Abstract
Background
Coronavirus disease 2019 (COVID‐19) and cancer are serious public health problems worldwide. However, little is known about the risk factors of in‐hospital mortality among COVID‐19 patients with and without cancer in Brazil. The objective of this study was to evaluate the risk factors of in‐hospital mortality among COVID‐19 patients with and without cancer and to compare mortality according to gender and topography during the year 2020 in Brazil.
Methods
This was a secondary data study of hospitalized adult patients with a diagnosis of COVID‐19 by real‐time polymerase chain reaction testing in Brazil. The data were collected from the Influenza Epidemiological Surveillance Information System.
Results
This study analyzed data from 322,817 patients. The prevalence of cancer in patients with COVID‐19 was 2.3%. COVID‐19 patients with neurological diseases and cancer had the most lethal comorbidities in both sexes. COVID‐19 patients with cancer were more likely to be older (median age, 67 vs 62 years; P < .001), to have a longer hospital stay (13.1 vs 11.5 days; P < .001), to be admitted to the intensive care unit (45.3% vs 39.6%; P < .001), to receive more invasive mechanical ventilation (27.1% vs 21.9%), and to have a higher risk of death (adjusted odds ratio [aOR], 1.94; 95% confidence interval [CI], 1.83‐2.06; P < .001) than those without cancer. Patients with hematological neoplasia (aOR, 2.85; 95% CI, 2.41‐3.38; P < .001) had a higher risk of mortality than those with solid tumors (aOR, 1.83; 95% CI, 1.72‐1.95; P < .001) in both sexes.
Conclusions
Brazilian COVID‐19 patients with cancer have higher disease severity and a higher risk of mortality than those without cancer.
Keywords: Brazil, cancer, coronavirus disease 2019 (COVID‐19), mortality, prevalence, risk factors
Short abstract
Patients with hematological neoplasia (adjusted odds ratio [aOR], 2.85; 95% confidence interval [CI], 2.41‐3.38; P < .001) have a higher risk of mortality than those with solid tumors (aOR, 1.83; 95% CI, 1.72‐1.95; P < .001) in both sexes.
Introduction
The emergence of coronavirus disease 2019 (COVID‐19) has caused an unprecedented public health crisis in the world since December 2019. Up to December 31, 2020, the World Health Organization reported more than 82 million confirmed cases and approximately 1.8 million deaths in the world. In the same period, Brazil reported more than 7.6 million confirmed cases and almost 195,000 deaths. 1 Cancer is also a serious public health problem, and it is increasing as a leading cause of mortality because of the aging population and the prevalence of important risk factors as well as a marked decline in mortality due to stroke and heart disease. 2 , 3
Patients with cancer have been associated with higher risks of viral infection, the development of COVID‐19–related complications, and mortality. 4 , 5 , 6 , 7 COVID‐19 patients with cancer are at increased risk for mortality according to age, gender, and comorbidities. 8 , 9 Male COVID‐19 patients with 10 or without cancer 11 , 12 have greater severity and mortality. Nevertheless, the prevalence of cancer in patients with COVID‐19 is usually low, approximately 2%, 13 and the mortality of COVID‐19 patients with and without cancer in developing countries such as Brazil remains poorly explored.
Furthermore, mortality due to COVID‐19 in patients with cancer could be heterogeneous. Cancer patients with different tumor types could have differing susceptibility to severe acute respiratory syndrome coronavirus 2 infections and mortality, so it would be not accurate to classify all patients with cancer as equally vulnerable. 9 In a pioneering Brazilian study, COVID‐19 patients with lung and hematological cancers were found to have a greater risk of death when they were undergoing oncological treatment. 14 However, these studies failed to determine the risk of mortality in COVID‐19 patients with cancer according to gender and in different topographies. Recently, 2 other Brazilian studies using a large nationwide surveillance database showed higher in‐hospital mortality for COVID‐19 patients who were from the northern and northeastern regions of the country, who were brown‐ and black‐skinned, 15 who were older, and who were admitted to the intensive care unit (ICU). 16 However, these studies did not evaluate the risk of mortality in COVID‐19 patients with cancer. Therefore, this study was aimed at evaluating the prevalence of patients with cancer as well as the severity and risk factors of in‐hospital mortality among COVID‐19 patients and at analyzing mortality according to gender and topography.
Materials and Methods
Study Design
This was a secondary data study of hospitalized adult patients with a diagnosis of COVID‐19 in Brazil. The data were retrieved from the Influenza Epidemiological Surveillance Information System (Sistema de Informação de Vigilância Epidemiológica da Gripe [SIVEP‐Gripe] in Portuguese). This system is used by the epidemiological surveillance of states and municipalities to insert the files of severe acute respiratory syndrome cases seen in hospitals and emergency care units (Unidades de Pronto Atendimento in Portuguese). Data were publicly available online at https://covid.saude.gov.br and were retrieved on January 17, 2021.
Patients were included in the study if they had COVID‐19 confirmed by real‐time polymerase chain reaction testing and were admitted between March 1 and December 31, 2020. Patients younger than 20 years, patients living outside Brazil, patients without discharge date data, and patients whose discharge date happened after December 31, 2020, were excluded from the analysis (Fig. 1).
