Abstract
Objective
We aimed to identify, appraise, synthesize, and contextualize rapidly emerging reports on medication taking (adherence) among patients with rheumatic diseases during the COVID‐19 pandemic.
Methods
We searched MEDLINE, EMBASE, and CINAHL for peer‐reviewed communications, letters, and articles published during the COVID‐19 pandemic evaluating medication taking among individuals with rheumatic diseases. We appraised assessment and reporting of medication adherence according to established definitions of 3 distinct problems of medication taking (i.e., noninitiation, poor implementation, and discontinuation) and pooled findings using random‐effects models.
Results
We included 31 peer‐reviewed studies in our synthesis from various jurisdictions, of which 25 described medication taking among rheumatology patients and 6 described medication prescribing among rheumatology providers. The pooled prevalence of overall medication nonadherence was 14.8% (95% confidence interval [95% CI] 12.3–17.2) and that of medication discontinuation (i.e., stopping of prescriptions) and poor implementation (i.e., not taking medication at the dose/frequency prescribed) as 9.5% (95% CI 5.1–14.0) and 9.6% (95% CI 6.2–13.0), respectively. Noninitiation (i.e., not starting/not filling new prescriptions) was not addressed.
Conclusion
Medication taking among individuals with rheumatic diseases during the COVID‐19 pandemic varies globally. Unclear reporting and extensive variation in research methods between studies create barriers to research replication, comparison, and generalization to specific patient populations. Future research in this area should use consistent and transparent approaches to defining and measuring medication taking problems to ensure that findings appropriately describe the epidemiology of medication adherence and have the potential to identify modifiable targets for improving patient care.
INTRODUCTION
In response to the COVID‐19 pandemic, international recommendations for rheumatic disease management were rapidly released, including those from the American College of Rheumatology (1), and the European Alliance of Associations for Rheumatology (2), all of which consistently call for adherence to medication therapies, including conventional synthetic, biologic, and targeted synthetic disease‐modifying antirheumatic drugs (csDMARDs, bDMARDs, tsDMARDs) as well as other therapies such as nonsteroidal antiinflammatory drugs (NSAIDs) (1, 2). Indeed, taking medications as prescribed is important to the management of rheumatic diseases across both routine and exceptional circumstances, such as the COVID‐19 pandemic, as patients rely on medications to relieve symptoms, perform daily life activities, and prevent irreversible joint and organ damage. However, as a result of the COVID‐19 pandemic, adherence with therapies among patients with rheumatic diseases has become increasingly complex due to health care system factors, such as the occurrence of medication shortages and limitations on nonessential health care visits. For example, hydroxychloroquine (HCQ), a csDMARD used to treat systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), was initially speculated to be a viable treatment for COVID‐19, leading to concerns regarding access for patients with rheumatic diseases. Early reports of successful treatment with HCQ (3) and emergency authorization by the US Food and Drug Administration led to critical shortages in medication availability (4). The therapeutic efficacy of interleukin‐6 inhibitor against COVID‐19 has also been discussed in a recent systematic review and meta‐analysis (5). Condition‐ and patient‐related factors may also place barriers to optimal rheumatic disease management and patients' medication taking as prescribed. Indeed, patients with rheumatic disease are known to be at higher risk of infection secondary to immune‐modulating treatments, immune‐dysfunction, and the presence of comorbidities (6). Therefore, concerns regarding immunosuppressive effects secondary to antirheumatic therapy may contribute to nonadherence to medications.
SIGNIFICANCE & INNOVATIONS.
We synthesized rapidly emerging reports during the COVID‐19 pandemic, including 25 on medication taking (adherence) among rheumatology patients and 6 on medication prescribing among providers.
We calculated pooled prevalence estimates of 9.5% for poor implementation, 9.6% for discontinuation, and 14.8% for medication nonadherence overall, suggesting that most patients with rheumatic disease are taking medications as prescribed during the COVID‐19 pandemic.
We identified a number of serious and persistent barriers (e.g., challenges obtaining medications, personal beliefs, and fears) to accessing medications and care identified by patient and providers.
Our findings reveal the necessity for researchers to use standardizing methods for assessing and measuring medication adherence to ensure that research findings have the potential to inform interventions and support patient care.
Since the start of the COVID‐19 pandemic, numerous reports have assessed experiences with medication taking among patients with rheumatic diseases. Accordingly, we conducted a rapid review to systematically identify studies, synthesize findings, and describe the data to date examining the medication taking experiences among this patient population as well as the prescribing experiences of rheumatology providers.
MATERIALS AND METHODS
Search strategy
We conducted a rapid review informed by guidelines from the Cochrane Rapid Reviews Methods Group (7). A search strategy was developed collaboratively with all authors, including an information scientist (UE), to identify peer‐reviewed studies on medication taking (adherence) among patients with rheumatic diseases during the COVID‐19 pandemic. Searches were performed by UE of the databases Ovid MEDLINE, Ovid EMBASE, and CINAHL (all from March 2020), using subject headings and keywords of unindexed terms related to “medication adherence,” “rheumatic diseases,” and “COVID‐19.” Additional resources were searched, including the Cochrane COVID‐19 Study Register, Epistemonikos COVID‐19 Living Overview of Evidence, and Prospero. The searches were conducted on January 13, 2021 (see Supplementary Tables 1, 2, and 3, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24744, for the complete search strategy).
Study selection
Two authors (NR and RG) screened titles and abstracts for peer‐reviewed concise communications, letters, and articles that fulfilled inclusion criteria: 1) observational design, 2) study sample including individuals with rheumatic disease (e.g., ankylosing spondylitis, psoriatic arthritis, RA, SLE) and/or health care providers, 3) evaluation of medication taking (adherence) among individuals with rheumatic disease, and 4) during the COVID‐19 pandemic. Any conflicts were resolved via consensus and/or consultation with the corresponding author (MADV). The Covidence platform was used to support the screening process.
