Abstract
Background
There is growing evidence regarding the venous thromboembolic (VTE) pathophysiology of coronavirus disease 2019 (COVID‐19). Several studies have reported varying incidences of this disease.
Objectives
The main purpose of this study was to determine the real incidence of deep or superficial vein thrombosis in COVID‐19. The study also aimed to identify risk and protective factors for VTE.
Methods
Patients were consecutively enrolled and assessed with a bilateral Duplex ultrasonography of lower limbs during hospitalization. The exam was repeated weekly until discharge, and then follow‐up for 1 month.
Results
Two‐hundred and thirty‐three patients were enrolled. Mean age was 54.4 years (SD 12.7) and 47.8% were female. About 127 patients (54.5%) had comorbidities. At enrollment, patients were normotensive and had normal saturation (95.6%—SD 1.6, with a respiratory rate of 19.1 rpm—SD 4.0), with 130 needing at least supplementary oxygen therapy (55.8%). About 147 patients (63.1%) had at least 1 Duplex ultrasonography study performed and 1.7% had 5 or more studies. One patient had a distal posterior tibial vein thrombosis, which showed signs of chronicity and was congruent with the patient history. Therefore, the incidence of thrombotic events was nearly zero.
Discussion
Our study results suggest that performing a Duplex Ultrasonography screening protocol in stable COVID‐19 patient populations, who may need hospitalization but are without symptoms of vein thrombosis, is not founded. We presumably emphasize the advantage of using intermediate LMWH doses as well as early walking in COVID‐19 patients.
Keywords: Coronavirus Disease 2019 (COVID‐19), deep vein thrombosis, duplex ultrasonography, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2), superficial vein thrombosis
Abbreviations
- COVID‐19
coronavirus disease 2019
- DVT
deep vein thrombosis
- GSV
great saphenous vein
- ICU
intensive care units
- IQR
interquartile range
- IRCU
intermediate respiratory care unit
- LMWH
low molecular weight heparin
- PE
pulmonary embolism
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SD
standard deviation
- SSV
small saphenous vein
- SVT
superficial vein thrombosis
- US
ultrasound
- VTE
venous thromboembolic
The coronavirus disease of 2019 (COVID‐19) is a viral illness caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Since it was first described, it has been linked to an increased incidence of venous thromboembolism (VTE), along with hemostatic abnormalities. 1 , 2 This has been suggested to be caused by inflammation, platelet activation, endothelial dysfunction, and other factors 3 , 4 that occur in COVID‐19 patients.
In view of these data, thromboprophylaxis was encouraged, seemingly associated with a lower mortality. 5 Nevertheless, a steep rise in incidence of VTE in intensive care units (ICU) 6 , 7 despite thromboprophylaxis prompted some authors to recommend therapeutic anticoagulation in COVID‐19 patients. 8
Recent reports, however, show a rising incidence of bleeding events in the context of anticoagulation and COVID‐19, 2 , 9 thus prompting a revision of the available evidence on anticoagulation and raising uncertainty on the proper prophylaxis for these patients. 10
Identifying the true incidence of VTE in these patients and establishing the optimal thromboprophylaxis remains thus vital. 9
In this manuscript, we aim to assess the incidence and risk or protective factors for developing deep vein thrombosis (DVT) or superficial vein thrombosis (SVT) in COVID‐19 patients.
Patients and Methods
Observational, prospective, and single‐center study to assess the baseline characteristics and incidence of DVT or SVT of patients consecutively admitted in a medical ward with a recent diagnosis of COVID‐19 pneumonia between February and March 2021. A total of 233 patients were included in the study.
The study is in accordance with the Declaration of Helsinki and was approved by the local Research Ethics Committee. Informed consent was obtained from each enrolled patient.
Patient Selection
Patients admitted to our hospital with confirmed COVID‐19 requiring admission to a medical ward were included. A monthly follow‐up was scheduled, after recruitment, by history review or telephonic contact. We excluded patients <18 years. A sample of patients who met these inclusion criteria were enrolled and prospectively studied.