Figure 1.
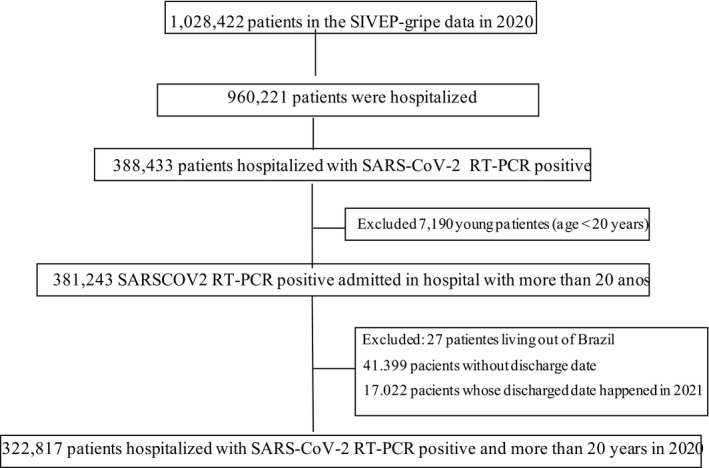
Flowchart of the SIVEP‐Gripe data used in this study. RT‐PCR indicates real‐time polymerase chain reaction; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; SIVEP‐Gripe, Sistema de Informação de Vigilância Epidemiológica da Gripe.
The following demographic, epidemiological, and clinical variables were evaluated: age at diagnosis, sex, self‐reported ethnicity/skin color (according to the Brazilian Institute of Geography and Statistics), self‐reported level of education, main symptoms of COVID‐19, and presence of comorbidities potentially associated with the severity of COVID‐19 (with an emphasis on cancer topographies). In addition, data were collected upon ICU admission on the following: mortality, need for respiratory support (noninvasive vs invasive), time in the ICU, hospital length of stay, and hospital mortality.
Statistical Analysis
Data were analyzed with SPSS version 24.0. The analysis was performed with valid data only. A descriptive analysis of the study population was performed with mean and standard deviation measures for continuous variables and with absolute and relative frequency distributions for categorical variables. The Kolmogorov‐Smirnov test was used to check the normal distribution of continuous variables. The t test was used to compare continuous variables, and the χ2 test was performed with the objective of comparing the categorical data. Differences were considered significant when the P value was <.05. A logistic regression analysis was used to explore the association between the comorbidities, with an emphasis on cancer topographies, and the risk of death. The variables whose associations with outcomes in univariate analyses exhibited P values < .15 were sequentially tested in a multivariate model; we started with the variable most strongly associated with the risk of death and continued until no other variable reached significance. 17 Variables with P < .05 were maintained in the final model.
Ethics
Ethics committee approval was not required because only secondary data available on the internet were used in this study.
Results
In total, 322,816 COVID‐19 patients were identified; 56.2% were men, the mean age was 61 ± 17 years, and the most frequent skin color was white (55% of cases). In the total population studied, 2.3% of the patients had a history of cancer. Comparing COVID‐19 patients with and without cancer showed that patients with cancer were older (median, 67 vs 62 years), and a majority of the patients with cancer were White (62.2% vs 54.8% of patients without cancer) with a level of education up to high school (53.0% vs 45.1% of patients without cancer; Table 1).
TABLE 1.
Demographic Characteristics of COVID‐19 Patients With and Without Cancer in Brazil in 2020
| Characteristic | All Patients (n = 322,816 [100%]) | Cancer (n = 7406 [2.3%]) | No Cancer (n = 315,410 [97.7%]) | P a |
|---|---|---|---|---|
| Age (n = 322,816) | ||||
| Mean ± SD, y | 61 ± 17 | 66 ± 16 | 61 ± 145 | <.001 |
| Median, y | 62 | 67 | 62 | |
| Age groups (n = 322,816), No. (%) | <.001 | |||
| 20‐39 y | 39,690 (12.3) | 421 (5.7) | 39,269 (12.5) | |
| 40‐49 y | 45,010 (13.9) | 627 (8.5) | 44,383 (14.1) | |
| 50‐59 y | 59,847 (18.5) | 1155 (11.6) | 58,692 (18.6) | |
| 60‐69 y | 68,881 (21.3) | 1969 (26.6) | 66,852 (21.2) | |
| 70‐79 y | 60,132 (18.6) | 1905 (25.7) | 58,227 (18.5) | |
| ≥80 y | 49,316 (15.3) | 1328 (17.9) | 47,988 (15.2) | |
| Sex (n = 322,774), No. (%) | <.001 | |||
| Male | 181,419 (56.2) | 3896 (52.6) | 177,523 (56.3) | |
| Female | 141,355 (43.8) | 3509 (47.4) | 137,846 (43.7) | |
| Ethnicity/skin color (n = 245,117), No. (%) | <.001 | |||
| White | 134,759 (55) | 3529 (62.2) | 131,230 (54.8) | |
| Brown‐skinned | 90,891 (37.1) | 1675 (29.5) | 89,216 (37.3) | |
| Black | 15,314 (6.2) | 376 (6.6) | 14,938 (6.2) | |
| Asian | 3539 (1.4) | 91 (1.6) | 3448 (1.4) | |
| Indigenous | 614 (0.3) | 610 (0.3) | 4 (0.1) | |
| Level of education (n = 115,730), No. (%) | <.001 | |||
| Illiterate | 6780 (5.9) | 153 (5.6) | 6627 (5.9) | |
| Up to high school | 52,360 (45.3) | 1458 (53) | 50,902 (45.1) | |
| High school | 37,056 (32) | 715 (26) | 36,341 (32.2) | |
| College or university | 19,534 (16.9) | 426 (15.5) | 19,108 (16.9) |
Abbreviations: COVID‐19, coronavirus disease 2019; SD, standard deviation.