Data extraction and appraisal
Two study authors (NR and JYP) extracted information on the type of publication (e.g., article, letter), country, study period, and study sample characteristics, including information on types of rheumatic disease, age, and sex. In extracting information on medication taking problems among rheumatology patients, we applied definitions by the International Society of Medication Adherence (8) that describe distinct problems of medication taking, corresponding to 3 types of medication nonadherence: 1) noninitiation, that is, not filling a prescription or starting a dosing regimen; 2) poor implementation of the dosing regimen, where scheduled doses are delayed or omitted; and 3) discontinuation or stopping of drug therapy. Instances when authors reported a cumulative measure not defined as any of the aforementioned types of medication taking problems were assigned as overall medication nonadherence. In addition to extracting and classifying medication taking according to each type of medication nonadherence, we also extracted corresponding information on the extent of the problem (e.g., percentage of patients), as well as potential reasons, when available. If a newer publication for the same study previously identified was found, only the newer results were extracted, unless the earlier publication reported information that was not reported in the more recent publication. With respect to articles describing prescribing by rheumatology providers, we extracted information on reported practice patterns (e.g., continuing, switching, delaying, discontinuing medications) and experiences (e.g., drug shortages) during the COVID‐19 pandemic.
We appraised the quality of medication adherence measurement and reporting using the International Society of Pharmacoeconomics and Outcomes Research Medication Compliance and Persistence Special Interest Group (9), as we have done in prior systematic reviews on medication adherence in rheumatic diseases (10, 11). We adapted and condensed this checklist, which aims to establish standards for reporting of medication adherence studies, to 10 priority items (e.g., clarity of study objectives, description of data collection methods/data source, and explicit definition of medication taking problem), awarding a single point for each criterion for a maximum score of 10.
Statistical analysis
We conducted a narrative synthesis of included studies. Where possible, we also pooled and reported the prevalence of medication nonadherence according to each type of medication taking problem (noninitiation, poor implementation, or discontinuation) as well as overall nonadherence using random‐effects models. We used the proportion (of patients) and corresponding 95% confidence intervals (95% CIs) as estimates. All data analysis was performed using Stata, version 15.
RESULTS
Our search identified 273 articles after the exclusion of duplicates (Figure 1). Of these, we identified 35 studies that met our inclusion criteria, including 25 assessing medication taking among rheumatology patients and 6 assessing medication prescribing among rheumatology providers.
Figure 1.
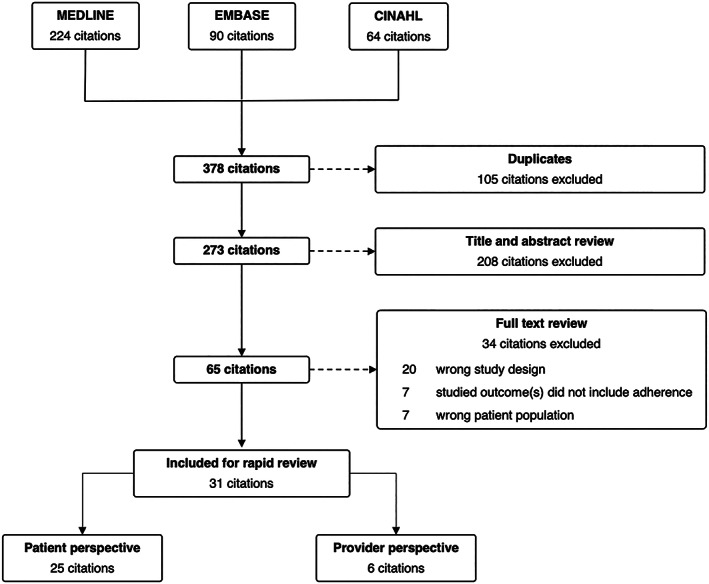
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flow chart.
Medication taking among rheumatology patients
We describe the characteristics of 25 included studies assessing medication taking in Table 1. Altogether, studies spanned several countries predominantly located across the Asian, European, North African, and North American continents. All of the studies were based on self‐report, with the majority based on surveys and 4 based on interviews.
Table 1.
Characteristics of included studies assessing medication taking among rheumatology patients*
| Medication taking | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author (ref.) | Publication type | Data collection | Study period | Country | Rheumatic disease | No. | Age | Female, % | No initiation | Poor implementation | Discontinuation | Reasons |
| Abualfadl et al, 2021 (39) | Article | Survey | 06/–/20 to 08/–/20 | Egypt | RA | 1,037 | Mean ± SD 44.2 ± 12.3 | 82.4 | ○ | ○ | ● | ○ |
|
Ciurea et al, 2021 (18) |
Article | Structured interview and survey | 01/01/20 to 06/3020 | Switzerland |
Axial SpA, RA, PsA |
666 | Mean ± SD: axial SpA: 47.1 ± 11.8; RA: 55.3 ± 13.2; PsA: 52.6 ± 10.7 | Axial SpA: 50.9, RA: 71.8, PsA: 49.6 | ○ | ● | ○ | ○ |
| Costantino et al, 2021 (12) | Article | Survey | 04/18/20 to 05/21/20 | France | SpA, RA, PsA | 655 | Mean ± SD 51.0 ± 13.4 | 61.8 | ○ | ● | ● | ● |
