Abstract
Objective
With a vaccine effectiveness of 95% for preventing coronavirus disease 2019 (COVID‐19), Pfizer‐BioNTech BNT162b2 (BNT162b2) was the first vaccine against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) to be approved. However, immunosuppressive therapy was an exclusion criterion in the phase 3 trial that led to approval. Thus, extrapolation of the trial results to patients with rheumatic diseases treated with immunosuppressive drugs warrants caution.
Methods
Patients with systemic lupus erythematosus (SLE; n = 61) and rheumatoid arthritis (RA; n = 73) were included from the COPANARD (Corona Pandemic Autoimmune Rheumatic Disease) cohort, followed since the beginning of the COVID‐19 pandemic. Patients received the BNT162b2 vaccine between December 2020 and April 2021. All patients had total antibodies against SARS‐CoV‐2 measured before vaccination and 1 week after the second vaccination (VITROS Immunodiagnostic Products).
Results
Of 134 patients (median age, 70 years), 77% were able to mount a detectable serological response to the vaccine. Among patients treated with rituximab, only 24% had detectable anti–SARS‐CoV‐2 antibodies in their serum after vaccination. The time since the last rituximab treatment did not seem to influence the vaccine response. No significant difference was observed between patients with RA or SLE when adjusting for treatment, and no correlation between antibody levels and age was detected (r = −0.12; P = 0.18).
Conclusion
Antibody measurements against SARS‐CoV‐2 in patients with RA and SLE after two doses of the BNT162b2 vaccine demonstrated that 23% of patients could not mount a detectable serological response to the vaccine. B cell–depleting therapy (BCDT) is of specific concern, and our findings call for particular attention to the patients receiving BCDT.
INTRODUCTION
The news regarding vaccines against severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) impacted the world in late 2020 and spiked global hope.
The Pfizer‐BioNTech BNT162b2 (BNT162b2) was the first vaccine against SARS‐CoV‐2 to be approved by the European Medicines Agency, with a high vaccine effectiveness of 95% for preventing coronavirus disease 2019 (COVID‐19) (1). Although the phase 3 BNT162b2 vaccine trial was promising, it excluded most patients with rheumatic diseases (RDs), and treatment with immunosuppressive therapy was a key exclusion criterion (1). Thus, extrapolation of the trial results to patients with RDs warrants caution.
Vaccine response can be assessed in different manners. A widely accessible way is by measuring antibodies against the vaccine (2). With the BNT162b2 vaccine, the humoral response correlated well with the cellular response (3). In a study of health care workers, 98% developed a significant antibody response against the SARS‐CoV‐2 spike protein after a two‐dose vaccination with BNT162b2 (4).
Most knowledge on vaccine response in patients with RDs originates from influenza and pneumococcal vaccines. Methotrexate impairs the antibody response in patients with RDs receiving the influenza vaccine. However, most patients generally achieved titers sufficient to be protected against infection (5). The majority of disease‐modifying rheumatic drugs (DMARDs) do not seem to influence the vaccine response (6). Still, a potential suboptimal response to vaccines after B cell–depleting therapy (BCDT) has been reported (7). The European League Against Rheumatism (EULAR) recommends that patients on BCDT, when possible, receive vaccination 6 months after or 1 month before BCDT (8). Whether these findings can be extrapolated to guide vaccination strategies for COVID‐19 remains uncertain (9).
Reports about the severity of COVID‐19 in patients with RDs have been conflicting (10, 11, 12, 13). However, the pandemic has prompted isolation and significant behavioral changes in this group (14), and immunosuppression used in the management of RDs potentially increases the patients’ vulnerability to infectious diseases (15). In Denmark, patients with RDs receiving immunosuppressants were quickly categorized by the government as having a higher risk for severe COVID‐19 than the general population. This patient group was, therefore, among the first in Denmark to receive the new vaccine.
Although data on immunosuppressed individuals following messenger RNA (mRNA) vaccination are emerging that demonstrate a reduced antibody response (16, 17), no studies on immunosuppressed patients with RDs receiving the complete two‐dose vaccination with the BNT162b2 vaccine have been published.
We aimed to explore the antibody response against SARS‐CoV‐2 after complete vaccination with BNT162b2 in 134 patients with systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) from the COPANARD (Corona Pandemic Autoimmune Rheumatic Disease) cohort (14).
