that samples with high CT (>32) values tend to give false‐negative results in different studies regardless of time and temperature. These samples may be considered as samples of subjects with a low viral load or as insufficiently taken swabs or samples contaminated during swab collection or in the laboratory environment. Since we may not know exactly which scenario was the reason, our recommendation would be to request new samples from patients with sample CTs above 32.
In Table 1, we observed significant differences between the results of the same day or consecutive days for a few samples, although the same expert operators performed the PCR procedures, we think this was due to manual errors.
We chose to analyze samples on two different platforms to evaluate the efficiency of the platforms we use in our routine laboratory. Platforms did not differ significantly over the course of the study.
In conclusion, it is best to store samples in VTM to get the most reliable results and to avoid test and sample repeats due to invalid results. Even under the best conditions where swab samples are taken into VTMs and stored after nucleic acid extraction, samples can only be stored at room temperature for up to 3 days without a reduction in viral load. Positive samples can be stored for at least 5 days at both room temperature and 4°C without loss of positivity. Samples with high viral loads with CTs below 25 can be stored at both room temperature and 4°C without loss of positivity for up to 12 days, however, samples with lower viral loads with CTs above 25 cannot be stored for 12 days. For samples with CT values above 32, we recommend repeating the sample to avoid misinterpretation of cases.
With this study, we tried to create a guide on under which conditions we can obtain more reliable results in routine laboratory studies of SARS‐CoV‐2.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Elif Yilmaz Gulec: Developed the protocol, abstracted and analyzed data, wrote the manuscript, and is the guarantor. Nevra P. Cesur: Contributed to the development of the protocol, methodology, review, and editing. Gonca Yesilyurt Fazlioglu: Contributed to the development of the protocol, methodology, review, and editing. Cemal Kazezoglu: contributed to the development of protocol and methodology.
ACKNOWLEDGMENTS
The present work was supported by Bioeksen AR‐GE teknolojileri Ltd Şti. The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication. Special thanks to Arzu Tanrıverdi and Ayhan Demir for their valuable contribution to this study. We would like to thank all COVID‐19 Diagnostic Center staff for their devoted efforts during the entire pandemic period.
Yilmaz Gulec E, Cesur NP, Yesilyurt Fazlioğlu G, Kazezoğlu C. Effect of different storage conditions on COVID‐19 RT‐PCR results. J Med Virol. 2021;93:6575‐6581. 10.1002/jmv.27204
Nevra P. Cesur, Gonca Yesilyurt Fazlioglu, and Cemal Kazezoglu are co‐authors.
This study was conducted in COVID‐19 Diagnostic Center, Health Sciences University, Istanbul Kanuni Sultan Suleyman Training and Research Hospital, Istanbul, Turkey.
REFERENCES
- 1. Lam WK, Zhong NS, Tan WC. Overview on SARS in asia and the world. Respirology. 2003;8(Suppl 1):S2‐S5. 10.1046/j.1440-1843.2003.00516.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID‐19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91‐98. 10.1016/j.jare.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382:1199‐1207. 10.1056/nejmoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Byambasuren O, Cardona M, Bell K, Clark J, McLaws ML, Glasziou P. Estimating the extent of asymptomatic COVID‐19 and its potential for community transmission: systematic review and meta‐analysis [published online ahead of print September 13, 2020]. J Assoc Med Microbiol Infect Dis Canada. 2020. 10.3138/jammi-2020-0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lippi G, Simundic AM, Plebani M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID‐19). Clin Chem Lab Med. 2020;58(7):1070‐1076. 10.1515/cclm-2020-0285 [DOI] [PubMed] [Google Scholar]
- 7. Da Costa JP, Barros H, Middleton J, et al. COVID‐19 testing: A reflection on test accuracy in the real world. 2020. http://asset.youoncdn.com/ab296ab30c207ac641882479782c6c34/7c558d9ed2c5af4f35a5812eee70ca3d.pdf
- 8. World Health Organization . Laboratory testing for coronavirus disease 2019 (COVID‐19) in suspected human cases. 2020;(March):1‐7.
- 9. Jensen C, Johnson FB. Comparison of various transport media for viability maintenance of herpes simplex virus, respiratory syncytial virus, and adenovirus. Diagn Microbiol Infect Dis. 1994;19(3):137‐142. 10.1016/0732-8893(94)90055-8 [DOI] [PubMed] [Google Scholar]
- 10. Druce J, Garcia K, Tran T, Papadakis G, Birch C. Evaluation of swabs, transport media, and specimen transport conditions for optimal detection of viruses by PCR. J Clin Microbiol. 2012;50(3):1064‐1065. 10.1128/JCM.06551-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perchetti GA, Nalla AK, Huang M, et al. Validation of SARS‐CoV‐2 detection across multiple specimen types. J Clin Virol. 2020;20:128104438. 10.1016/j.jcv2020104438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kiran U, Gokulan CG, Kuncha SK, et al. Easing diagnosis and pushing the detection limits of SARS‐CoV‐2. Biol Methods Protoc. 2021;5(1):1‐5. 10.1093/biomethods/bpaa017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rogers AA, Baumann RE, Borillo GA, et al. Evaluation of transport media and specimen transport conditions for the detection of SARS‐CoV‐2 by use of real‐time reverse transcription‐PCR. J Clin Microbiol. 2020;58(8):1‐5. 10.1128/JCM.00708-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agaoglu BN, Yıldız J, Akgun Dogan O, et al. COVID‐19 PCR test performance for samples stored at ambient temperature [published online ahead of print June 18, 20120]. bioRxiv. 2020. 10.1101/2020.06.15.153882 [DOI] [Google Scholar]
- 15. Basso D, Aita A, Navaglia F, et al. SARS‐CoV‐2 RNA identification in nasopharyngeal swabs: issues in pre‐analytics. Clin Chem Lab Med. 2020;58(9):1579‐1586. [DOI] [PubMed] [Google Scholar]
- 16. Moore C, Corden S, Sinha J, Jones R. Dry cotton or flocked respiratory swabs as a simple collection technique for the molecular detection of respiratory viruses using real‐time NASBA. J Virol Methods. 2008;153(2):84‐89. 10.1016/j.jviromet.2008.08.001 [DOI] [PubMed] [Google Scholar]