|
Fragoulis et al, 2021 (19) |
Letter | Structured interview |
04/14/20 to 04/22/20 |
Greece | IA, MCTD, SLE, SSc, vasculitis, APS, SS, PMR, other | 500 | Mean ± SD 53.7 ± 15.3 | 73.2 | ○ | ○ | ● | ● |
|
George et al, 2021 (20) |
Article | Survey | 03/29/20 to 05/26/20 | US | RA, PsA, AS, SLE | 1,517 | Mean ± SD 55.1 ± 11.7 | 88.3 | ○ | ○ | ● | ● |
|
Glintborg et al, 2021 (15) |
Article | Survey | 03/–/20 to 06/–/20 | Denmark | RA, PsA, axial SpA, other | 12,789 | ≤39: 8%; 40–59: 35%; 60–79: 53%, ≥80: 4% | 65 | ○ | ● | ● | ● |
| Hassen et al, 2020 (22) | Letter, article | Survey | 03/–/20 to 04/–/20 | Saudi Arabia | RA, SLE, BD, SpA, SS, other | 637 |
Mean ± SD 35.7 ± 9.6 |
87.3 | ○ | ● | ● | ● |
| Kavadichanda et al, 2021 (32) | Article | Structured interview |
04/15/20 to 04/30/20 |
India | SLE, IIM, MCTD, SSc, other CTDs | 373 |
35 (IQR 25–44) |
88.7 | ○ | ○ | ● | ● |
| Khabbazi et al, 2020 (29) | Letter | Interview | 07/10/20 to 07/24/20 | Iran | RA, SpA, SLE, APS, BD, vasculitis, UIA, IIM, SSc, SS, other | 858 | Mean ± SD 48.8 ± 13.4 | 68.9 | ○ | ● | ● | ● |
|
Koker et al, 2020 (26) |
Article | Survey | 05/01/20 to 05/20/20 | Turkey | JIA, autoinflammatory diseases, CTDs, vasculitis | 414 | Mean ± SD 12.5 ± 4.7 | 54.1 | ○ | ○ | ● | ● |
|
López‐Medina et al, 2021 (31) |
Letter | Survey | 04/25/20 to 05/05/20 | Spain | SpA, PsA, RA, systemic autoimmune diseases, fibromyalgia, OA, other | 644 | Missing data | Missing data | ○ | ● | ● | ● |
| Maldonado et al, 2021 (24) | Article | Survey | 05/08/20 to 06/01/20 | US | SLE, RA, JIA, DM, PM, sarcoidosis, other | 361 | 42 (IQR 23–58) | 88 | ○ | ● | ● | ● |
| Michaud et al, 2020 (28) | Article | Survey | 03/25/20 to 04/01/20 | US | RA, OA, SLE, other | 530 | Mean ± SD 65.0 ± 10.9 | 84.4 | ○ | ● | ● | ● |
|
Murray et al, 2021 (30) |
Article | Survey | 04/28/20 to 05/05/20 | Ireland | RA, SpA, CTD, vasculitis, other | 1,381 | <40: 24.1%; 41–60: 59.2%; >60: 16.7% | 87.8 | ○ | ● | ● | ● |
| O'leary et al, 2020 (40) | Letter | Missing data | Ireland | Missing data | 398 | Missing data | Missing data | ○ | ○ | ● | ○ | |
| Pineda‐Sic et al, 2021 (21) | Letter | Survey | 05/14/20 to 05/25/20 | Mexico | RA, SLE, SS, axial SpA, PsA, IIM, scleroderma, vasculitis, other | 345 | <30: 13.9%; 30–60: 65.8%; >60: 20.3% | 90.2 | ○ | ● | ● | ● |
|
Ramirez et al, 2020 (17) |
Article | Survey | 02/–/20 to 04/–/20 | Italy | SLE | 417 | <30: 12%; 31–40: 20%; 41–50: 25% >50: 43% | 91 | ○ | ● | ● | ● |
|
Rathi et al, 2020 (23) |
Article | Survey | 04/–/20 to 05/–/20 | India | SLE | 1,040 | Mean ± SD 27.5 ± 19.1 | 9:1 (F:M ratio) | ○ | ● | ● | ● |
|
Rosenbaum et al, 2020 (13) |
Letter | Survey | 04/10/20 to 05/07/20 | US, 64 other countries | AS, other | 2,992 | Median: F: 53; M: 54 | 61.4 | ○ | ● | ● | ○ |
|
Roux et al, 2020 (16) |
Letter | Survey | 04/10/20 to 04/21/20 | France | SpA | 609 | Mean ± SD 45 ± 11 | 76 | ○ | ● | ● | ○ |
| Schmeiser et al, 2020 (41) | Letter | Survey |
03/16/20 to 04/03/20 |
Germany | RA, SpA, PsA, SLE, SSc, PMR, SS, GPA, PM, EGPA, other | 656 | <30: 7%; 30–60: 52%; >60: 23% | Missing data | ○ | ○ | ● | ○ |
|
Seyahi et al, 2020 (25) |
Article | Survey | Missing data | Turkey | RA, CTD, SpA, BD, FMF, vasculitis | 771 | 42 (range 16–81) | – | ○ | ● | ● | ● |
| Singh and Edwards, 2020 (14) | Article | Survey | 04/–/20 to 06/–/20 | US | Gout | 122 | Mean ± SD 54.2 ± 13.8 | 35 | ○ | ● | ● | ○ |
| Ziadé et al, 2020 (27) | Article | Survey | 05/08/20 to 05/22/20 | 14 Arab countries† | Missing data | 2,163 | Mean ± SD 40.0 ± 11.9 | 72.3 | ○ | ○ | ● | ● |
| Zen et al, 2020 (42) | Article | Survey | 04/09/20 to 04/25/20 | Italy | SLE, SSc, RA, AAV, IIM | 916 | Mean ± SD 53.6 ± 14.3 | 78.6 | ○ | ○ | ● | ○ |
○ = not reported; ● = reported. AAV = ANCA‐associated vasculitis; APS = antiphospholipid syndrome; AS = ankylosing spondylitis; BD = Behçet's disease; CTD = connective tissue disease (e.g., systemic lupus erythematosus [SLE], SSc [systemic sclerosis], SS [Sjögren's syndrome]); DM = dermatomyositis; EGPA = eosinophilic granulomatosis with polyangiitis; FMF = familial Mediterranean fever; GPA = granulomatosis with polyangiitis; IA = inflammatory arthritis; IIM = idiopathic inflammatory myopathies; IQR = interquartile range; JIA = juvenile idiopathic arthritis; MCTD = mixed connective tissue diseases; OA = osteoarthritis; PM = polymyositis; PMR = polymyalgia rheumatica; PsA = psoriatic arthritis; RA = rheumatoid arthritis; ref. = reference; SpA = spondyloarthritis; UIA = undifferentiated inflammatory arthritis.
