Abstract
Acute kidney injury (AKI) may develop in patients with coronavirus disease 2019 (COVID‐19) and is associated with in‐hospital death. We investigated the incidence of AKI in 223 hospitalized COVID‐19 patients and analyzed the influence factors of AKI. The incidence of cytokine storm syndrome and its correlation with other clinicopathologic variables were also investigated. We retrospectively enrolled adult patients with virologically confirmed COVID‐19 who were hospitalized at three hospitals in Wuhan and Guizhou, China between February 13, 2020, and April 8, 2020. We included 124 patients with moderate COVID‐19 and 99 with severe COVID‐19. AKI was present in 35 (15.7%) patients. The incidence of AKI was 30.3% for severe COVID‐19 and 4.0% for moderate COVID‐19 (p < 0.001). Furthermore, cytokine storm was found in 30 (13.5%) patients and only found in the severe group. Kidney injury at admission (odds ratio [OR]: 3.132, 95% confidence interval [CI]: 1.150–8.527; p = 0.025), cytokine storm (OR: 4.234, 95% CI: 1.361–13.171; p = 0.013), and acute respiratory distress syndrome (ARDS) (OR: 7.684, 95% CI: 2.622–22.523; p < 0.001) were influence factors of AKI. Seventeen (48.6%) patients who received invasive mechanical ventilation developed AKI, of whom 64.7% (11/17) died. Up to 86.7% of AKI patients with cytokine storms may develop a secondary bacterial infection. The leukocyte counts were significantly higher in AKI patients with cytokine storm than in those without (13.0 × 10⁹/L, interquartile range [IQR] 11.3 vs. 8.3 × 10⁹/L, IQR 7.5, p = 0.005). Approximately 1/6 patients with COVID‐19 eventually develop AKI. Kidney injury at admission, cytokine storm and ARDS are influence factors of AKI. Cytokine storm and secondary bacterial infections may be responsible for AKI development in COVID‐19 patients.
Keywords: acute kidney injury, COVID‐19, cytokine storm, secondary bacterial infections
1. INTRODUCTION
The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pandemic has so far spread to more than 200 countries in the world, infected more than three million persons globally, and caused more than 210,000 deaths globally, 1 surpassing the previous severe acute respiratory syndrome (SARS) epidemic and posing a severe worldwide public health threat. 2 Coronavirus disease 2019 (Covid‐19), caused by SARS‐CoV‐2 has prominent respiratory involvement characterized by the symptoms of viral pneumonia such as fever, fatigue, dry cough, and lymphopenia. 3 Although the lungs are considered as the major targeted organ by SARS‐CoV‐2 with diffuse alveolar damage, SARS‐CoV‐2 may also cause damage to other organs such as the heart, liver, and kidneys. Patients could eventually succumb to multiple organ failure, shock, acute respiratory distress syndrome (ARDS), heart failure, and renal failure. 4 , 5 , 6
SARS‐CoV‐2 has been shown to directly attack the kidneys, possibly by binding to converting enzyme‐2 (ACE2) on the renal cell membrane. 7 The presence of ACE2 in extrapulmonary tissues could explain the multiorgan dysfunction caused by COVID‐19. The kidneys have one of the highest content of ACE2, which SARS‐CoV‐2 spike protein directly recognizes and binds to. 8 A postmortem study found that clusters of coronavirus particles with distinctive spikes were in the tubularepithelium and podocytes. Diffuse proximal tubule injury with loss of brushborder, and even frank necrosis were observed in COVID‐19 cases. 9 A recent meta‐analysis showed that COVID‐19 patients with severe acute kidney injury (AKI) were at significant risk for mortality (relative risk: 4.19, 95% confidence interval [CI]: 3.31−5.31). 10 In 701 patients with COVID‐19 from Wuhan, the epicenter of SARS‐CoV‐2 outbreak, Cheng et al. found that 5.1% of the patients developed AKI, which was associated with a significantly elevated risk of in‐hospital death. 11
A prominent feature of COVID‐19 in a sizable proportion of SARS‐CoV‐2 infected persons is development of ARDS. Elevated production of proinflammatory cytokines/chemokines or even hypercytokinemia, also known as cytokine storm, may develop in SARS‐CoV‐2 infections and contributes to acute lung injury and development of ARDS. 12 , 13 And bacterial infection may also aggravate the patient's condition and increase the difficulty of treatment. 14 , 15 Exaggerated, excessive synthesis of interleukin 6 (IL‐6) leads to an acute severe systemic inflammatory response and also can activate the coagulation pathway and vascular endothelial cells, leading to multiorgan dysfunction within a short time. 16 , 17 In our clinical experience, the sudden massive surge in IL‐6 levels makes it very difficult for patients with COVID‐19 to recover from kidney injury. However, currently, there is no study on the role of cytokine storm in kidney injury.
In the current retrospective study, we investigated the incidence of AKI in hospitalized patients with COVID‐19 from three medical centers in and beyond Wuhan and analyzed the influence factors of AKI in COVID‐19 patients. We further studied the incidence of cytokine storm syndrome and its correlation with other clinicopathologic variables.
2. METHODS
2.1. Participants
This retrospective cohort study enrolled adult patients (≥18 years) with virologically confirmed COVID‐19, who were hospitalized at Zhongnan Hospital, Wuhan University, Wuhan, China, Leishenshan Hospital, Wuhan, China and Jiangjunshan Hospital, Guiyang, Guizhou, China. COVID‐19 was diagnosed by the China National Health Commission Coronavirus Pneumonia Prevention and Control Program (7th edition). We excluded patients who required maintenance hemodialysis as well as patients with incomplete clinical records. Severe COVID‐19 was defined according to the WHO's interim guidelines. 18
The study protocol was approved by the Chinese ethics committees of the authors' affiliated institutions (No. 2020020 and No. 2020020). Due to the retrospective nature of the study, informed consent was waived by the ethics committees. Patients provided written informed consent to the collection of their urine samples. Patient data were anonymized in this paper.