PATIENTS AND METHODS
Patients
Patients with SLE or RA in the outpatient clinic at the Department of Rheumatology at Aarhus University Hospital (AUH) were selected for this study. Patients were chosen from the COPANARD cohort (14) and had been followed at AUH since the pandemic's first wave. A total of 134 individuals from this cohort received BNT162b2 vaccination between late December 2020 and April 2021 and were included in this study.
The inclusion criterion for the patients with SLE was fulfillment of the American College of Rheumatology (ACR) 1982 revised classification criteria for SLE. The inclusion criteria for patients with RA were fulfillment of either the 1987 ACR or the 2010 ACR/EULAR Classification Criteria and treatment with either a biological or small molecule DMARD. All patients resided in the same geographical region of Denmark (Central and North Region).
Vaccination
All patients were vaccinated via the public vaccine services following the national vaccine program. Patients were offered the BNT162b2 vaccine, with 21 (interquartile range, 21‐24) days between the two vaccinations.
Blood samples
Blood samples were collected and analyzed at the Department of Clinical Microbiology at AUH. Samples were collected prior to vaccination and 1 week after the second vaccination.
SARS‐CoV‐2 antibody testing
The serum was tested for antibodies against SARS‐CoV‐2 using the VITROS Immunodiagnostic Products Anti‐SARS‐CoV‐2 Total test (Ortho Clinical Diagnostics).
The analyses were performed by experienced staff at the Department of Clinical Microbiology at AUH on the VITROS® 5600 according to the manufacturer’s instructions. The assay is a commercial SARS‐CoV‐2 double antigen sandwich chemiluminescent immunoassay, which detects total antibodies captured by recombinant SARS‐CoV‐2 spike S1 protein coated in a microtiter well. A one‐level calibration is lot specific and links the sample signal to a cutoff value. A signal/cutoff (S/CO) of 1 or more was considered positive, and a S/CO of less than 1 was considered negative.
Results were based on a single test result. Performance characteristics of the VITROS SARS‐CoV‐2 total antibody chemiluminescent immunoassay have been determined in a Danish validation study (18). The assay had a sensitivity of 95.3% (95% confidence interval [CI], 90.7‐97.7) and a specificity of 100% (95% CI, 99.4‐100). No cross‐reactivity was observed.
Statistics
All values reported are medians with interquartile ranges (IQRs) unless otherwise stated. The statistical significance of differences was assessed using the Mann‐Whitney nonparametric test for continuous variables and Pearson’s χ2 test for categorical variables. Univariate and multivariate logistic regression analyses were performed with presence of SARS‐CoV‐2 antibodies after vaccination as the dependent variable.
Ethics
Patients were offered participation in the study after informed consent. The project was approved by The Danish Data Protection Agency (1‐16‐02‐19‐21). The Central Denmark Region Committee on Health Research Ethics was consulted concerning the present study (1‐10‐72‐1‐21). The study was conducted according to the Declaration of Helsinki.
RESULTS
Patients
We included 134 patients in the study, including 73 patients (54%) with RA and 61 patients (46%) with SLE, with a median age of 70 (69‐74) years and 60 (46‐67) years, respectively (Table 1). Patients with RA were significantly older than the patients with SLE (P < 0.001). The majority of patients were female (96 [72%]) and white (133 [99%]). Patients with RA were predominantly treated with tumor necrosis factor (TNF) inhibitors (49%), rituximab (21%), and anti–IL‐6 therapy (11%), with approximately half of the patients (55%) being treated in combination with methotrexate. All patients with SLE were antinuclear antibody positive (61 [100%]), and 19 (31%) had kidney affection. Patients with SLE were primarily treated with hydroxychloroquine (37 [61%]), prednisolone (29 [48%]), azathioprine (15 [25%]), and mycophenolate (16 [26%]).
Table 1.