χ2 test.
As for symptoms related to COVID‐19, the most frequent were cough (79.7%), dyspnea (79.6%), and fever (71.6%). COVID‐19 patients without cancer showed a higher frequency of most symptoms (P < .001). Most patients had chronic heart disease (66.5%) and diabetes (52.2%), and with the exception of pulmonary disease and immunosuppression, comorbidities were more frequently reported in noncancer patients (P < .001). Of all patients, 39.7% were admitted to the ICU, and 22.1% of the patients were subjected to invasive mechanical ventilation. Overall in‐hospital mortality was 37%, and ICU mortality was 64%. COVID‐19 patients with cancer had longer hospital stays than those without cancer (13.1 vs 11.5 days; P < .001). The overall in‐hospital mortality rate was 37.0%, and it was higher among patients with cancer (60.5% vs 36.5%; P < .001; Table 2). COVID‐19 patients with cancer more frequently needed admission to the ICU (45.3% vs 39.6%) and invasive ventilatory support (27.1% vs 21.9%) than patients with cancer (P < .001; Table 2). There were temporal trends of higher mortality, more frequent admissions to the ICU, and a greater need for invasive respiratory support in COVID‐19 patients with cancer in comparison with those without cancer (P < .001; Fig. 2).
TABLE 2.
Symptoms, Comorbidities, In‐Hospital Mortality, Admissions to the ICU, and Respiratory Support Among COVID‐19 Patients With and Without Cancer in Brazil in 2020
| Characteristic | All Patients (n = 322,816 [100%]), No. (%) | Cancer (n = 7406 [2.3%]), No. (%) | No Cancer (n = 315,410 [97.7%]), No. (%) | P a |
|---|---|---|---|---|
| Symptoms | ||||
| Cough (n = 289,197) | 230,361 (79.7) | 4398 (69.7) | 225,963 (79.6) | <.001 |
| Dyspnea (n = 289,120) | 230,078 (79.6) | 5094 (77.7) | 224,984 (86) | <.001 |
| Fever (n = 283,316) | 202,929 (71.6) | 3949 (63.2) | 198,980 (71.8) | <.001 |
| Oxygen saturation < 95% (n = 273,849) | 193,997 (70.8) | 4747 (73.8) | 189,250 (70.8) | <.001 |
| Respiratory distress (n = 268,673) | 183,762 (68.4) | 4053 (66.3) | 179,709 (68.4) | <.001 |
| Weakness (n = 125,913) | 35,651 (28.3) | 774 (29.7) | 34,877 (28.3) | <.001 |
| Odynophagia (n = 238,012) | 54,384 (22.8) | 773 (14) | 53,651 (23) | <.001 |
| Diarrhea (n = 236,197) | 44,387 (18.8) | 891 (16.8) | 43,496 (18.8) | <.001 |
| Loss of taste (n = 122,357) | 17,487 (14.3) | 201 (8.2) | 17,286 (14.4) | <.001 |
| Loss of smell (n = 122,756) | 17,428 (14.2) | 197 (8) | 17,286 (14.3) | <.001 |
| Vomit (n = 230,995) | 24,932 (10.8) | 676 (13) | 24,256 (10.7) | <.001 |
| Abdominal pain (n = 121,679) | 8491 (7) | 243 (9.7) | 8248 (6.9) | <.001 |
| Comorbidities | ||||
| Chronic heart disease (n = 179,251) | 119,413 (66.5) | 2405 (47.5) | 116,738 (67) | <.001 |
| Diabetes (n = 167,078) | 87,201 (52.2) | 1561 (33.1) | 85,640 (52.7) | <.001 |
| Obesity (n = 137,262) | 20,992 (15.3) | 257 (6.3) | 20,735 (15.6) | <.001 |
| Renal disease (n = 138,617) | 14,916 (10.8) | 460 (10.8) | 14,456 (10.8) | .458 |
| Neurological disease (n = 138,924) | 14,340 (10.3) | 320 (7.6) | 14,020 (10.4) | <.001 |
| Pulmonary disease (n = 138,481) | 13,683 (9.9) | 521 (12.3) | 13,162 (9.8) | <.001 |
| Immunosuppression (n = 136,393) | 9569 (7) | 1846 (39.6) | 7723 (5.9) | <.001 |
| Asthma (n = 136,605) | 8962 (6.6) | 129 (3.1) | 8833 (6.7) | <.001 |
| Liver disease (n = 134,674) | 3161 (2.3) | 146 (3.6) | 3015 (2.3) | <.001 |
| Hospitalization (n = 322,816) | ||||
| In‐hospital mortality | 119,545 (37) | 4479 (60.5) | 115,066 (36.5) | <.001 |
| Length of hospital stay, mean, d | 11.5 | 13.1 | 11.5 | <.001 |
| ICU admission and mortality | ||||
| Admission to ICU (n = 293,441) | 116,640 (39.7) | 3159 (45.3) | 113,481 (39.6) | <.001 |
| In‐ICU mortality (n = 106,401) | 68,052 (64) | 2392 (57.1) | 65,660 (64.2) | <.001 |
| Length of ICU stay, mean, d | 11.1 | 10.9 | 11.1 | .526 |
| Respiratory support (n = 281,050) | <.001 | |||
| None | 72,245 (22.3) | 1480 (22.3) | 70,765 (25.8) | |
| Noninvasive | 146,806 (52.2) | 3360 (50.6) | 143,446 (52.3) | |
| Invasive | 61,999 (22.1) | 1802 (27.1) | 60,197 (21.9) |
Abbreviations: COVID‐19, coronavirus disease 2019; ICU, intensive care unit.