2.2. Data collection
We reviewed the clinical charts, nursing records, laboratory results, and chest computed tomography scans of all patients using the hospitals' electronic records system. Epidemiological, clinical, imaging, serological records, and treatment and outcomes data were collected. To ensure accuracy, four researchers independently reviewed and checked the data form.
2.3. Definitions
AKI was defined by the Kidney Disease: Improving Global Outcomes (KDIGO) guideline as one of the following: (1) an increase in SCr by ≥0.3 mg/dl (≥26.5 mol/l) within 48 h; (2) an increase in SCr to ≥1.5 times baseline within the previous 7 days; (3) urine volume ≤0.5 ml/kg/h for 6 h. 19 AKI stage of diagnosis and peak SCr were determined relative to baseline SCr according to the Acute Kidney Injury Network (AKIN) criteria (urine output was not used): stage 1, an increase in SCr by ≥0.3 mg/dl or 0.5‐ to <2‐fold increase and stage 2, a 2‐ to <3‐fold increase; stage 3, a ≥3‐fold increase, or SCr ≥4.0 mg/dl after a rise of at least 0.5 mg/dl or acute dialysis requirement. Scr was measured at least twice weekly during hospitalization. Newly emerging kidney disease was defined as the presence of abnormal symptoms or laboratory findings related to kidney diseases at admission in a patient with no previous history of chronic kidney disease (CKD). CKD was defined as the estimated glomerular filtration rate (eGFR)<60 ml/min per 1.73 m2 or urine microalbumin‐creatinine ratio ≥30 μg/mg at least 3 months before admission. 21 eGFR was calculated using a simplified Modification of Diet in Renal Disease (MDRD) equation. 29 Severe COVID‐19 was defined as any of the following conditions: (1) respiratory rate ≥30 breaths/min, (2) oxygen saturation ≤93% in the resting state, (3) arterial oxygen partial pressure (PaO2)/fractional inspired oxygen (FiO2) ratio ≤300 mmHg, and (4) a 50% increase in chest radiologic abnormalities in 24–48 h 22 Cytokine storm syndrome was diagnosed when IL‐6 levels surged over 100‐fold than the normal value within 48 h and lung and other organ (at least two organs) functional deterioration was present.
2.4. Enzyme‐linked immunosorbent assay (ELISA)
Thirty‐one urine samples were collected from COVID‐19 patients without a history of kidney disease or abnormality of urinary sediment test at admission. The urine samples were centrifuged at 1000 g for 5 min and the supernatants were stored at −20°C for 1 week, and then stored at −80°C. The tubular injury marker, human neutrophil gelatinase‐associated lipocalin (NGAL) (ab119600; Abcam PLC) and kidney injury factor‐1 (KIM‐1) (ab235081; Abcam PLC), and the glomerular injury marker‐urinary transferrin (u‐TF) (ab217780; Abcam PLC), and urinary microalbumin (ab108788; Abcam PLC) were quantified by ELISA according to the manufacturer's instructions. Urine samples were diluted in 1:50 and 1:5000 before an ELISA assay to fit the concentrations of respective proteins in the linear range of the standard curve. All the detection results were expressed in absolute terms and also normalized against the urinary creatinine concentration.23
2.5. Statistical analysis
The primary outcome of the study was the incidence of AKI in patients with COIVD‐19. Statistical analysis was done with SPSS 23 (SPSS Inc.). Analyses were descriptive in nature. Summary tabulations included the number of observations; median, interquartile range (IQR) for continuous variables; number and percentage per category for categorical data. Categorical data were compared using the χ 2 test. Continuous data were compared using Student's t test. Nonnormal distributed continuous data were compared using the Mann–Whitney–Wilcoxon test. Risk factors of AKI were identified by univariate and multivariate analysis and the factors with p < 0.1 entered the multivariate analysis. All p values were two‐tailed and p < 0.05 indicated a significant statistical difference. Association rule analysis: support ≥10%, credibility ≥80%, Apriori algorithm.
3. RESULTS
3.1. Patient demographic and baseline data
This study included a total of 223 patients, 52 patients from Zhaongnan Hospital of Wuhan University, 70 from Leishenshan Hospital, and 101 patients from Jiangjunshan Hospital. The study flowchart is shown in Figure 1, patient demographic and baseline characteristics in Table 1, and laboratory findings in Table 2. A total of 256 patients were diagnosed with COVID‐19 in these institutions during the review period. Five cases requiring maintenance hemodialysis were excluded. In addition, we excluded 12 patients under 18 years of age and 16 patients with incomplete clinical information. Finally, 223 patients with COVID‐19 were included in the current study. Their median age was 55 (IQR: 30) years, and 58.3% of patients were male. One hundred twenty‐four (55.6%) patients had moderate COVID‐19 and 99 (44.4%) had severe COVID‐19. Patients with moderate COVID‐19 were significantly younger (p < 0.001) and had significantly lower rates of underlying conditions (p < 0.001). In addition, 30 (30.3%) of patients who all had severe COVID‐19 received mechanical ventilation.
Figure 1.
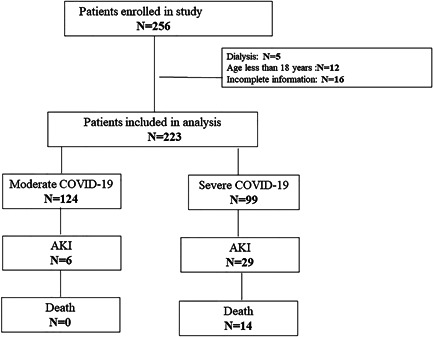
The study flowchart
Table 1.