Demographics, disease characteristics, and treatment details for the 134 patients included in the study
| SLE | RA | |
|---|---|---|
| Patients included, n | 61 | 73 |
| Female sex, n (%) | 41 (77.1) | 49 (67.1) |
| Age, median (IQR), years | 60.2 (46.3‐67.1) | 70.3 (66.9‐73.5) |
| BMI, median (IQR), kg/m2 | 24.7 (22.5‐27.5) | 26.4 (23.3‐28.9) |
| Disease duration, median (IQR), years | 15 (7‐30) | 19 (10‐24) |
| Charlson score, median (IQR) | 3 (2‐4) | 4 (3‐5) |
| Smoking status, % | ||
| Active | 8.3 | 9.9 |
| Previous | 43.3 | 60.5 |
| Never | 48.3 | 29.6 |
| Hypertension, n (%) | 19 (31.2) | 33 (45.2) |
| ACE‐inhibitor or AT2‐antagonist treatment, n (%) | 28 (45.9) | 33 (45.2) |
| SARS‐CoV‐2 antibody positive prevaccination, n (%) | 1/50 (2) | 0/65 (0.0) |
| White, n (%) | 60 (98.3) | 73 (100) |
| RA | ||
| ACPA positivity, n (%) | ‐ | 63/72 (87.5) |
| IgM‐RF positivity, n (%) | ‐ | 58 (79.5) |
| Erosive disease on X‐ray, n (%) | ‐ | 63 (86.3) |
| DMARD, n (%) | ‐ | |
| Methotrexate | ‐ | 40 (54.8) |
| Salazopurine | ‐ | 2 (2.7) |
| Hydroxychloroquine | ‐ | 1 (1.4) |
| Prednisone | ‐ | 8 (11) |
| Leflunomide | ‐ | 5 (6.9) |
| Azathioprine | ‐ | 2 (2.7) |
| Biologics and small molecules | ||
| Number of biologics tried, median (IQR) | ‐ | 2 (1‐3) |
| TNF‐inhibitors, n (%) | ‐ | 36 (49.3) |
| Rituximab, n (%) | ‐ | 15 (20.6) |
| JAK inhibitor, n (%) | ‐ | 8 (11) |
| Anti–IL‐6, n (%) | ‐ | 8 (11) |
| Abatacept, n (%) | ‐ | 6 (8.2) |
| SLE | ||
| ACR classification criteria, n (%) | ||
| Malar rash | 31 (50.8) | ‐ |
| Discoid rash | 4 (6.6) | ‐ |
| Photosensitivity | 25 (41) | ‐ |
| Oral ulcers | 15 (24.6) | ‐ |
| Nonerosive arthritis | 53 (86.9) | ‐ |
| Pleuritis or pericarditis | 17 (27.9) | ‐ |
| Renal disorder | 19 (31.2) | ‐ |
| Neurologic disorder | 5 (8.2) | ‐ |
| Hematologic disorder | 52 (85.3) | ‐ |
| Immunologic disorder | 55 (90.2) | ‐ |
| Positive antinuclear antibody | 61 (100) | ‐ |
| SLICC/ACR Damage Index, median (IQR) | 1 (1‐2) | ‐ |
| Treatment | ||
| Hydroxychloroquine, n (%) | 37 (60.7) | ‐ |
| Prednisone, n (%) | 29 (47.5) | ‐ |
| Prednisone dose mg, median (IQR) | 5 (5‐7.5) | ‐ |
| Azathioprine, n (%) | 15 (24.6) | ‐ |
| Mycophenolate mofetil, n (%) | 16 (26.2) | ‐ |
| Methotrexate, n (%) | 6 (9.8) | ‐ |
| Rituximab, n (%) | 2 (3.3) | ‐ |
| Belimumab, n (%) | 3 (4.9) | ‐ |
| Other (privigen, tacrolimus, and taltz), n (%) | 5 (8.2) | ‐ |
| No treatment, n (%) | 6 (9.8) | ‐ |
Abbreviation: ACE, angiotensins‐converting enzyme; ACPA, anti–citrullinated protein antibody; ACR, American College of Rheumatology; anti–IL‐6, interleukin 6 inhibitor; AT2, angiotensin II receptor; BMI, body mass index; DMARD, disease‐modifying rhematic drug; IgM‐RF, IgM rheumatoid factor; IQR, interquartile range; JAK, Janus kinase; RA, rheumatoid arthritis; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; SLE, systemic lupus erythematosus; SLICC/ACR, Systemic Lupus International Collaborating Clinics/American College of Rheumatology; TNF, tumor necrosis factor.