Iraq, Saudi Arabia, Egypt, Morocco, Algeria, Kuwait, Lebanon, Jordan, Tunisia, United Arab Emirates, Syria, Libya, Palestine, Oman, Qatar, other.
The quality appraisal score of included studies is reported in Supplementary Table 4, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24744. The majority of studies clearly described their aims, their study sample, the timeframe for data collection, and methods for determining medication taking problems. All studies described their methods for data collection and appropriately presented findings on medication taking; however, the majority of studies did not define medication taking problems (nonadherence) and none used accepted definitions. Overall, definitions of medication nonadherence varied between reports, so that meaningfully synthesizing findings was challenging.
Twelve studies (n = 22,842) reported the overall prevalence of nonadherence to therapy, which ranged from 6.5% to 34.2% of patients. Pooling the crude estimates of overall nonadherence resulted in a prevalence of 14.8% (95% CI 12.3–17.2) (Figure 2A). No studies reported on noninitiation (i.e., not starting/not filling new prescriptions). Six studies (n = 3,884) reported the overall prevalence of poor implementation (i.e., not taking medication at the dose/frequency prescribed), which ranged from 2.7% to 16.4%. Pooling the crude estimates of poor implementation resulted in a prevalence of 9.5% (95% CI 5.1–14.0) (Figure 2B). Ten studies (n = 7,087) reported the overall prevalence of medication discontinuation (i.e., stopping prescription), which ranged from 2% to 31.4%. Pooling the crude estimates of discontinuation resulted in a prevalence of 9.6% (95% CI 6.2–13.0) (Figure 2C). Six studies (12, 13, 14, 15, 16, 17) additionally reported on specific counts based on medication type (i.e., csDMARDs, bDMARDs, tsDMARDs, corticosteroids, NSAIDs, etc.), with 1 reporting on HCQ specifically (17). Three of these studies (12, 14, 16) provided specific counts for the type of medication change (i.e., dose increase/decrease, frequency changes). Two studies (15, 18) collected repeated measures of medication taking (i.e., >1 timepoint) within their sample.
Figure 2.
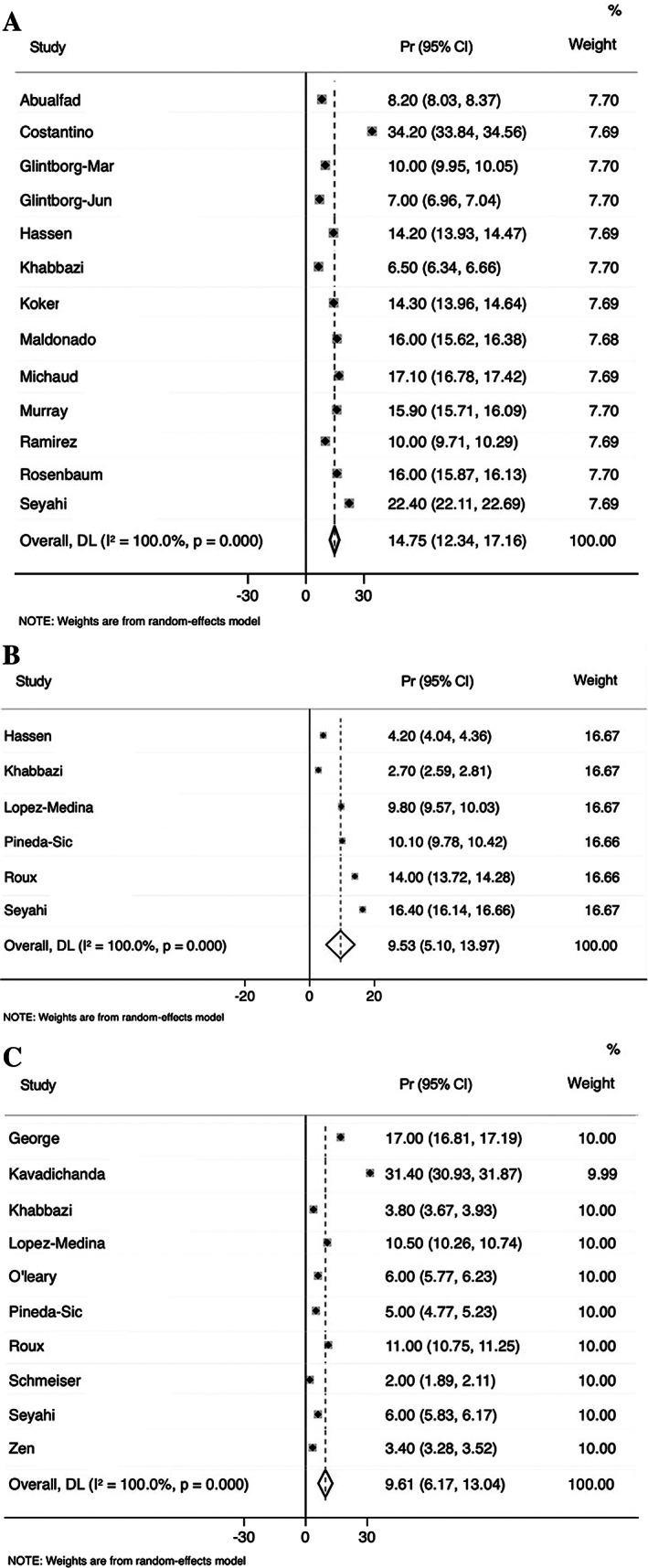
Forest plots: A, overall medication nonadherence, B, poor implementation, and C, discontinuation among rheumatology patients during the COVID‐19 pandemic. 95% CI = 95% confidence interval; Pr = prevalence.