Initial Patient Assessment
We registered demographic data: age, sex, weight. Medical history: comorbidities and medications. Risk factors for VTE: previous personal or family history of VTE, pregnancy/puerperium, oral contraceptives, hormone replacement therapy, autoimmune disease, recent surgery—last 3 months—, recent immobilization, thrombophilia—lupus anticoagulant, beta2 glycoprotein, anticardiolipin, factor V Leiden, prothrombin gene mutation, antithrombin deficiency, protein C, protein S and methyl tetrahydrofolate reductase‐, malignancy— active or in remission—, obesity— as define by the WHO (a BMI > 30), venous insufficiency, active chemotherapy—. Imaging tests performed: duplex lower extremity ultrasound performed during follow‐up and prior to discharge; other radiology exams. Physical exam. Laboratory tests: creatinine, urea, hemoglobin, white blood cells, platelets, D‐dimer, INR. Variables correlated to therapy: type of anticoagulation, dose, duration. Variables correlated to follow‐up: end of symptoms, destination, and date of discharge.
Ultrasound Data Collection
Internal Medicine physicians performed initial and follow‐up bilateral whole‐leg ultrasound (US) examination in all admitted patients. These physicians had a long‐standing experience in US (more than 10 vascular examinations performed per week and more than 5 years of experience in performing and interpreting US examinations). The patient was positioned in the supine position, with the head raised around 30°, and the investigated leg was externally rotated at the hip and knee flexion up to 15°. In all cases, a whole‐leg US protocol was performed, and the following veins were scanned transversally over their entire length, with probe compression applied at 1–2 cm intervals: common femoral vein, femoral vein, popliteal veins, anterior, and posterior tibial veins, peroneal veins, medial and lateral gastrocnemius veins, soleal veins, the saphenofemoral/popliteal junctions, the trunk of the great saphenous vein (GSV) and small saphenous vein (SSV), and where thrombosis was suspected based on physical exam (pain and swelling). If needed, augmentation maneuvers were performed to find a spontaneous or reverse‐flow intraluminal color filling while investigating common femoral vein, superficial femoral vein, and popliteal veins bilaterally.
The study was performed using a GE VENUE GO ultrasound system, with a linear transducer (5–10 MHz) (General Electrics Healthcare, Madrid, Spain).
Outcome Measures and Definitions
The diagnosis of venous thrombosis was confirmed by the presence of a noncompressible or partially compressible area in the course of an identified vein. Then a longitudinal scan was performed to confirm the presence of thrombosis and/or to obtain a Doppler spectral waveform if needed in nondiagnostic transverse exams (i.e. difficulty visualizing the vein, residual thrombosis,…).
Our aim was to know the real incidence of DVT in patients admitted in a pandemic emergency hospital. The risk factors associated with the presence of thrombosis were assessed. Baseline and follow‐up studies were compared by report.
With all these data, we intended to identify areas of improvement in the care of patients with VTE.
Statistical Analysis
Baseline characteristics are presented as mean and standard deviation (SD) or median and interquartile range (IQR) for continuous variables and count and proportions for categorical variables. Analyses were conducted with the statistical IBM SPSS software v25.0 (SPSS Inc., Chicago, IL).
Results
A total of 280 patients were screened between February and March 2021 (Figure 1). Five patients were excluded due to absence of consecutive recruitment and 42 rejected after careful informed consent was delivered. About 233 patients were enrolled, summarized in Table 1. The mean age was 54.4 years (SD 12.7) and 47.8% were female. Also, 54.5% patients had an underlying medical condition. At enrollment, patients were normotensive and had normal saturation (95.6%—SD 1.6, with a respiratory rate of 19.1 rpm—SD 4.0), with 55.8% needing at least supplementary oxygen therapy. Mean lymphocyte count was 1.11 x109 (SD 0.6, Normal Value‐NV: 1.0–4.0), CRP was 66.0 mg/dL (SD 63.7, NV: 0–10), LDH was 368.3 U/L (SD 322.4; NV: 140–280), D‐dimer 729.7 ng/mL (SD 1182.6, NV: <400) at admission and D‐dimer of 1723.6 ng/mL (SD 9378.8) at 1 week follow‐up.
Figure 1.