Demographic and baseline characteristics of COVID‐19 patients
| All | Moderate COVID‐19 | Severe COVID‐19 | Z/χ 2 value (p value) | |
|---|---|---|---|---|
| Number | 223 | 124 | 99 | |
| Age (years) | 55 (30.0) | 40.0 (25.0) | 67.0 (21.0) | −9.711 (<0.001) a |
| Sex: Male (n, %) | 130 (58.3%) | 66 (53.2%) | 64 (64.6%) | 2.729 (0.099) b |
| Hospital location | ||||
| Wuhan | 122 (54.7%) | 39 (31.5%) | 83 (83.8%) | 60.971 (<0.001) b |
| Not in Wuhan | 101 (45.3%) | 85 (68.5%) | 16 (16.2%) | |
| Traveling or residing in Wuhan | 160 (71.7%) | 71 (57.3%) | 89 (89.9%) | 28.935 (<0.001) b |
| Underlying diseases (n, %) | ||||
| Hypertension | 63 (34.1%) | 20 (17.2%) | 43 (62.3%) | 43.017 (<0.001) b |
| Diabetes | 31 (16.8%) | 12 (10.3%) | 19 (27.5%) | 10.166 (0.001) b |
| Coronary heart disease | 7 (3.8%) | 2 (1.7%) | 5 (7.2%) | 2.371 (0.124) c |
| Hyperuricemia or gout | 3 (1.6%) | 3 (2.6%) | 0 (0.0%) | 1.057 (0.304) c |
| Nervous system disease | 8 (4.3%) | 2 (1.7%) | 6 (8.7%) | 3.537 (0.060) c |
| Bronchitis or asthma | 3 (1.6%) | 3 (2.6%) | 0 (0.0%) | 1.057 (0.304) c |
| Hepatic disease | 8 (4.3%) | 6 (5.2%) | 2 (2.9%) | 0.131 (0.718) c |
| Chronic kidney disease | 5 (2.7%) | 1 (0.8%) | 4 (5.8%) | 1.358 (0.244) c |
| Others | 38 (20.5%) | 29 (25.0%) | 9 (13.0%) | 3.621 (0.057) b |
| No comorbidities | 63 (34.1%) | 56 (48.3%) | 7 (10.1%) | 29.402 (<0.001) b |
| Initial symptoms | ||||
| Fever (temperature ≥37.3°C) | 148 (66.4%) | 60 (48.4%) | 88 (88.9%) | 40.456 (<0.001) b |
| Cough | 169 (75.8%) | 70 (56.5%) | 99 (100%) | 56.889 (<0.001) b |
| Sputum | 64 (28.7%) | 38 (30.6%) | 26 (26.2%) | 0.517 (0.472) b |
| Dyspnea | 104 (46.6%) | 5 (4.0%) | 99 (100%) | 203.719 (<0.001) b |
| Fatigue | 73 (32.7%) | 45 (36.3%) | 28 (28.3%) | 1.603 (0.205) b |
| Diarrhoea | 26 (11.7%) | 12 (9.7%) | 14 (14.1%) | 1.065 (0.302) b |
| Myalgia | 31 (13.9%) | 18 (14.5% | 13 (13.1%) | 0.088 (0.766) b |
| Pharyngalgia | 30 (13.5%) | 22 (17.7%) | 8 (8.1%) | 4.413 (0.036) b |
| No symptom | 10 (4.5%) | 10 (8.1%) | 0 (0.0%) | 6.582 (0.01) c |
| Time to admission since symptom onset (days) | 10.0 (11.0) | 9.0 (9.0) | 10.0 (14.0) | −1.665 (0.096) |
| Outcome: Death | 20 (9.0%) | 0 (0.0%) | 20 (20.2%) | 27.519 (<0.001) b |
| Antiviral therapies | ||||
| Arbidol | 150 (67.3%) | 56 (45.1%) | 94 (94.9%) | 61.973 (<0.001) b |
| Ribavirin | 80 (35.9%) | 4 (3.2%) | 76 (79.8%) | 129.421 (<0.001) b |
| Lopinavir/ritonavir | 101(45.3%) | 78 (62.9%) | 23 (23.2%) | 34.964 (<0.001) b |
| Favipiravir | 21 (9.4%) | 19 (15.3%) | 2 (2%) | 11.420 (0.001) b |
| Lianhua Qingwen | 16 (7.2%) | 13 (10.5%) | 3 (3%) | 6.194 (0.013) b |
| Invasive ventilator treatment | 30 (13.5%) | 0 (0.0%) | 30 (30.3%) | 43.417 (<0.001) b |
Note: Data are expressed as median (IQR) or n (%).
Abbreviations: COVID‐2019, coronavirus disease 2019; IQR, interquartile range.
Mann–Whitney U test.
Pearson χ 2 test.
Continuity correction χ 2 value.
Table 2.