The majority of studies reported some findings related to reasons for medication taking changes. Glintborg et al (15) performed multivariable logistical regression analyses and found that male sex (odds ratio [OR] 1.51 [95% CI 1.21–1.89], P < 0.001), age ≥80 years compared to ≤39 years (OR 0.11 [95% CI 0.006–0.52], P = 0.03), lower education (OR 0.56 [95% CI 0.45–0.69], P < 0.001), working (OR 1.52 [95% CI 1.16–1.99], P = 0.003), and use of bDMARDs (OR 1.86 [95% CI 1.02–3.81], P = 0.05) were associated with patients changing at least 1 medication due to fear of COVID‐19. Fragoulis et al (19) performed univariable logistical regression analyses and found that therapy discontinuation due to fear of immunosuppression was associated with unemployment (OR 9.19 [95% CI 1.30–64.7], P = 0.03) and chronic obstructive pulmonary disease (OR 27.53 [95% CI 3.17–239.1], P = 0.003). George et al (20) performed univariable logistical regression analyses and found that participants who stopped a DMARD were more likely to avoid office visits (OR 1.46 [95% CI 1.04–2.04], P = 0.03) and to report that telehealth options were not available to them (OR 2.26 [95% CI 1.25–4.08], P < 0.01). Additionally, some studies examined issues related to medication taking, which included lack of medication availability (15, 19, 21, 22, 23), medication shortages (12, 17, 20, 24, 25, 26, 27), difficulty obtaining medication (20, 23, 25, 27, 28) or an infusion (20, 23, 24), fear of medication‐related immunosuppression (15, 19, 20, 24, 29, 30), fear of contracting COVID‐19 (12, 15, 21, 26, 27, 31), experiencing symptoms of respiratory infection/COVID‐19 (12, 19, 20, 29, 30), financial concerns/challenges paying for medication (20, 21, 24, 32), fear of referral to clinic/hospital (29), challenges accessing public transport (32), a prescriber (24), or a pharmacy (24), and not prioritizing medication taking (24). Three studies (17, 30, 31) noted whether medication changes were patient or prescriber initiated. However, most studies did not adequately describe how identified medication taking issues related to the specific types of nonadherence, and therefore these results were challenging to synthesize.
Medication prescribing among rheumatology providers
We describe the characteristics of 6 included studies assessing medication prescribing in Table 2. Most studies (33, 34, 35, 36, 37) reported on medication shortages, specifically HCQ, which ranged from 38.9% (34) to 71% (36), and tocilizumab, which ranged from 8.9% (34) to 14% (35). Several studies (33, 34, 35) reported on providers avoiding starting or switching to a new DMARD during the COVID‐19 pandemic, ranging from 20% (34) to 74% (35). Two of these studies specifically reported on providers' reluctance to start or switch to a bDMARD or tsDMARD, which ranged from 57.6% (33) to 74% (35), and some reasons for this, including patients' fear to start these medications and decreased patient access to rheumatology care (e.g., screening procedures) (33). Overall, 4 studies (33, 34, 35, 38) provided specific counts for the type of medication change based on medication type. Of these, 3 (33, 34, 38) reported on bDMARD medication prescribing, with 50% (38) to 84.2% (34) remaining unchanged. Additionally, 3 studies (33, 35, 38) reported providers reducing the dose/frequency of steroids, ranging from 23% (35) to 56% (38), and 3 studies (34, 35, 38) reported providers reducing the dose/frequency of NSAIDs, ranging from 11% (38) to 15% (35). Overall, measures of prescribing practices varied between reports, so that comparing findings was challenging.
Table 2.
Characteristics of included studies assessing medication prescribing among rheumatology providers
| Study | Publication type | Data collection | Study period | Location | Provider | No. | Age, no. years (%) or mean ± SD | Female, % |
|---|---|---|---|---|---|---|---|---|
| Akintayo et al, 2021 (33) | Article | Survey | 04/28/20 to 05/05/20 | 20 pan‐ African countries* | Rheumatologists | 554 | 42.6 ± 11.2 | 72 |
|
Batu et al, 2020 (34) |
Article | Survey | 05/–/20 | 70 countries | Rheumatologists | 493 | <35 (10.8), 35–44 (34.7), 45–65 (49.9) >65 (4.7) | 67 |
| Dejaco et al, 2021 (35) | Article | Survey | 05/13/20 to 06/17/20 | 58 countries† | Rheumatologists | 1,286 | <30 (4.7), 30–39 (24.9), 40–49 (29.5), 50–59 (26.2), 60–69 (12.8), ≥70 (2.0) | 62.9 |
| Mehta et al, 2020 (38) | Article | Survey | 04/08/20 to 05/04/20 | US | Rheumatologists | 271 | 25–34 (18.8), 35–44 (33.6), 45–54 (19.6), 55–64 (14.0), >65 (14.0) | 57.6 |
| Mehta et al, 2020 (36) | Article | Survey | 04/08/20 to 04/27/20 | 61 countries (US, Europe, other) | Rheumatologists | 506 | Missing data | 51.6 |
| Ziadé et al, 2020 (37) | Article | Survey | 05/09/20 to 05/24/20 | 15 Arab countries‡ | Rheumatologists | 858 | 25–34 (24), 35–44 (37), 45–54 (21), 55–64 (15), 65–74 (3) | 60 |
Algeria, Egypt, Libya, Morocco, Tunisia, Sudan, Benin, Ghana, Nigeria, Senegal, Mali, Ivory Coast, Cameroon, Kenya, Madagascar, Mauritius, Mozambique, Tanzania, South Africa, Zambia.