χ2 test.
Figure 2.
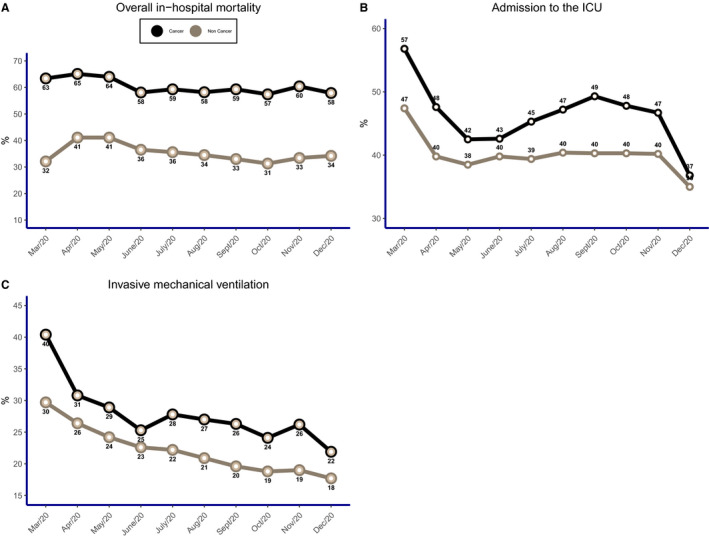
Percent distributions according to cancer patients versus noncancer patients: (A) overall in‐hospital mortality (P < .001), (B) admission to the ICU (P < .001), and (C) invasive mechanical ventilation (P < .001). ICU indicates intensive care unit.
When all patients were evaluated by different comorbidities, the percentual of death for COVID‐19 patients was 25% and 43% without comorbidities and with at least 1 comorbidity, respectively. Different comorbidities were associated with different chances of death, with neurological disease, cancer, chronic renal disease, chronic hepatic disease, and chronic pulmonary disease being the 5 most lethal comorbidities reported in sequence in both sexes and when they were analyzed separately (Fig. 3).
Figure 3.
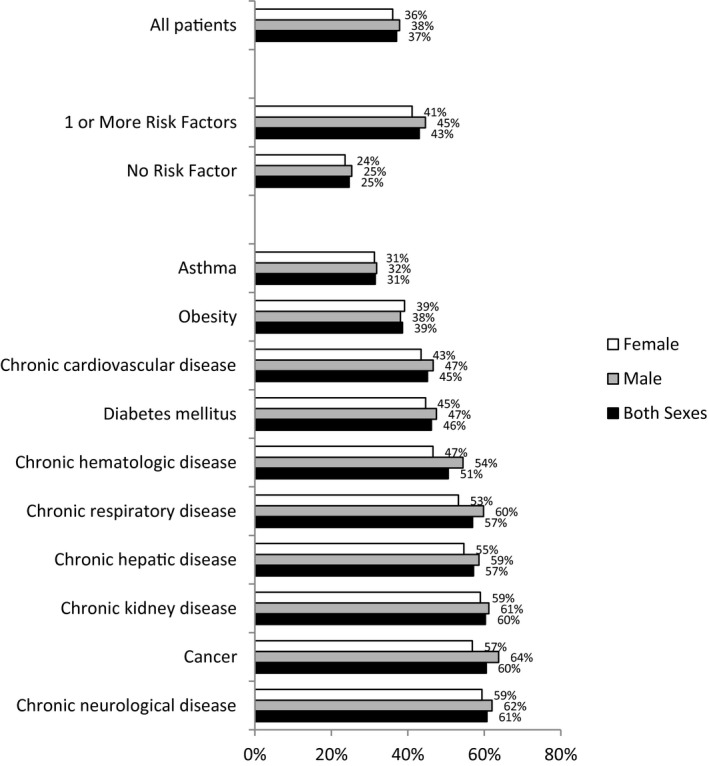
Percent distributions of the lethality of different comorbidities according to sex.
An analysis of the adjusted risk of mortality of COVID‐19 patients showed that patients with cancer had a 1.94 times greater risk of death (95% confidence interval [CI], 1.83‐2.06; P < .001). When they were stratified by sex, a high risk of mortality in patients with cancer was also observed among men (adjusted odds ratio [aOR], 1.78; 95% CI, 1.64‐1.94; P < .001) and among women (aOR, 2.02; 95% CI, 1.86‐2.19; P < .001; Table 3).