Total SARS‐CoV‐2 antibodies
Of 134 patients, 31 (23%) had undetectable antibodies against SARS‐CoV‐2, which were measured 8 (7, 8, 9, 10, 11) days after the second vaccination with the BNT162b2 vaccine (Figure 1A). Fewer patients with RA (49 [67%]) than those with SLE (54 [89%]) had measurable antibodies against SARS‐CoV‐2. However, the difference observed with diagnosis was nonsignificant when adjusting for treatment with rituximab (P = 0.28; Supplemental Table 1). We did not observe a difference in antibody levels between the two patient groups (P = 0.08) (Figure 1B). No correlation was observed between antibody serum level and age (r = −0.12; P = 0.18) (Supplemental Figure 1).
Figure 1.
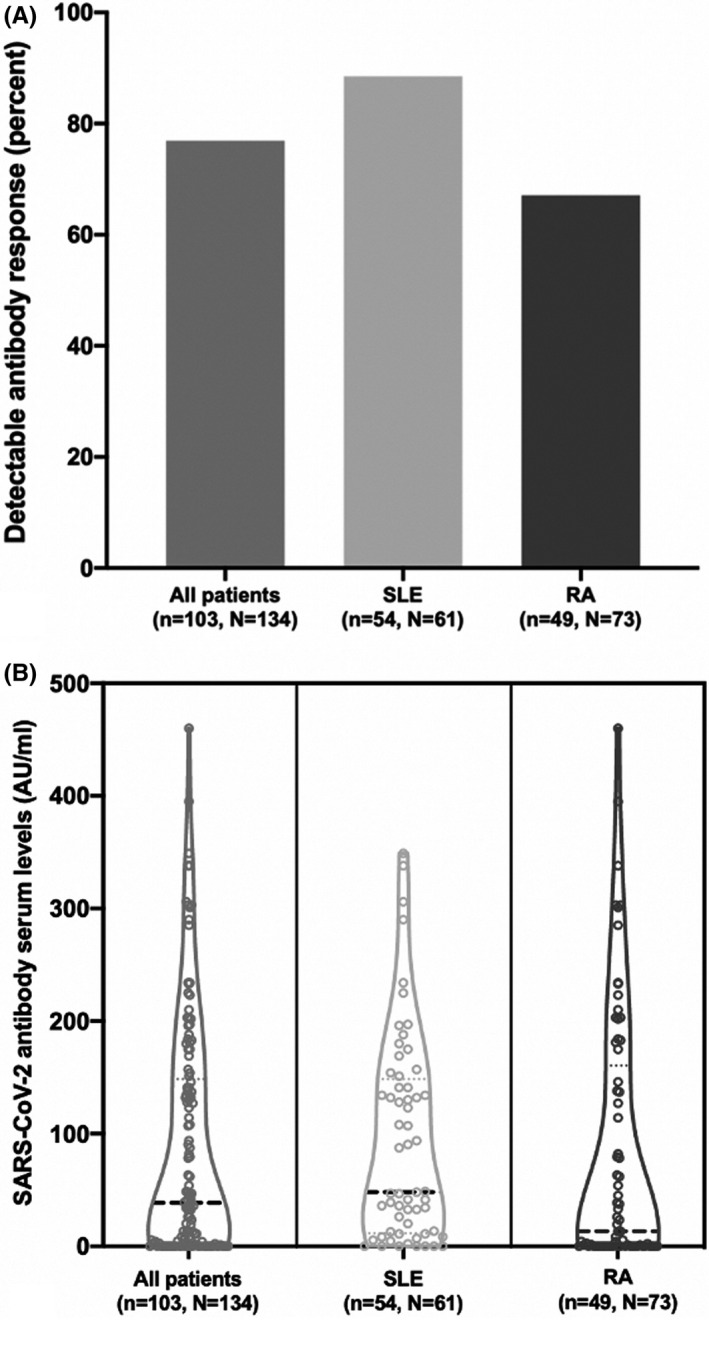
Antibody response against messenger RNA coronavirus disease 2019 (COVID‐19) vaccine Pfizer‐BioNTech BNT162b2 1 to 2 weeks after the vaccine in patients with rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). (A) Percentage of patients with positive severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) antibody results after vaccination. (B) Levels of SARS‐CoV‐2 antibodies in serum after vaccination. One outlier, a patient with lupus, with antibody levels of 1120 arbitrary units (AU)/ml, is removed from the display. The patient was the only patient with positive antibody levels prior to vaccination.