Romania, Italy, Netherlands, Germany, France, Spain, Denmark, Austria, UK, Greece, Switzerland, Portugal, Croatia, Turkey, Sweden, Ireland, Finland, Norway, Hungary, Slovenia, Belgium, Albania, Georgia, Israel, Lebanon, Cyprus, Czech Republic, Latvia, Montenegro, Russian Federation, Bulgaria, Serbia, Belarus, San Marino, North Macedonia.
Levant countries: Iraq, Jordan, Lebanon, Palestine, Syria; Gulf countries: Saudi Arabia, Kuwait, Oman, Qatar, United Arab Emirates; North African countries: Algeria, Egypt, Libya, Morocco, Tunisia.
DISCUSSION
There has been a rapid emergence of peer‐reviewed reports describing medication taking among patients with rheumatic diseases during the COVID‐19 pandemic, highlighting the importance of medication adherence in rheumatology care. Systematically identifying and synthesizing these reports, our rapid review identified 31 studies of medication taking during the COVID‐19 pandemic, including 25 from the perspective of patients with rheumatic disease and 6 from the perspective of health care providers prescribing medication for rheumatic disease treatment. Altogether, with a pooled prevalence of 9.5% for poor implementation, 9.6% for discontinuation, and 14.8% for medication nonadherence overall, the results suggest that most patients with rheumatic disease are taking medications as prescribed during the COVID‐19 pandemic. Nevertheless our narrative synthesis identified a number of serious barriers to accessing medications and care from both patient and provider perspectives.
Synthesizing findings across studies to generate pooled estimates on the prevalence of medication nonadherence provides an indicator of the extent of potential medication taking problems and can help to identify target areas for intervention with respect to different types of medication taking problems. Only 3 studies (15, 19, 20) in our review conducted regression analyses to identify factors associated with medication nonadherence, with only 1 examining multivariable analyses and reporting that patients who changed the dose of at least 1 of their medications due to fear of COVID‐19 were more frequently male, treated with bDMARDs, age ≤39 years (compared to ≥80 years), higher educated, and working. Also important is identifying reasons for nonadherence, including systemic barriers (e.g., medication shortages, challenges obtaining medications) as well as individual barriers (e.g., personal beliefs, fears, or capacity to prioritize medication taking), to accessing medications and medical care identified in our review. Moreover, characterizing the impact of the COVID‐19 pandemic on how rheumatology providers approach medication prescribing is essential to understanding the context for changes in medication taking among patients, including whether they are patient or provider initiated.
To ensure that emerging research findings can both be replicated and compared across studies, it is critical that researchers use consistent and transparent approaches to defining and measuring medication taking problems. Specifically, we recommend that researchers distinguish between the specific types of medication taking problems (8) (i.e., noninitiation, poor implementation, and discontinuation) to ensure that reporting on this topic appropriately describes the extent of this issue and identifies modifiable targets and areas of intervention that may better support patient care. Moreover, systematically reporting specific counts based on medication type and medication taking change (i.e., dose increase/decrease, frequency changes) as well as collecting repeated measures of medication taking (i.e., >1 timepoint) within their sample can help provide a more thorough understanding of the phenomenon and add rigor to study findings.
Strengths and limitations of our study warrant discussion. To ensure a thorough literature search, we developed our search strategy in collaboration with an information scientist who executed all database searches. We also took a structured approach to data extraction, particularly in using definitions by the International Society of Medication Adherence (8) as a framework to identify and describe 3 distinct problems of medication taking, corresponding to 3 types of medication nonadherence, that is, noninitiation, poor implementation, and early discontinuation. Nonetheless, our systematic review may be vulnerable to publication bias, given that this limitation is inherent to systematic reviews. With the exception of 1 study that was translated from German (39, 40, 41, 42), our included studies were published in English, and relevant publications in other languages may have been missed. In addition, results of our meta‐analyses suggested heterogeneity between studies, reflecting the wide variation in approaches to defining and measuring medication taking problems, and we caution the interpretation of pooled estimates. Moreover, the variation in reported prevalence of nonadherence and the range of issues impacting medication taking during the COVID‐19 pandemic demonstrate the relevance of examining adherence by jurisdiction, given regional differences in health care systems and pandemic management.
Altogether, our rapid review shows that medication taking among individuals with rheumatic disease during the COVID‐19 pandemic varies globally. As the COVID‐19 pandemic progresses, assessing its impact on health care provision, particularly among vulnerable patient populations, is needed. Moreover, researchers in this area must ensure that research findings have the potential to inform patient care through standardizing methods used to assess and measure medication adherence.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. De Vera had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Rebić, De Vera.
Acquisition of data
Rebić, Ellis, De Vera.
Analysis and interpretation of data
Rebić, Park, Garg, Ellis, Kelly, Davidson, De Vera.
Supporting information
Disclosure Form
Supplementary Table 1 Database(s): Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process, In‐Data‐Review & Other Non‐Indexed Citations, Daily and Versions(R)
Supplementary Table 2. Database(s): Ovid Embase (Search run: January 13, 2021)
Supplementary Table 3. Database(s): CINAHL (Search run: January 13, 2021)
Supplementary Table 4. Quality assessment of included studies on medication taking among rheumatology patients using an adapted Checklist from the International Society of Pharmacoeconomics and Outcomes Research Medication Compliance and Persistence Special Interest Group.
Ms. Rebić is a recipient of a Canadian Institutes of Health Research Canada Graduate Scholarship Doctoral Award. Dr. De Vera holds a Tier 2 Canada Research Chair and is a recipient of a Scholar Award from the Michael Smith Foundation for Health Research.
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Facr.24744&file=acr24744‐sup‐0001‐Disclosureform.pdf.