TABLE 3.
Risk of Mortality for COVID‐19 Patients in Brazil in 2020
| Variable | Univariate Analysis | P | Multivariate Analysis | P |
|---|---|---|---|---|
| Cancer (both sexes) a | ||||
| Yes | 2.66 (2.54‐2.80) | <.001 | 1.94 (1.83‐2.06) | <.001 |
| No | 1.0 | 1.0 | ||
| Cancer (male) b | ||||
| Yes | 2.96 (2.77‐3.16) | <.001 | 1.78 (1.64‐1.94) | <.001 |
| No | 1.0 | 1.0 | ||
| Cancer (female) c | ||||
| Yes | 2.39 (2.239‐2.56) | <.001 | 2.02 (1.86‐2.19) | <.001 |
| No | 1.0 | 1.0 |
Abbreviation: COVID‐19, coronavirus disease 2019.
Adjusted by age, pulmonary disease, hematological disease, and cardiovascular disease.
Adjusted by age, schooling, neurological disease, renal disease, pulmonary disease, hematological disease, and cardiovascular disease.
Adjusted by neurological disease, pulmonary disease, hematological disease, and cardiovascular disease.
When we considered the topography of cancer, after adjustments, patients with hematological cancer had a 2.85 times higher risk of death (95% CI, 2.41‐3.38; P < .001), and those with solid tumors had a 1.83 times higher risk (95% CI, 1.72‐1.95; P < .001; Table 4). A graphical representation of the aORs for the 10 most frequent cancer topographies in this study is shown in Figure 4.
TABLE 4.
Risk of Mortality for COVID‐19 Patients With Different Types of Cancer in Brazil in 2020
| Variable | Univariate Analysis | P | Multivariate Analysis | P |
|---|---|---|---|---|
| Cancer (both sexes) a | ||||
| Hematological | 3.27 (2.86‐2.80) | <.001 | 2.85 (2.41‐3.38) | <.001 |
| Solid tumors | 2.59 (2.46‐2.72) | 1.83 (1.72‐1.95) | ||
| No | 1.0 | 1.0 | ||
| Cancer (male) b | ||||
| Hematological | 3.24 (2.70‐3.90) | <.001 | 2.90 (2.30‐3.70) | <.001 |
| Other solid tumors | 2,92 (2.72‐3.14) | 1.83 (1.57‐1.86) | ||
| No | 1.0 | 1.0 | ||
| Cancer (female) c | ||||
| Hematological | 3.32 (2.73‐4.03) | <.001 | 2.67 (2.12‐3.39) | <.001 |
| Other solid tumors | 2.29 (2.13‐2.46) | 1.87 (1.71‐2.04) | ||
| No | 1.0 | 1.0 |
Abbreviation: COVID‐19, coronavirus disease 2019.
Adjusted by age, pulmonary disease, hematological disease, and cardiovascular disease.
Adjusted by age, schooling, neurological disease, renal disease, pulmonary disease, hematological disease, and cardiovascular disease.
Adjusted by neurological disease, pulmonary disease, hematological disease, and cardiovascular disease.
Figure 4.
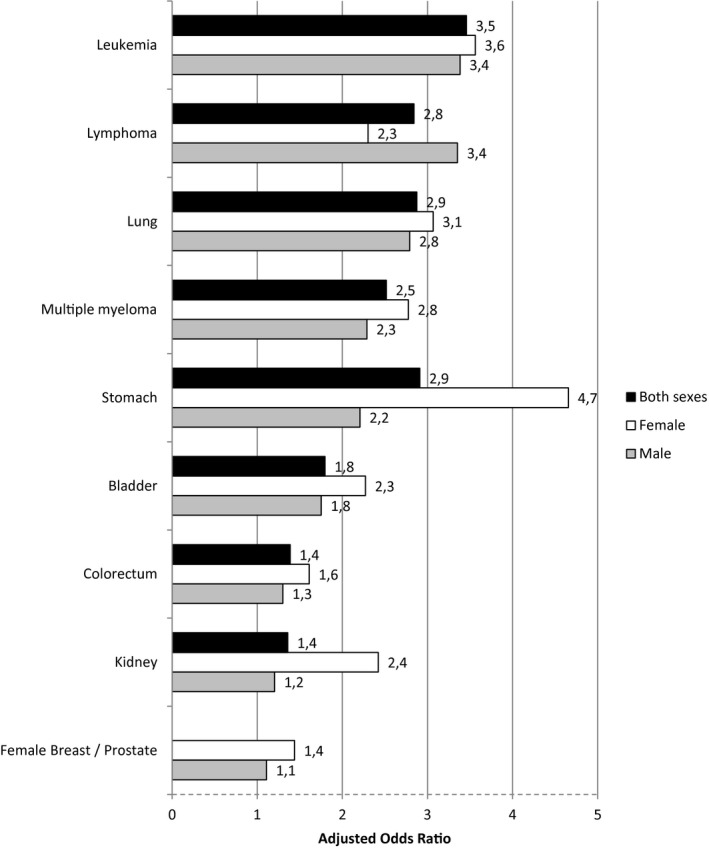
Adjusted odds ratios and 95% confidence intervals for mortality in patients with coronavirus disease 2019 according to the topography of cancer.