Laboratory findings of COVID‐19 patients
| All patients | Moderate COVID‐19 | Severe COVID‐19 | Z/χ 2 value (p value) | |
|---|---|---|---|---|
| Blood routine | ||||
| Leucocyte count (×10⁹/L) | 5.7 (3.2) | 5.5 (2.0) | 6.7 (6.0) | −2.690 (0.007) |
| Lymphocyte count (×10⁹/L) | 1.3 (0.8) | 1.5 (1.0) | 1.1 (1.0) | −5.544 (<0.001) |
| Haemoglobin (g/dl) | 129.4 (27.2) | 140.0 (25.0) | 121.0 (18.0) | −7.783 (<0.001) |
| Platelets (×109/L) | 201.0 (71.0) | 206.0 (81.0) | 200.0 (59.0) | −2.060 (0.039) |
| Blood biochemistry | ||||
| ALT (U/L) | 22.0 (23.3) | 23.0 (24.0) | 21.0 (20.0) | −2.141 (0.032) |
| AST (U/L) | 21.0 (14.0) | 23.0 (12.0) | 21.0 (19.0) | −3.053 (0.002) |
| LDH (U/L) | 175.0 (66.0) | 170.5 (63.0) | 208.0 (169.0) | −2.644 (0.008) |
| d‐dimer (mg/L) | 0.7 (2.5) | 0.4 (0.3) | 1.9 (4.9) | −8.058 (<0.001) |
| Total bilirubin (μmol/L) | 11.3 (7.9) | 11.4 (8.1) | 10.9 (7.7) | −0.776 (0.438) |
| CK (U/L) | 61.0 (42.0) | 62.0 (42.0) | 53.0 (47.0) | −1.070 (0.284) |
| CKMB (U/L) | 8.0 (7.0) | 7.0 (5.0) | 12.0 (13.5) | −2.851 (0.004) |
| K (mmol/L) | 4.2 (0.6) | 4.2 (0.7) | 4.1 (0.6) | −2.630 (0.009) |
| Na (mmol/L) | 140.5 (3.9) | 140.9 (4.0) | 139.2 (2.6) | −3.208 (0.001) |
| Cl (mmol/L) | 101.4 (3.1) | 101.7 (3.5) | 100.9 (3.5) | −1.815 (0.070) |
| CRP (mg/L) | 12.0 (26.0) | 4.0 (14.2) | 29.6 (60.9) | −4.957 (<0.001) |
| IL‐6 (pg/ml) | 5.8 (60.5) | 1.5 (3.7) | 30.1 (162.7) | −6.641 (<0.001) |
| Cytokine storm syndrome | 30 (13.5%) | 0 (0.0%) | 30 (30.3%) | 43.417 (<0.001) a |
| Kidney function estimation | ||||
| eGFR (ml/min/1.73 m2) | 102.1 (26.5) | 110.5 (19.5) | 88.1 (43.6) | −8.560 (<0.001) |
| Scr (μmol/L) | 61.9 (26.5) | 61.4 (21.8) | 65.0 (38.6) | −1.504 (0.133) |
| BUN (mmol/L) | 4.6 (3.4) | 3.6 (2.1) | 6.6 (4.8) | −8.217 (<0.001) |
| Cystatin C (mg/L) | 0.77 (0.2) | 0.8 (0.2) | 1.0 (0.4) | −4.429 (<0.001) |
| Urine sediment examination | ||||
| Urinary protein: positive | 44 (19.7%) | 9 (7.3%) | 35 (35.4%) | 27.436 (<0.001) a |
| Urinary erythrocyte: positive | 55 (24.7%) | 23 (18.5%) | 32 (32.3%) | 2.660 (0.103) |
| Urinary leukocyte: positive | 60 (26.9%) | 21 (16.9%) | 39 (39.4%) | 8.013 (0.005) a |
| Acute kidney injury | 35 (15.7%) | 5 (4.0%) | 30 (30.3%) | 28.713 (<0.001) a |
| Stage 1 | 18 (8.1%) | 5 (4.0%) | 13 (13.1%) | 6.142 (0.013) a |
| Stage 2 | 7 (3.1%) | 0 | 7 (7.0%) | 9.052 (0.003) a |
| Stage 3 | 10 (4.5%) | 0 | 10 (10.1%) | 10.861 (0.001) b |
Note: Data are expressed as median (IQR) or n (%).
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CK, creatine kinase; CKMB, isoenzyme of creatine kinase; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate. eGFR is tested by MDRD Study Equation; IL‐6, interleukin‐6; IQR, interquartile range; LDH, lactate dehydrogenase; Scr, serum creatinine.
Pearson χ 2 test.
Continuity correction chi‐square value, non‐label is used Mann–Whitney U test.
3.2. Incidence of AKI and kidney disease
AKI was present in 35 (15.7%) patients with COVID‐19, including stage 1 in 18, stage 2 in 7, and stage 3 in 10 patients. The incidence of AKI was noticeably higher in patients with severe than patients with moderate COVID‐19 (30.3% vs. 4%) (p < 0.001). In addition, COVID‐19 patients with AKI had a significantly higher rate of pre‐existing hypertension compared with patients with no AKI (57.1% vs. 21.9%, p < 0.001) (Table 3). Nine (36%) of 25 COVID‐19 patients with no clinical kidney abnormality has early damage of renal cells characterized by the expression of early biomarkers for kidney injury, including 1 with both high albumin‐to‐creatinine ratio (ACR) and KIM‐1, 2 with solely high KIM‐1, and 6 patients with elevated human neutrophil gelatinase‐associated lipocalin (NGAL) (Supporting Information Table).
Table 3.
Subgroup analysis of COVID‐19 patients with and without AKI (NO CKD, n = 5)
| AKI | Non‐AKI | Total | Z/χ 2 value (p value) | |
|---|---|---|---|---|
| Number | 35 | 183 | 218 | |
| Hospital location | ||||
| Wuhan | 29 (82.9%) | 91 (49.7%) | 120 (55.0%) | 13.032 (<0.001) |
| Not in Wuhan | 6 (17.1%) | 92 (50.3%) | 98 (45.0%) | |
| Underlying diseases | ||||
| Hypertension | 20 (57.1%) | 40 (21.9%) | 60 (27.5%) | 18.338 (<0.001) |
| Diabetes | 7 (20.0%) | 23 (12.6%) | 30 (13.8%) | 0.813 (0.367) |
| COVID‐19 severity | ||||
| Moderate | 5 (14.3%) | 118 (64.5%) | 123 (56.4%) | 30.107 (<0.001) |
| Severe | 30 (85.7%) | 65 (35.5%) | 95 (43.6%) | |
| Leucocyte count (×10⁹/L) | 10.7 (10.6) | 6.78 (3.87) | 7.2 (4.3) | −4.439 (<0.001)* |
| Leucocyte count (>10 × 10⁹/L) | 20 (57.1%) | 37 (20.2%) | 57 (26.1%) | 20.744 (<0.001) |
| IL‐6 (pg/ml) | 247.1 (1042.4) | 4.3 (23.2) | 6.1 (66.8) | −4.891 (<0.001)* |
| Cytokine storm | 18 (51.4%) | 11 (6.0%) | 29 (13.3%) | 48.685 (<0.001) |
| Mortality | 14 (40.0%) | 5 (2.7%) | 19 (8.7%) | 46.713 (<0.001) |
| Mechanical ventilation | 17 (48.6%) | 12 (6.6%) | 29 (13.3%) | 41.399 (<0.001) |
Note: Data are expressed as n (%), or n/N (%). p < 0.05 was considered statistically significant.