REFERENCES
- 1. Mikuls TR, Johnson SR, Fraenkel L, Arasaratnam RJ, Baden LR, Bermas BL, et al. American College of Rheumatology guidance for the management of rheumatic disease in adult patients during the COVID‐19 pandemic: version 1. Arthritis Rheumatol 2020;72:e1–12. [DOI] [PubMed] [Google Scholar]
- 2. Landewe RB, Machado PM, Kroon F, Bijlsma HW, Burmester GR, Carmona L, et al. EULAR provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS‐CoV‐2. Ann Rheum Dis 2020;79:851–8. [DOI] [PubMed] [Google Scholar]
- 3. Sarzi‐Puttini P, Giorgi V, Sirotti S, Marotto D, Ardizzone S, Rizzardini G, et al. COVID‐19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol 2020;38:337–42. [PubMed] [Google Scholar]
- 4. Jakhar D, Kaur I. Potential of chloroquine and hydroxychloroquine to treat COVID‐19 causes fears of shortages among people with systemic lupus erythematosus. Nat Med 2020;26:632. [DOI] [PubMed] [Google Scholar]
- 5. Coomes EA, Haghbayan H. Interleukin‐6 in Covid‐19: a systematic review and meta‐analysis. Rev Med Virol 2020;30:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hsu CY, Ko CH, Wang JL, Hsu TC, Lin CY. Comparing the burdens of opportunistic infections among patients with systemic rheumatic diseases: a nationally representative cohort study. Arthritis Res Ther 2019;21:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garritty C, Gartlehner G, Nussbaumer‐Streit B, King VJ, Hamel C, Kamel C, et al. Cochrane Rapid Reviews Methods Group offers evidence‐informed guidance to conduct rapid reviews. J Clin Epidemiol 2021;130:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol 2012;73:691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peterson A, Nau D, Cramer J, Brenner J, Gwadry‐Sridhar F, Nichol M. A checklist for medication compliance studies using retrospective databases. Value Health 2007;10:3–12. [DOI] [PubMed] [Google Scholar]
- 10. De Vera MA, Marcotte G, Rai S, Galo JS, Bhole V. Medication adherence in gout: a systematic review. Arthritis Care Res (Hoboken) 2014;66:1551–9. [DOI] [PubMed] [Google Scholar]
- 11. Mehat P, Atiquzzaman M, Esdaile JM, Aviña‐Zubieta A, De Vera MA. Medication nonadherence in systemic lupus erythematosus: a systematic review. Arthritis Care Res (Hoboken) 2017;69:1706–13. [DOI] [PubMed] [Google Scholar]
- 12. Costantino F, Bahier L, Tarancón LC, Leboime A, Vidal F, Bessalah L, et al. COVID‐19 in French patients with chronic inflammatory rheumatic diseases: clinical features, risk factors and treatment adherence. Joint Bone Spine 2021;88:105095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosenbaum JT, Hamilton H, Choi D, Weisman MH, Reveille JD, Winthrop KL. Biologics, spondylitis and COVID‐19. Ann Rheum Dis 2020;79:1663. [DOI] [PubMed] [Google Scholar]
- 14. Singh JA, Edwards NL. Gout management and outcomes during the COVID‐19 pandemic: a cross‐sectional internet survey. Ther Adv Musculoskelet Dis 2020;12:1759720X20966124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Glintborg B, Jensen DV, Engel S, Terslev L, Jensen MP, Hendricks O, et al. Self‐protection strategies and health behaviour in patients with inflammatory rheumatic diseases during the COVID‐19 pandemic: results and predictors in more than 12 000 patients with inflammatory rheumatic diseases followed in the Danish DANBIO registry. RMD Open 2021;7:e001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roux CH, Brocq O, Gerald F, Pradier C, Bailly L. Impact of home confinement during the COVID‐19 pandemic on medication use and disease activity in spondyloarthritis patients [letter]. Arthritis Rheumatol 2020;72:1771–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ramirez GA, Gerosa M, Beretta L, Bellocchi C, Argolini LM, Moroni L, et al. COVID‐19 in systemic lupus erythematosus: data from a survey on 417 patients. Semin Arthritis Rheum 2020;50:1150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ciurea A, Papagiannoulis E, Bürki K, von Loga I, Micheroli R, Moller B, et al. Impact of the COVID‐19 pandemic on the disease course of patients with inflammatory rheumatic diseases: results from the Swiss Clinical Quality Management cohort. Ann Rheum Dis 2021;80:238. [DOI] [PubMed] [Google Scholar]
- 19. Fragoulis GE, Evangelatos G, Arida A, Bournia VK, Fragiadaki K, Karamanakos A, et al. Treatment adherence of patients with systemic rheumatic diseases in COVID‐19 pandemic. Ann Rheum Dis 2021;80:e60. [DOI] [PubMed] [Google Scholar]
- 20. George MD, Venkatachalam S, Banerjee S, BAker JF, Merkel PA, Gavigan K, et al. Concerns, healthcare use, and treatment interruptions in patients with common autoimmune rheumatic diseases during the COVID‐19 pandemic. J Rheumatol 2021;48:603–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pineda‐Sic RA, Galarza‐Delgado DA, Serna‐Pena G, Castillo‐Torres SA, Flores‐Alvarado DE, Esquivel‐Valerio JA, et al. Treatment adherence behaviours in rheumatic diseases during COVID‐19 pandemic: a Latin American experience. Ann Rheum Dis 2021;80:e85. [DOI] [PubMed] [Google Scholar]
- 22. Hassen LM, Almaghlouth IA, Hassen IM, Daghestani MH, Almohisen AA, Alqurtas EM, et al. Impact of COVID‐19 outbreak on rheumatic patients' perceptions and behaviors: a cross‐sectional study. Int J Rheum Dis 2020;23:1541–1549. [DOI] [PubMed] [Google Scholar]
- 23. Rathi M, Singh P, Bi HP, Shivanna A, Kavadichanda C, Tripathy SR, et al. Impact of the COVID‐19 pandemic on patients with systemic lupus erythematosus: observations from an Indian inception cohort. Lupus 2020;30:158–64. [DOI] [PubMed] [Google Scholar]