Discussion
In this study, data were analyzed from 322,816 hospitalized COVID‐19 patients in Brazil during the year 2020, and it showed that cancer was one of the worst comorbidities related to death in COVID‐19 patients in Brazil, even after adjustments for age, sex, and comorbidities. COVID‐19 patients with cancer had greater disease severity, they were associated with being older, more often admitted to the ICU, received more invasive mechanical ventilation, and had higher mortality rates than noncancer patients during the entire evaluated period.
The frequency of COVID‐19 patients with cancer has ranged from 0.8% to 10.6% of cases in different studies. 18 , 19 , 20 , 21 , 22 A meta‐analysis showed a frequency of approximately 2% for cancer in COVID‐19 patients, especially in large studies with more than 100 patients included. 13 Fifteen percent to 17.7% of COVID‐19 patients with cancer needed ICU admission, and 10% to 19.3% required invasive mechanical ventilation. 4 , 8 A systematic review and meta‐analysis showed that the need to be admitted to the ICU was more often reported in patients with cancer than those without cancer (3220 events; RR, 1.56; 95% CI, 1.31‐1.87; P < .0001). 23 In this study, we identified 2.3% of the cases as being COVID‐19 patients with cancer, and they had longer hospital stays, a higher frequency of ICU admissions (45%), and a greater need for mechanical ventilation support (27%), albeit with lower ICU mortality in comparison with those without cancer. Furthermore, the symptoms of COVID‐19 patients could not be differentiated between patients with cancer and patients without cancer. The clinical diagnosis of COVID‐19 is a challenge because it is often superimposed on the symptoms of neoplasia and/or treatment toxicity. In Latin America (including Brazil), the infrastructure of diagnosis, treatment, and support for patients with cancer is considered insufficient because of low funding and the heterogeneous distribution of resources and services 24 ; this could explain why patients with cancer have had later health assistance.
Patients with cancer have higher mortality in comparison with COVID‐19 patients without cancer. In a previous study, the risk of death in the hospital for COVID‐19 patients with cancer was higher because of older age, male sex, chronic renal disease, and obesity. 25 Cancer patients with COVID‐19 also had an independent risk factor of mortality, with an almost 2‐fold higher risk than COVID‐19 patients without cancer, even after adjustments for age, gender, and different comorbidities. 26 In our analysis, female patients had a slightly higher risk of death than male patients after adjustments because breast cancer, reported in 15% of cases, was the most common topography identified.
COVID‐19 patients with different tumor types have distinct vulnerabilities and mortalities. Hematological malignancies were associated with greater severity and mortality 8 in comparison with patients with solid tumors or COVID‐19 patients without cancer, 27 but recent chemotherapy incurred an increased risk of death during COVID‐19–associated hospital admissions. 28 In a Brazilian study, patients with lung or hematological cancer and patients under oncological treatment had a higher risk of death among patients with COVID‐19. 14 Mortality due to COVID‐19 in patients with cancer could be heterogeneous for different kinds of primary tumors, and individualized strategies for preventing or reducing the risk of mortality in COVID‐19 patients with cancer are necessary.
Patients with cancer have more vulnerable clinical conditions, which are associated with greater severity and lethality due to COVID‐19. 8 They are often older, immunosuppressed, and poorly nourished and have different comorbidities and adverse effects caused by oncological treatment. 5 , 7 , 8 , 14 Hematological malignancies, associated with greater susceptibility due to immune system impairment, have been associated with a poor prognosis for COVID‐19 patients with cancer. 8 , 14 , 28 Oncological treatments have also been related to the risk of mortality due to COVID‐19. 8 , 14 Although oncological patients have not been included in clinical trials that have evaluated different vaccines against COVID‐19, 29 , 30 , 31 American and European oncological societies have recommended that patients with cancer be vaccinated when vaccines are available. 32 , 33 So, it would be desirable for oncological patients to have priority in different immunization programs because they are more susceptible to COVID‐19 and other infectious diseases.
Besides that, there was a negative impact of the COVID‐19 pandemic on screening, diagnosis, and treatment for patients with cancer in 2020. 34 There may have been progression of cancer or impaired survival if treatments were delayed. Oncological procedures were reduced or postponed during the pandemic. 35 , 36 Also, there were delays because patients were afraid of the pandemic, there were travel restrictions and quarantines, and they experienced worsening clinical conditions. 37 As a matter of fact, the COVID‐19 pandemic affected oncologists' decision‐making for cancer because they used less chemotherapy, immune checkpoint inhibitors, and steroids and recommended second‐ or third‐line therapies, especially in metastatic staging. 38
This study has some limitations. First, the SIVEP‐Gripe national data do not include information about staging, clinical performance, or oncological treatment; these aspects are very important for determining the prognosis in patients with cancer. Second, the quality of the data on the original forms from which the data were obtained may be open to criticism. However, as all cases of suspected COVID‐19 should have been notified in Brazil, we can expect that in‐hospital cases were better informed because it was necessary to access a free real‐time polymerase chain reaction swab test for each patient. Third, patient comorbidities were not defined by clear criteria, so we cannot be sure about levels of severity and treatment. However, this register is highly representative of the Brazilian population with COVID‐19 as a whole, and its analysis could be very important for improving epidemiological knowledge of COVID‐19 patients who have different conditions (ie, frequency and risk of death). This information could provide important support for better decision‐making by governments, institutions, and/or health professionals during the COVID‐19 pandemic.