Abbreviations: COVID‐2019, coronavirus disease 2019; IL‐6, interleukin‐6.
*Mann–Whitney U test, non‐label is used Pearson Χ 2 test.
3.3. Influence factors of AKI in COVID‐19
Our univariate analysis showed that older and male patients, patients with major chronic diseases, including hypertension and cardiovascular disease, were at significantly increased risk for in‐hospital AKI (Table 4). Other AKI risks included COVID severity, lymphopenia, leukocytosis, and elevated CRP, d‐dimer, IL‐6, kidney injury at admission, and development of cytokine storm and ARDS.
Table 4.
Univariate logistic analysis of influence factors of AKI in COVID‐19 patients
| OR (95% CI) | p value | |
|---|---|---|
| Hospital location: Wuhan | 5.163 (2.053–12.984) | <0.001 |
| Sex: Female | 0.347 (0.150–0.801) | 0.013 |
| Age: <65 years | 0.213 (0.101–0.449) | <0.001 |
| Disease severity: severe | 10.850 (4.030–29.213) | <0.001 |
| Traveling or residing in Wuhan | 5.197 (1.533–17.623) | 0.008 |
| Major chronic disease | 7.333 (2.811–19.133) | <0.001 |
| Hypertension | 7.093 (2.911–17.282) | <0.001 |
| CHD | 8.747 (1.847–41.415) | 0.006 |
| Kidney injury at admission | 6.833 (2.943–15.866) | <0.001 |
| Hepatic disease | 4.421 (1.909–10.239) | 0.001 |
| White blood cell count | 1.202 (1.113–1.299) | <0.001 |
| Lymphocyte count | 0.096 (0.037–0.245) | <0.001 |
| CRP | <0.001 | |
| CRP(Q1‐Q2) | 0.652 (0.104–4.087) | 0.648 |
| CRP(Q2‐ Q3) | 1.742 (0.393–7.73) | 0.465 |
| CRP(≥Q3) | 8.903 (2.416–32.811) | 0.001 |
| D‐dimmer | 1.060 (1.029–1.092) | <0.001 |
| IL6 | <0.001 | |
| IL6 (Q1–Q2) | 0.608 (0.095–3.882) | 0.599 |
| IL6 (Q2–Q3) | 1.667 (0.366–7.586) | 0.509 |
| IL6 (≥Q3) | 8.455 (2.216–32.25) | 0.002 |
| Cytokine storm | 15.441 (6.379–37.376) | <0.001 |
| ARDS | 11.736 (5.182–26.580) | <0.001 |
Abbreviations: AKI, acute kidney injury; ARDS, acute respiratory distress syndrome; CHD, coronary heart disease; CI, confidence interval; CRP, C‐reactive protein; IL‐6, interleukin‐6; OR, odds ratio.
Our logistic regression multivariate analysis revealed that female patients were an independent negative risk factor in the occurrence of AKI (OR: 0.288, 95% CI: 0.095–0.869; p = 0.027). Evidence of kidney injury at admission was associated with more than a threefold increased risk of AKI (OR: 3.132, 95% CI: 1.150–8.527; p = 0.025). The presence of cytokine storm was associated with more than a fourfold increased risk of AKI (OR: 4.234, 95% CI: 1.361–13.171; p = 0.013). In addition, patients with ARDS had a more than sevenfold increased risk of AKI (OR: 7.684, 95% CI: 2.622–22.523; p < 0.001) (Table 5).
Table 5.
Multivariate logistic analysis of influence factors of AKI in COVID‐19 patients
| OR (95% CI) | p value | |
|---|---|---|
| Sex: female versus male | 0.288 (0.095–0.869) | 0.027 |
| Kidney injury at admission: yes versus no | 3.132 (1.150–8.527) | 0.025 |
| Cytokine storm: yes versus no | 4.234 (1.361–13.171) | 0.013 |
| ARDS: yes versus no | 7.684 (2.622–22.523) | <0.001 |
Abbreviations: AKI, acute kidney injury; CI, confidence interval; OR, odds ratio.
3.4. Cytokine storm syndrome
The median baseline levels of IL‐6 were 5.8 pg/ml (IQR: 60.5) in the study cohort and were significantly higher in patients with severe (30.1 pg/ml, IQR: 162.7) compared with moderate (1.5 pg/ml, IQR: 3.7) (p < 0.001) COVID‐19. Overall, cytokine storm syndrome was observed in 30 (13.5%) patients, and only in those with severe COVID‐19. Additionally, COVID‐19 patients with AKI had significantly higher IL‐6 levels and rate of cytokine storm compared with the non‐AKI group (p < 0.001) (Table 3). Furthermore, our association rule analysis showed that cytokine storm exhibited a strong correlation and collinearity with leukocyte counts, lymphocyte counts, IL‐6 levels, d ‐dimer, and positive contact history (Figure 2). Similar results were observed when we analyzed AKI and newly emerging kidney disease in COVID‐19 patients (Table 6).
Figure 2.
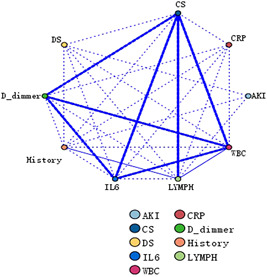
Association rules analysis of risk factors of AKI occurrence in COVID‐19 patients used to identify internal relations among items predicting AKI in COVID‐19 patients. The diameter of the lines corresponds to the degree of correlation. AKI, acute kidney injury; CHD, coronary heart disease, History, Wuhan contact history; CRP, C‐reactive protein; CS, cytokine storm; DS, disease severity; IL‐6, interleukin‐6; LYMPH#, lymphocyte count, WBC, white blood cell count
Table 6.