- 24. Maldonado D, Tu E, Mahmood S, Wahezi DM, Darapaneni R, Sima N, et al. Association of medication access difficulty and COVID‐19–related distress with disease flares in rheumatology patients during the COVID‐19 pandemic. Arthritis Care Res (Hoboken) 2021;73:1162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seyahi E, Poyraz BC, Sut N, Akdogan S, Hamuryudan V. The psychological state and changes in the routine of the patients with rheumatic diseases during the coronavirus disease (COVID‐19) outbreak in Turkey: a web‐based cross‐sectional survey. Rheumatol Int 2020;40:1229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koker O, Demirkan FG, Kayaalp G, Cakmak F, Tanatar A, Karadag SG, et al. Does immunosuppressive treatment entail an additional risk for children with rheumatic diseases? A survey‐based study in the era of COVID‐19. Rheumatol Int 2020;40:1613–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ziadé N, el Kibbi L, Hmamouchi I, Abdulateef N, Halabi H, Hamdi W, et al. Impact of the COVID‐19 pandemic on patients with chronic rheumatic diseases: a study in 15 Arab countries. Int J Rheum Dis 2020;23:1550–7. [DOI] [PubMed] [Google Scholar]
- 28. Michaud K, Wipfler K, Shaw Y, Simon TA, Cornish A, England BR, et al. Experiences of patients with rheumatic diseases in the United States during early days of the COVID‐19 pandemic. ACR Open Rheumatol 2020;2:335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khabbazi A, Kavandi H, Paribanaem R, Khabbazi R, Malek Mahdavi A. Adherence to medication in patients with rheumatic diseases during COVID‐19 pandemic. Ann Rheum Dis 2020. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 30. Murray K, Quinn S, Turk M, O'Rourke A, Molloy E, O'Neill L, et al. COVID‐19 and rheumatic musculoskeletal disease patients: infection rates, attitudes and medication adherence in an Irish population. Rheumatology (Oxford) 2021;60:902–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. López‐Medina C, Ladehesa‐Pineda L, Gómez‐García I, Puche‐Larrubia MA, Sequi‐Sabater JM, Armenteros‐Ortiz P, et al. Treatment adherence during the COVID‐19 pandemic and the impact of confinement on disease activity and emotional status: a survey in 644 rheumatic patients. Joint Bone Spine 2021;88:105085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kavadichanda C, Shah S, Daber A, Bairwa A, Mathew A, Dunga S, et al. Tele‐rheumatology for overcoming socioeconomic barriers to healthcare in resource constrained settings: lessons from COVID‐19 pandemic. Rheumatology (Oxford) 2021;60:3369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Akintayo RO, Akpabio AA, Kalla AA, Dey D, Migowa AN, Olaosebikan H, et al. The impact of COVID‐19 on rheumatology practice across Africa. Rheumatology (Oxford) 2021;60:392–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Batu ED, Lamot L, Sag E, Ozen S, Uziel Y. How the COVID‐19 pandemic has influenced pediatric rheumatology practice: results of a global, cross‐sectional, online survey. Semin Arthritis Rheum 2020;50:1262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dejaco C, Alunno A, Bijlsma JW, Boonen A, Combe B, Finckh A, et al. Influence of COVID‐19 pandemic on decisions for the management of people with inflammatory rheumatic and musculoskeletal diseases: a survey among EULAR countries. Ann Rheum Dis 2021;80:518–26. [DOI] [PubMed] [Google Scholar]
- 36. Mehta B, Moezinia CJ, Jannat‐Khah D, Gibofsky A, Tornberg H, Pearce‐Fisher D, et al. Hydroxychloroquine and chloroquine in COVID‐19: a survey of prescription patterns among rheumatologists. J Clin Rheumatolgy 2020;26:224‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ziadé N, Hmamouchi I, el Kibbi L, Abdulateef N, Halabi H, Abutiban F, et al. The impact of COVID‐19 pandemic on rheumatology practice: a cross‐sectional multinational study. Clin Rheumatol 2020;39:3205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mehta B, Jannat‐Khah D, Mancuso CA, Bass AR, Moezinia CJ, Gibofsky A, et al. Geographical variations in COVID‐19 perceptions and patient management: a national survey of rheumatologists. Semin Arthritis Rheum 2020;50:1049–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abualfadl E, Ismail F, Shereef RRE, Hassan E, Tharwat S, Mohamed EF, et al. Impact of COVID‐19 pandemic on rheumatoid arthritis from a multi‐centre patient‐reported questionnaire survey: influence of gender, rural–urban gap and north–south gradient. Rheumatol Int 2021;41:345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O'leary A, Urbaniak A, Phelan M, Harty L. Compliance with immunosuppression for rheumatic disease during the COVID‐19 pandemic. Ir Med J 2020;113:1–2.32298555 [Google Scholar]
- 41. Schmeiser T, Broll M, Dormann A, Frabel C, Hermann W, Hudowenz O, et al. [A cross sectional study on patients with inflammatory rheumatic diseases in terms of their compliance to their immunsuppressive medication during COVID‐19 pandemic]. Z Rheumatol 2020;79:379–84. In German. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zen M, Fuzzi E, Astorri D, Saccon F, Padoan R, Ienna L, et al. SARS‐CoV‐2 infection in patients with autoimmune rheumatic diseases in northeast Italy: a cross‐sectional study on 916 patients. J Autoimmun 2020;112:102502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form
Supplementary Table 1 Database(s): Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process, In‐Data‐Review & Other Non‐Indexed Citations, Daily and Versions(R)
Supplementary Table 2. Database(s): Ovid Embase (Search run: January 13, 2021)
Supplementary Table 3. Database(s): CINAHL (Search run: January 13, 2021)
Supplementary Table 4. Quality assessment of included studies on medication taking among rheumatology patients using an adapted Checklist from the International Society of Pharmacoeconomics and Outcomes Research Medication Compliance and Persistence Special Interest Group.