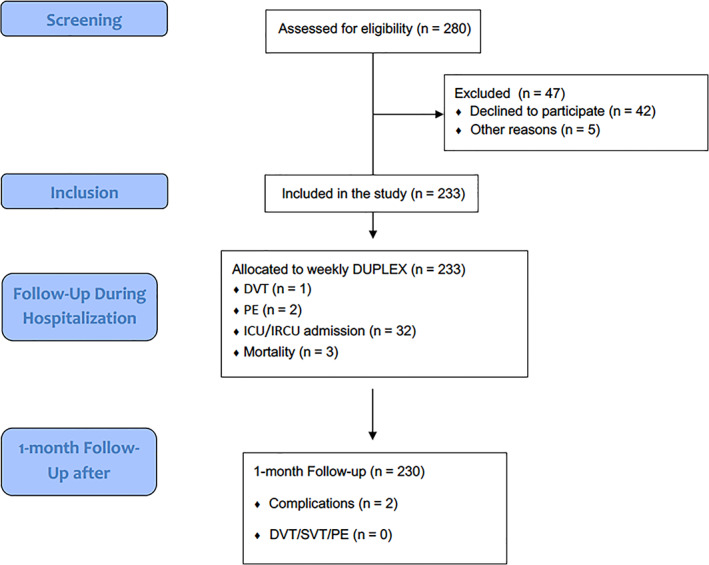
STROBE flow diagram.
Table 1.
Demographics and Clinical Characteristics of Patients Included (N = 233)
| Demographics | N (%) |
|---|---|
| Gender (female)—N (%) | 111 (47.8) |
| Age (years) mean (SD) | 54.4 (12.7) |
| Race | |
| Caucasian—N (%) | 133 (57.3) |
| Latin—N (%) | 89 (38.4) |
| Arab—N (%) | 6 (2.3) |
| Past medical history | N (%) |
| Hypertension—N (%) | 57 (24.5) |
| Dyslipidemia—N (%) | 53 (22.7) |
| Diabetes Mellitus—N (%) | 30 (3.3) |
| Obesity—N (%) | 54 (23.2) |
| Smoking habit—N (%) | 17 (7.3) |
| Cardiovascular disease—N (%) | 7 (3.0) |
| Pulmonary disease—N (%) | 34 (14.6) |
| Previous Thromboembolic Disease—N (%) | 2 (0.9) |
| Familiar Thromboembolic Disease—N (%) | 6 (2.6) |
| Malignancy—N (%) | 3 (1.3) |
| Systemic Autoimmune Disease—N (%) | 4 (1.7) |
| Phyisical exam | |
| SBP (mmHg) mean (SD) | 121.7 (18.0) |
| Heart rate (bpm) mean (SD) | 84.6 (14.2) |
| Temperature (°C) mean (SD) | 36.8 (0.7) |
| SO2 (%) mean (SD) | 95.6 (1.6) |
| Respiratory rate (rpm) mean (SD) | 19.1 (4.0) |
| Weight (kg) mean (SD) | 81.1 (16.7) |
| Height (cm) mean (SD) | 166.2 (10.1) |
| Oxygen (lpm) mean (SD) | 1.6 (2.7) |
| Laboratory results—Mean (SD) | |
| WBC × 109/L (SD) | 6.2 (2.7) |
| Lymphocite × 109/L (SD) | 1.1 (0.6) |
| Platelets × 109/L (SD) | 224.1 (100.8) |
| Creatinine—mg/dL (SD) | 1.11 (4.4) |
| AST—U/L (SD) | 53.7 (38.6) |
| Total bilirrubin—mg/dL (SD) | 0.7 (0.4) |
| LDH—U/L (SD) | 368.3 (322.4) |
| CK—U/L (SD) | 180.1 (256.3) |
| D‐dimer—ng/mL (SD) | 729.7 (1182.6) |
| D‐dimer at 1 week from admission—ng/mL (SD) | 1723.6 (9378.8) |
| PCT—ng/mL (SD) | 0.1 (0.1) |
| C‐Reactive Protein—mg/dL (SD) | 66.0 (63.7) |
| IL‐6—pg/mL (SD) | 152.3 (314.3) |
| Imaging modalities | 89 (92.7) |
| Chest X‐ray—Normal—N (%) | 5 (2.1) |
| Chest X‐ray—Infiltrates—N (%) | 228 (97.9) |
| Chest X‐ray—Involvement >50%—N (%) | 141 (65.6) |
| Duplex Ultrasound—DVT—N (%) | 1 (0.4) |
| Number of Duplex Ultrasound performed during follow‐up | 3 (1.3) |
| 0—N (%) | |
| 1—N (%) | 147 (63.1) |
| 2—N (%) | 64 (27.5) |
| 3—N (%) | 13 (5.6) |
| 4—N (%) | 2 (0.9) |
| 5 or more—N (%) | 4 (1.7) |
| Computed Tomography Angiography—PE—N (%) | 2 (0.9) |
| Therapy | |
| Remdesivir—N (%) | 8 (3.4) |
| Antibiotics—N (%) | 28 (12.3) |
| Tocilizumab—N (%) | 7 (3.0) |
| Dexamethasone 6 mg—N (%) | 175 (75.1) |
| Dexamethasone 20 mg—N (%) | 38 (16.3) |
| Low molecular weight heparin | |
| Prophylactic dose | 196 (84.1) |
| Intermediate dose | 24 (10.3) |
| Anticoagulation dose | 6 (2.6) |
| Oxygen—N (%) | |
| Nasal Cannula to Non‐rebreather mask—N (%) | 100 (53.2) |
| High flow nasal cannula or non‐invasive ventilation—N (%) | 23 (12.2) |
| Mechanical ventilation—N (%) | 7 (3.7) |
| Follow‐up | |
| Admission to an Intermediate Respiratory Care Unit or Intensive Care Unit—N (%) | 32 (13.9) |
| Mortality—N (%) | 3 (1.3) |
| Length of stay—N of days (SD) | 7.1 (34.3) |
| Complications at 1 month—N (%) | 2 (0.9) |
| DVT or PE diagnosis at 1 month from hospital discharge—N (%) | 0 (0.0) |
CK, creatine kinase; DVT, deep vein thrombosis; IL‐6, Interleukin 6; LDH, lactate dehydrogenase; N, number; PCT, procalcitonin; PE, pulmonary embolism; SBP, systolic blood pressure; SD, standard deviation.