Results of association rule based on Apriori algorithm
| Consequent | Antecedent | Support % | Confidence % |
|---|---|---|---|
| LYMPH | AKI + History | 14.8 | 93.9 |
| DS | AKI + History | 14.8 | 90.9 |
| LYMPH | AKI + DS | 13.9 | 93.6 |
| DS | AKI + LYMPH + History | 13.9 | 93.6 |
| LYMPH | AKI + DS + History | 13.5 | 96.7 |
| History | AKI + CHD | 11.2 | 88.0 |
| LYMPH | AKI + CHD | 11.2 | 84.0 |
| DS | AKI + CHD | 11.2 | 80.0 |
Abbreviations: AKI, acute kidney injury; CHD, coronary heart disease; history, traveling or residing history in Wuhan; DS, disease severity; LYMPH, lymphocyte absolute value.
3.5. Mechanically ventilated COVID‐19 patients with AKI
Seventeen (48.6%) patients who received invasive mechanical ventilation developed AKI, and 64.7% (11/17) of them died. Compared with non‐ventilated patients with AKI, mechanically ventilated patients with AKI had significantly higher IL‐6 levels and rate of cytokine storm (Figure 3). Mechanically ventilated AKI patients also had significantly higher CRP levels, d‐dimer levels, and leukocyte counts (p < 0.05). The trend chart of each patient showed that the order of occurrence of ventilator treatment, cytokine storm, and AKI followed a certain pattern (Figure 4) in which serum creatinine transiently fell after initiation of ventilation, but rose again sharply upon development of a cytokine storm.
Figure 3.
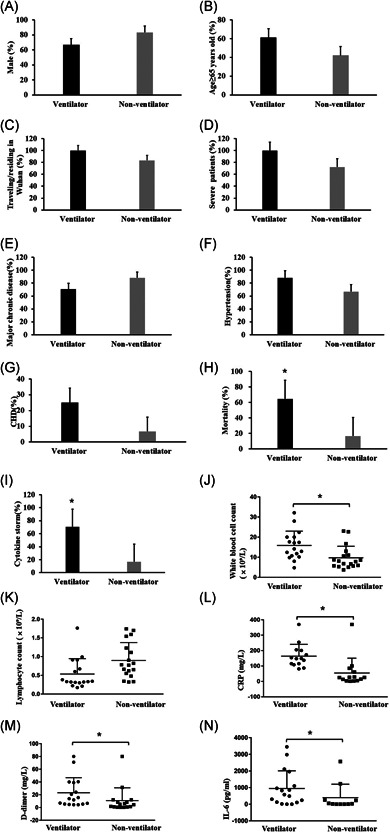
Characteristics comparison of 35 AKI patients with or without ventilator treatment. (A) Sex: male; (B) age ≥65 years; (C) major chronic disease; (D) hypertension; (E) coronary heart disease; (F) severe patients; (G) death; (H) cytokine storm; (I) white blood cell count; (J) lymphocyte count; (K) C‐reactive protein; (L) d‐dimer; (M) interleukin‐6. *p < 0.05 ventilator versus nonventilator. AKI, acute kidney injury
Figure 4.
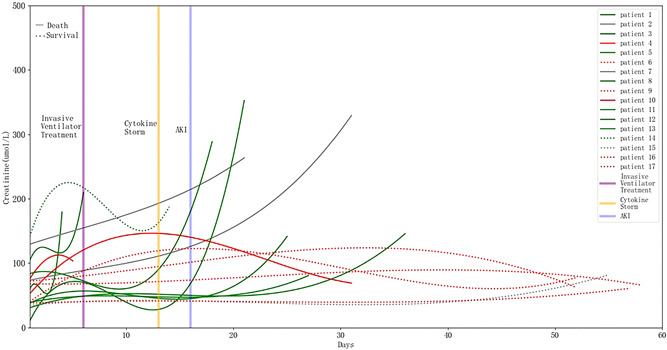
Trend of creatinine levels change in patients diagnosed with COVID‐19, who were admitted to the hospital with acute kidney injury later and treated with an invasive ventilator (n = 17). The solid line represents the dead patients (n = 11) who died, and the dotted line surviving patients (n = 6). The blue vertical line represents the median time of AKI occurrence (16 days), and the orange vertical line the median time of cytokine storm occurrence (13 days). The purple vertical line represents the median time of initiating invasive ventilator treatments (6 days). The cytokine storm occurred 3 days before AKI, and patients died or were discharged on average 6 days after AKI. AKI, acute kidney injury
3.6. Secondary bacterial infection
Of the COVID‐19 patients with AKI, 57.1% (20/35) patients were susceptible to bacterial infection characterized by elevated leukocyte counts (>10 × 10⁹/L). Their leukocyte counts were significantly higher than that of patients with no AKI (p < 0.001) (Table 3). Compared with AKI patients with normal leukocyte counts, those who suffered AKI and secondary bacterial infection were older (age ≥65 years old: 70% vs. 33.3%, p = 0.031), more likely to develop cytokine storm syndrome (65% vs. 13.3%, p = 0.002) and receive invasive mechanical ventilation (70% vs. 20.0%, p = 0.003), and had higher mortality (60% vs. 13.3%, p = 0.005). Besides this, AKI patients with bacterial infection had higher leukocyte counts (16.9 × 10⁹/L, IQR: 9.8 vs. 6.7 × 10⁹/L, IQR: 2.7, p < 0.001), IL‐6 (488.0 pg/ml, IQR: 1104.9 vs. 6.6 pg/ml, IQR: 299.8, p = 0.013) and d ‐dimer (12.1 mg/L, IQR: 33.7 vs. 3.5 mg/L, IQR: 8.4, p = 0.006), all of which are also the correlation factors of the cytokine storm. Up to 86.7% of AKI patients with cytokine storms may develop a secondary bacterial infection. The percentage was significantly higher when compared with the noncytokine storm group (86.7% vs. 35%, p = 0.002). The leukocyte counts were also significantly higher in AKI patients with cytokine storm than those without (13.0 × 10⁹/L, IQR: 11.3 vs. 8.3 × 10⁹/L, IQR: 7.5, p = 0.005).