This is the largest population‐based study conducted in Brazil that has evaluated prevalence, temporal trends, morbidity, length of hospital stay, cancer topographies, and mortality risk among COVID‐19 patients with and without cancer throughout the year 2020. Previous population‐based studies in this country failed to determine these aspects involving COVID‐19 patients with cancer, who must be considered a priority for receiving immunizations, including the vaccine against COVID‐19, as well as diagnostic procedures and oncological modalities of treatment during the COVID‐19 pandemic.
In conclusion, this Brazilian study showed that COVID‐19 patients with cancer were older, had greater disease severity, stayed longer in the hospital, had a greater need for ICU admission, and had more need for invasive mechanic ventilation during their hospital stay beyond all oncological support in the year 2020. Furthermore, COVID‐19 patients with cancer and hematological topographies had a higher risk of death comparing with those without cancer and those with solid tumors, respectively, even with adjustments for age, sex, and different comorbidities.
Funding Support
No specific funding was disclosed.
Conflict of Interest Disclosures
The authors made no disclosures.
Author Contributions
Guilherme Jorge Costa: Literature search, study design, data collection, data analysis, data interpretation, and writing. Carla Rameri Alexandre Silva de Azevedo: Literature search, data interpretation, and review and editing. José Iran Costa Júnior: Literature search, data interpretation, and review and editing. Anke Bergmann: Literature search, data analysis, data interpretation, and review and editing. Luiz Claudio Santos Thuler: Literature search, study design, data analysis, data interpretation, and review and editing. All the authors have participated directly, written the manuscript, approved the version to be published, and agreed to be accountable for all aspects of the work.
Costa GJ, de Azevedo CRAS, Júnior JIC, Bergmann A, Thuler LCS. Higher severity and risk of in‐hospital mortality for COVID‐19 patients with cancer during the year 2020 in Brazil: A countrywide analysis of secondary data. Cancer. 2021. 10.1002/cncr.33832
References
- 1. World Health Organization . Accessed January 17, 2021. https://www.who.int/
- 2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209‐249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7‐33. doi: 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 4. De Melo AC, Thuler LCS, Da Silva JL, et al. Cancer in patients with COVID‐19: a report from the Brazilian National Cancer Institute. PLoS One. 2020;15:0241261. doi: 10.1371/journal.pone.0241261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meng Y, Meng Y, Lu W, et al. Cancer history is an independent risk factor for mortality in hospitalized COVID‐19 patients: a propensity score–matched analysis. J Hematol Oncol. 2020;13:75. doi: 10.1186/s13045-020-00907-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Santos CS, Morales CM, Álvarez ED, Castro CÁ, Robles AL, Sandoval TP. Determinants of COVID‐19 disease severity in patients with underlying rheumatic disease. Nat Med. 2020;39:2789‐2796. doi: 10.1007/s10067-020-05301-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID‐19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907‐1918. doi: 10.1016/S0140-6736(20)31187-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang K, Sheng Y, Huang C, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID‐19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:904‐913. doi: 10.1016/S1470-2045(20)30310-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee LYW, Cazier JB, Starkey T, Turnbull CD, Kerr R, Middleton G. COVID‐19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;6736:1919‐1926. doi: 10.1016/S0140-6736(20)31173-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park R, Chidharla A, Mehta K, Sun W, Wulff‐Burch E, Kasi A. Sex‐bias in COVID‐19–associated illness severity and mortality in cancer patients: a systematic review and meta‐analysis. EClinicalMedicine. 2020;26:100519. doi: 10.1016/j.eclinm.2020.100519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jin J, Bai P, He W, et al. Gender differences in patients with COVID‐19: focus on severity and mortality. Front Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pradhan A, Olsson PE. Sex differences in severity and mortality from COVID‐19: are males more vulnerable? Biol Sex Differ. 2020;11:53. doi: 10.1186/s13293-020-00330-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Desai A, Sachdeva S, Parekh T, Desai R. COVID‐19 and cancer: lessons from a pooled meta‐analysis. JCO Glob Oncol. 2020;6:557‐559. doi: 10.1200/go.20.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aparecida G, Feriani D, Leonardo I, et al. Differences in mortality of cancer patients with COVID‐19 in a Brazilian cancer center. Semin Oncol. Published online February 1, 2021. doi: 10.1053/j.seminoncol.2021.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baqui P, Bica I, Marra V, Ercole A, van der Schaar M. Ethnic and regional variations in hospital mortality from COVID‐19 in Brazil: a cross‐sectional observational study. Lancet Glob Health. 