Regarding the imaging studies, all the included patients had a chest X‐ray, 97.9% of the patients had infiltrates, and 65.6% had more than 50% of lung involvement. About the Duplex ultrasonography study, 3 patients were discharged before the first ultrasound, 63.1% had at least 1 study performed and 1.7% had 5 or more studies.
One patient had a distal tibial posterior vein thrombosis, which showed signs of chronicity and was congruent with the patient history.
Ninety‐seven percent of the patients received low‐molecular‐weight heparin (LMWH) therapy, 12.9% above standard prophylactic dose. About 13.9% were admitted to an intermediate respiratory care unit (IRCU) or intensive care unit (IC), and the mortality rate was 1.3%. Mean hospital admission was 7.1 days (SD 34.3). After 1‐month follow‐up, no patients had evidence of vein thrombosis. Two patients were admitted to the emergency department due to dyspnea, ruling out vein thrombosis or pulmonary embolism (PE), receiving immediate discharge. There were no bleeding events in our cohort.
Discussion
Our study provides an ample population of COVID‐19 patients obtained consecutively, excluding only those who were underage or declined participation, thus presenting a real‐life group of hospitalized COVID‐19 patients.
In our population, the incidence of thrombotic events was nearly zero, in contrast to the reported incidence in the literature, which ranges from 14 to approximately 30% in some series. 1 , 2 , 6 Only one patient in our population presented DVT. Upon revision of his past medical history, it became clear that he had suffered from a DVT the year before. Its characteristics were consistent with the current findings and thus was classified as a chronic DVT and not as an acute event.
This low incidence could be explained by different theories. First of all, the patient population included in our study presented, in average, had a less severe form of SARS‐CoV‐2 pneumoniae than those of other published studies. This is confirmed by the percentage of patients admitted into an intensive or intermediate care unit (13.9%), in contrast to percentages of up to 32% in some series. 11 , 12
Besides, our population was also on the younger side of the average age reported in previous series, according to a meta‐analysis reviewing the incidence of VTE in hospitalized patients with COVID‐19. 2 These two factors led to an early mobilization of all patients in our population when possible. Since being bed‐ridden during hospitalization has been linked to a higher risk for VTE, 13 an early mobilization would be desirable in COVID‐19 patients with the ability to do so. Moreover, our specialized hospital for COVID‐19, with long corridors design, is perfectly suitable for this purpose.
Another point is the administration of steroids. It has already been described that dexamethasone reduces mortality in patients with SARS‐CoV‐2 pneumonia requiring respiratory support. 14 In our population, over 90% of all patients underwent treatment with dexamethasone. Perhaps, in addition to lowering the mortality, this therapy could reduce the rate of other complications, feasibly by reducing inflammation. Further research is needed to increase the available evidence.