4. DISCUSSION
This retrospective cohort study analyzed 233 COVID‐19 patients from three hospitals, two in Wuhan, Hubei province, and one in Guizhou province, and showed that approximately one in six patients with COVID‐19 developed AKI. Kidney injury at admission, cytokine storm, and ARDS were significant influence factors of AKI development in COVID‐19 patients. More intriguingly, these patients developed AKI at a median duration of 3 weeks from admission, coinciding with the hyperimmune response phase of the infection. More than half of the patients who received invasive mechanical ventilation developed AKI. Moreover, 64.5% of patients with AKI died within a median of 4 days.
AKI has been reported in patients with severe COVID‐19 24 and in 3.6%–15% of those with SARS‐CoV‐2 infection, 25 , 26 but over 25% of those with ARDS. 25 These studies suggested that the kidneys are among the target organs in SARS‐CoV‐2 infection. However, the mechanism of renal involvement is unclear. SARS‐CoV‐2 was detected in urine samples of COVID‐19 patients. 2 Kidney specimens from autopsy shows the SARS‐CoV‐2 can directly infect human kidney tubules, and the nucleocapsid protein antigens could be seen in kidney tissues. 27 , 28 However, the presence of SARS‐CoV‐2 in renal tissues cannot fully explain the development of AKI in COVID‐19 patients. The kidney is affected by crosstalk with other organs (such as the lungs), drug use, prerenal factors, and other factors that can cause AKI, which are important to identify. In our cohort study, the ratio of moderate to severe cases was 1.25:1 compared to 5–6:1 reported by others, 29 suggesting a more uniform distribution of severity, and 15.7% of the patients subsequently developed AKI. It is noteworthy that approximately one‐third of severe COVID‐19 cases developed AKI, and almost half of them eventually succumbed to the disease, similar to previous reports on SARS and MERS‐CoV infections. 30
Our sample size and representative cohort lend substantial strength to our study. Bei et al. reported 75.4% of renal complications, but Wang et al. reported only 18% of renal complications and no AKI in COVID‐19. Guizhou has a population of 36.22 million, and Wuhan has a permanent population of 11.21 million. We have included all the adult patients of COVID‐19 in Guizhou Province, 121 cases, 31 although the incidence of severe cases is 21% (unpublished data, Corresponding author, Dr. Zhou) similar to the overall data of China. The reason for our enrollment strategy was that Wuhan, but not Guizhou province, faced serious medical crowding in the early stage of COVID‐19. Guizhou Province initiated the first‐level response to major public health emergencies on January 24th, fell to the third level 32 days later, and was the first province in China to fully return to work and study on March 16th. Given the limitations of retrospective studies, the ability to include data collected in several different centers and provinces may eliminate confounding factors, such as medical crowding. Our results showed that 36.7% of the patients had kidney‐related diseases during the course of the disease, and 15.7% of the patients developed AKI. The total mortality rate of AKI was 40% in COIVD‐19.
The rapid evolution of moderate to severe COVID‐19 may be attributed to a cytokine storm. In our study, cytokine storm syndrome was diagnosed when IL‐6 levels rose over 100‐fold of the normal value within 48 h, accompanied by lung and other organ functional deterioration (at least two organs). We developed this diagnosis upon detection of at least one cytokine with a sharp rise (at least 100‐fold), accompanied by noticeable organ damage or deterioration. We did not exclude patients with sepsis. Our strict approach may have missed some patients with milder cytokine storms, but ensured indisputable detection of a cytokine storm when existed. We treated this phenomenon as a sub‐variable to be included in risk analysis. In our study, kidney injury at admission, cytokine storm and ARDS are influence factors of AKI in patients with COVID‐19. The occurrence of ARDS in patients with COVID‐19 is similar to that in patients with SARS.
Invasive mechanical ventilation is a known risk factor for AKI in critically ill patients, 32 including those with viral infections. 33 AKI emerges as a new concern in critically ill COVID‐19 patients on invasive mechanical ventilation. Anecdotal reports show that continuous renal replacement therapy (CRRT) machines are also in a severe shortage after ventilators, indicating that AKI accounts for marked consumption of medical resources. At the same time, these patients suffer from cytokine storms, adverse effects of ventilators, ARDS, lung‐kidney crosstalk, heart‐kidney crosstalk, and other complications. It is essential to delineate the characteristics of this subgroup of COVID‐9 patients to make advance preparations, like ensuring the availability of CRRT. In our study, we focused on the analysis of the characteristics of patients with AKI on mechanical ventilation. We found that sex, age, disease severity, and underlying conditions do not affect the occurrence of AKI, but mechanically‐ventilated patients with AKI are in a more severe inflammatory state and show a very high mortality rate (64.7%). Of note, the cytokine storm occurs on average 7 days after receiving invasive mechanical ventilation during hospitalization. We found that AKI patients with cytokine storm had significantly higher leukocyte counts than patients without cytokine storm. Based on these findings, it is conceivable that not only the viral infection but also secondary bacterial infections are responsible for the cytokine storm in COIVD‐19 patients with AKI, especially for individuals with long‐term weakened or compromised immunity. Hence, appropriate use of prophylactic antibiotics and immune regulation may help avoid the occurrence of cytokine storms and improve the prognosis of COIVD‐19 patients with AKI.
To our knowledge, this is the largest retrospective cohort study with AKI as the endpoint, including 223 inpatients from three centers. The inclusion of cases outside of Hubei Province makes the study less susceptible to the confounding factor of medical crowding. Our case ratio of moderate and severe patients is 1.25:1, which makes the attribution analysis of AKI more rational.