2020;8:e1018‐e1026. doi: 10.1016/S2214-109X(20)30285-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ranzani OT, Bastos LSL, Gelli JGM, et al. Characterisation of the first 250,000 hospital admissions for COVID‐19 in Brazil: a retrospective analysis of nationwide data. Lancet Respir Med. 2021;9:407‐418. doi: 10.1016/S2213-2600(20)30560-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:1‐8. doi: 10.1186/1751-0473-3-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khan MMA, Khan MN, Mustagir G, Rana J, Islam MS, Kabir MI. Effects of underlying morbidities on the occurrence of deaths in COVID‐19 patients: a systematic review and meta‐analysis. J Glob Health. 2020;10:020503. doi: 10.7189/jogh.10.020503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Suleyman G, Fadel RA, Malette KM, et al. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan Detroit. JAMA Netw Open. 2020;3:e2012270. doi: 10.1001/jamanetworkopen.2020.12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yehia BR, Winegar A, Fogel R, et al. Association of race with mortality among patients hospitalized with coronavirus disease 2019 (COVID‐19) at 92 US hospitals. JAMA Netw Open. 2020;3:e2018039. doi: 10.1001/jamanetworkopen.2020.18039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kabarriti R, Brodin NP, Maron MI, et al. Association of race and ethnicity with comorbidities and survival among patients with COVID‐19 at an urban medical center in New York. JAMA Netw Open. 2020;3:e2019795. doi: 10.1001/jamanetworkopen.2020.19795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liang WH, Guan WJ, Li CC, et al. Clinical characteristics and outcomes of hospitalised patients with COVID‐19 treated in Hubei (epicentre) and outside Hubei (non‐epicentre): a nationwide analysis of China. Eur Respir J. 2020;55:2000562. doi: 10.1183/13993003.00562-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Giannakoulis VG, Papoutsi E, Siempos II. Effect of cancer on clinical outcomes of patients with COVID‐19: a meta‐analysis of patient data. JCO Glob Oncol. 2020;6:799‐808. doi: 10.1200/go.20.00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goss PE, Lee BL, Badovinac‐Crnjevic T, et al. Planning cancer control in Latin America and the Caribbean. Lancet Oncol. 2013;14:391‐436. doi: 10.1016/S1470-2045(13)70048-2 [DOI] [PubMed] [Google Scholar]
- 25. Lunski MJ, Burton J, Tawagi K, et al. Multivariate mortality analyses in COVID‐19: comparing patients with cancer and patients without cancer in Louisiana. Cancer. 2021;127:266‐274. doi: 10.1002/cncr.33243 [DOI] [PubMed] [Google Scholar]
- 26. Navaratnam AV, Gray WK, Day J, Wendon J, Briggs TWR. Patient factors and temporal trends associated with COVID‐19 in‐hospital mortality in England: an observational study using administrative data. SSRN Electron J. 2020;2600:3706052. doi: 10.2139/ssrn.3706052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Suárez G, Oncol JH, Suárez JG, et al. Impact of hematologic malignancy and type of cancer therapy on COVID‐19 severity and mortality: lessons from a large population‐based registry study. J Hematol Oncol. 2020;13:133. doi: 10.1186/s13045-020-00970-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee LYW, Cazier JB, Starkey T, et al. COVID‐19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21:1309‐1316. doi: 10.1016/S1470-2045(20)30442-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kitchin N, Absalon J, Gurtman A, et al. Safety and immunogenicity of two RNA‐based Covid‐19 vaccine candidates. N Engl J Med. 2020;383:2439‐2450. doi: 10.1056/NEJMoa2027906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383:2603‐2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384:403‐416. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cancer and COVID‐19 vaccination: recommendations of the NCCN COVID‐19 Vaccination Advisory Committee. National Comprehensive Cancer Network. Published June 9, 2021. Accessed March 20, 2021. https://www.nccn.org/docs/default‐source/covid‐19/2021_covid‐19_vaccination_guidance_v3‐0.pdf?sfvrsn=b483da2b_60 [Google Scholar]
- 33. Garassino MC, Vyas M, de Vries EGE, et al. The ESMO call to action on COVID‐19 vaccinations and patients with cancer: Vaccinate. Monitor. Educate. Ann Oncol. 2021;32:579‐581. doi: 10.1016/j.annonc.2021.01.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Raymond E, Thieblemont C, Alran S, Faivre S. Impact of the COVID‐19 outbreak on the management of patients with cancer. Target Oncol. 2020;15:249‐259. doi: 10.1007/s11523-020-00721-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sud A, Jones ME, Broggio J, et al. Collateral damage: the impact on outcomes from cancer surgery of the COVID‐19 pandemic. Ann Oncol. 2020;31:1065‐1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maringe C, Spicer J, Morris M, et al. The impact of the COVID‐19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population‐based, modelling study. Lancet Oncol. 2020;21:1023‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Quaquarini E, Saltalamacchia G, Presti D, et al. Impact of COVID‐19 outbreak on cancer patient care and treatment: data from an outpatient oncology clinic in Lombardy (Italy). Cancers (Basel). 2020;12:2941. doi: 10.3390/cancers12102941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ürün Y, Hussain SA, Bakouny Z, et al. Survey of the impact of COVID‐19 on oncologists' decision making in cancer. JCO Glob Oncol. 2020;6:1248‐1257. doi: 10.1200/GO.20.00300 [DOI] [PMC free article] [PubMed] [Google Scholar]