Nonetheless, almost all of our patients (97%) were under treatment with LMWH, with an ample majority receiving standard doses (84% of the total), in line with current evidence supporting the use of prophylaxis at standard doses in hospitalized COVID‐19 patients. 9
All these arguments might have had a role in decreasing the incidence of VTE complications in our population compared to other series.
Nevertheless, in our cohort, 2 patients were diagnosed of subsegmental PE, with no evidence of vein thrombosis on follow‐up, confirmed by two different operators. Several theories have suggested that PE seen in COVID‐19 correspond to microthrombus of small‐caliber pulmonary arteries, and not always from an embolic origin in extremity veins. 4
Our findings are novel and useful, since they suggest a lower incidence of DVT or SVT than previously reported, with possible explanations.
We acknowledge some study limitations. First of all, the expert sonographers performed all ultrasound scans in a small, but consecutive sample of confirmed COVID‐19 patients. Another limitation is that this study was performed in a single center, which limits the generalizability of our results, and ought to be validated in future studies.
Although these limitations are important, we believe that our study results support the current recommendation of using dexamethasone and prophylactic LMWH doses. 9 , 14
Conclusion
In conclusion, our study results suggest that performing a Duplex Ultrasonography screening protocol in stable COVID‐19 patient populations, who may need hospitalization but are without symptoms of vein thrombosis, is not found. We presumably emphasize the advantage of using intermediate LMWH doses as well as early walking in COVID‐19 patients.
The authors have declared no conflicts of interest.
All authors have contributed to this work. Conception and design: YTC, LOO. Analysis and interpretation: YTC, LOO. Data collection: YTC, RC, CM, FD, MM, AC, BA, LOO. Writing the article: YTC, LOO. Critical revision of the article: YTC, RC, CM, FD, MM, AC, BA, JM, LOO. Final approval of the article: YTC, RC, CM, FD, MM, AC, BA, JM, LOO. Statistical analysis: YTC. Overall responsibility: YTC, LOO.
All authors read and approved the final manuscript. This work has not been supported by public grants or financial support. No sources of funding were used to assist in the preparation of this study. Each author certifies that he has no commercial associations that might pose a conflict of interest in connection with the submitted article. We certify that this research was conducted in conformity with ethical principles of our institution. This work, figures, and tables, have not been previously published and reproduced from another source.
Yale Tung Chen, principal investigator of the study, had full access to all data and takes responsibility for the integrity and the accuracy of the data analysis.
References
- 1. Jiménez D, García‐Sanchez A, Rali P, et al. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and meta‐analysis. Chest 2021; 159:1182–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Demelo‐Rodríguez P, Cervilla‐Muñoz E, Ordieres‐Ortega L, et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID‐19 pneumonia and elevated D‐dimer levels. Thromb Res 2020; 192:23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bikdeli B, Madhavan MV, Jimenez D, et al. COVID‐19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow‐up: JACC state‐of‐the‐art review. J Am Coll Cardiol 2020; 75:2950–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kyriakoulis KG, Kokkinidis DG, Kyprianou IA, et al. Venous thromboembolism in the era of COVID‐19. Phlebology 2021; 36:91–99. [DOI] [PubMed] [Google Scholar]
- 5. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020; 18:1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res 2020; 191:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost 2020; 18:1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Llitjos JF, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID‐19 patients. J Thromb Haemost 2020; 18:1743–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sadeghipour P, Talasaz AH, Rashidi F, et al. Effect of intermediate‐dose vs standard‐dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID‐19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA 2021; 325:1620–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Al‐Samkari H, Karp Leaf RS, Dzik WH, et al. COVID‐19 and coagulation: bleeding and thrombotic manifestations of SARS‐CoV‐2 infection. Blood 2020; 136:489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA 2020; 323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kearon C, Ageno W, Cannegieter SC, Cosmi B, Geersing GJ, Kyrle PA. Categorization of patients as having provoked or unprovoked venous thromboembolism: guidance from the SSC of ISTH. J Thromb Haemost 2016; 14:1480–1483. [DOI] [PubMed] [Google Scholar]
- 14. Horby P, Lim WS, Emberson JR, et al. RECOVERY collaborative group. Dexamethasone in hospitalized patients with Covid‐19. N Engl J Med 2021; 384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]