Our study has some limitations. First, we underestimated the impact of cytokine storms throughout the study because our diagnostic criteria for cytokine storms were conservative. Second, as limited data were available on the proportion of CRRT in patients with AKI, its impact on AKI was not studied. Last, interpretation of our findings might be limited by the small sample size. However, by including all adult patients in the three designated hospitals for COVID‐19, we believe our study population is representative of cases diagnosed and treated in China.
In conclusion, approximately one in six patients with COVID‐19 eventually develops AKI. Kidney injury on admission, development of cytokine storm, and ARDS during the course of the disease are influence factors of AKI development and could be used to stratify COVID‐19 patients to mitigate AKI risk. Cytokine storm and secondary bacterial infections may be responsible for AKI development in COVID‐19 patients.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Yu Meng designed the study. Rong‐Hou Zhou,Zhi‐Yong Peng and Hai‐Yan Yin collected the data. Xia‐Qing Li, Han Liu and Yin Guan performed the statistical analysis. Xia‐Qing Li and Han Liu wrote the paper. Xia‐Qing Li, Wen‐Yong Gao, Xiao Yang, Dian‐Shuang Xu prepared the figures and tables. Lilach O. Lerman, and Xing‐Dong Cai edited and revised the manuscript. All authors read and approved the submitted version.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The authors greatly appreciate all of the hospital staff and the 22nd batch of Guangdong medical team supporting Hubei province for their efforts in recruiting and treating patients and thank all patients involved in this study. The authors also thanks Yuan Chen and Nan‐Feng Mo for collating the data. This study was supported by the Guizhou Science and Technology Project (QKHZC[2020]4Y002), the Guiyang Science and Technology Project (ZKXM[2020]4‐1), the National Natural Science Foundation (grants 81772046 and 81971816), and the Special Project for Significant New Drug Research and Development in the Major National Science and Technology Projects of China (2020ZX09201007).
Li X, Liu H, Meng Y, et al. Critical roles of cytokine storm and secondary bacterial infection in acute kidney injury development in COVID‐19: A multi‐center retrospective cohort study. J Med Virol. 2021;93:6641‐6652. 10.1002/jmv.27234
Xia‐Qing Li and Han Liu contributed equally to this study.
Contributor Information
Yu Meng, Email: mengy@jnu.edu.cn.
Zhi‐Yong Peng, Email: pengzy5@hotmail.com.
Hou‐Rong Zhou, Email: zhr1974@163.com.
REFERENCES
- 1. WHO Situation Reports . April 28, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200430-sitrep-101-covid-19.pdf?sfvrsn=2ba4e093_2
- 2. Wang L, Li X, Chen H, et al. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol. 2020;51:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England). 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England). 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ding Y, He L, Zhang Q, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS‐CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203(2):622‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wrapp D, Wang N, Corbett KS, et al. Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science (New York, NY). 2020;367(6483):1260‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID‐19 in China. Kidney Int. 2020;98(1):219‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ali H, Daoud A, Mohamed MM, et al. Survival rate in acute kidney injury superimposed COVID‐19 patients: a systematic review and meta‐analysis. Ren Fail. 2020;42(1):393‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in‐hospital death of patients with COVID‐19. Kidney Int. 2020;97(5):829‐838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID‐19. Journal of Infection. 2020;80 (6):607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mehta P, McAuley DF, Brown M, et al . COVID‐19: consider cytokine storm syndromes and immunosuppression. The Lancet. 2020;395 (10229):1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rawson TM, Moore LSP, Zhu N, et al. Bacterial and fungal co‐infection in individuals with coronavirus: a rapid review to support COVID‐19 antimicrobial prescribing. Clin Infect Dis. 2020;71:2459‐2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li X, Wang L, Yan S, et al. Clinical characteristics of 25 death cases with COVID‐19: a retrospective review of medical records in a single medical center, Wuhan, China. Int J Infect Dis. 2020;94:128‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao M. Cytokine storm and immunomodulatory therapy in COVID‐19: role of chloroquine and anti‐IL‐6 monoclonal antibodies. Int J Antimicrob Agents. 2020;55:105982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76(1):16‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. Interim guidance. https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf. Accessed February 4, 2020.
- 19. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179‐c184. [DOI] [PubMed] [Google Scholar]
- 20. Kidney Disease : Improving Global Outcomes (KDIGO) CKD Work Group: KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease.Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011). 2013;3(1):19–62. [Google Scholar]
- 21. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Health Commission of the People's Republic of China : Diagnosis and treatment protocols of pneumonia caused by a novel coronavirus (Trial Version 7), 2020. http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf
- 23. Han WK, Wagener G, Zhu Y, et al. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol. 2009;4(5):873‐882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8:475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020; 323(11):1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Diao B, Wang C, Wang R, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) Infection. Nat Commun. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Farkash EA Wilson AM Jentzen JM. Ultrastructural evidence for direct renal infection with SARS‐CoV‐2. J Am Soc Nephrol. 2020; 31:1683‐1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chu KH, Tsang WK, Tang CS, Acute renal impairment in coronavirus‐associated severe acute respiratory syndrome [DOI] [PMC free article] [PubMed]
- 31. Guizhou Provincial Health Commission Accessed February 14, 2020. http://www.gzhfpc.gov.cn/xwzx_500663/yqtb/202002/t20200215_49183203.html
- 32. van den Akker JP, Egal M, Groeneveld AB. Invasive mechanical ventilation as a risk factor for acute kidney injury in the critically ill: a systematic review and meta‐analysis. Crit Care. 2013;17(3):R98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martin‐Loeches I, Papiol E, Rodríguez A, et al. Acute kidney injury in critical ill patients affected by influenza A (H1N1) virus infection. Crit Care. 2011;15(1):R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