Treatment influences on antibody response
We observed patients without a detectable vaccine response in all treatment groups except belimumab. This was most pronounced in the rituximab group, in which only 4 of 17 (24%) responded (Figures 2A and 2B). In a univariate model, only hydroxychloroquine and rituximab were significantly associated with no response to the vaccine. However, rituximab treatment alone remained significantly associated with no antibody vaccine response after a multivariate analysis adjusting for diagnosis and hydroxychloroquine (odds ratio, 0.07; 95% CI, 0.02‐0.26) (Supplemental Table 1). For all biologic DMARD (bDMARD) treatments (except abatacept), the combination with methotrexate decreased the number of responders, although this was not statistically significant (Supplemental Figures 2A and 2B).
Figure 2.
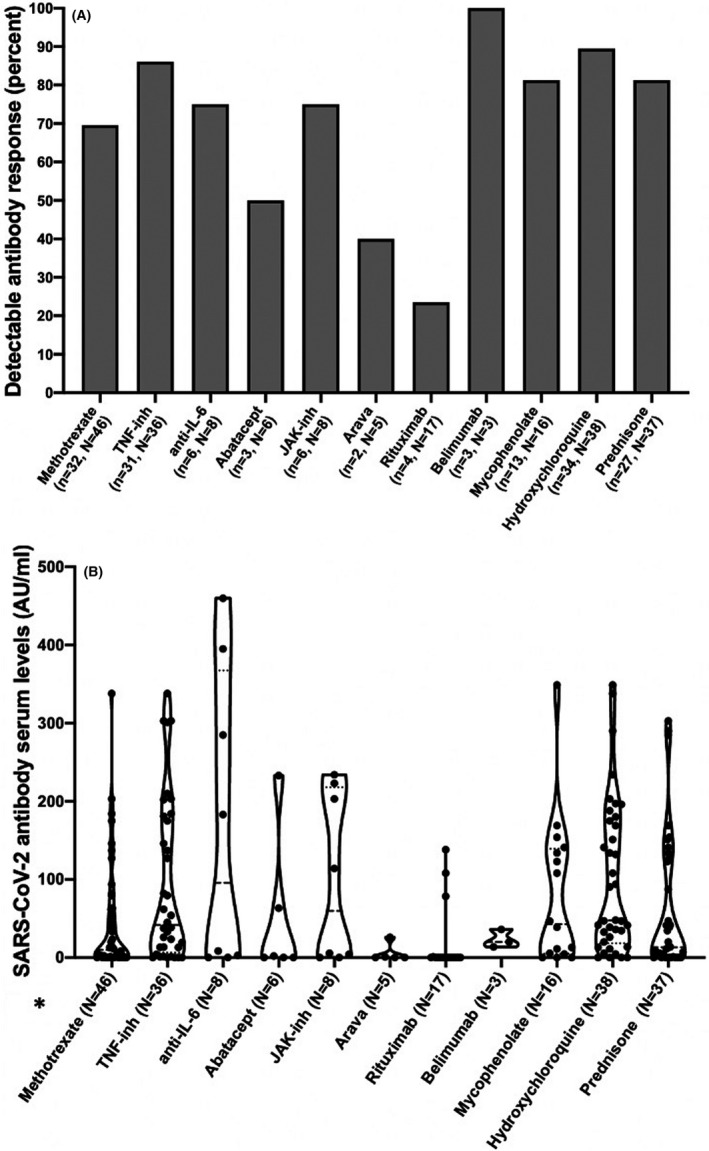
Antibody response against messenger RNA coronavirus disease 2019 (COVID‐19) vaccine Pfizer‐BioNTech BNT162b2 (BNT162b2) depending on treatment in patients with rheumatoid arthritis and systemic lupus erythematosus. (A) Number of patients with detectable antibodies against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) after vaccination depending on treatment with different disease‐modifying antirheumatic drugs (DMARDs). (B) Serum antibody levels against SARS‐COV‐2 after vaccination with BNT162b2 depending on treatment. Patients receiving more than one drug can be represented more than once in the figure. One outlier, a patient with lupus, with antibody levels of 1120 arbitrary units (AU)/ml, is removed from the display in B (treated with prednisolone). The patient was the only patient with positive antibody levels prior to vaccination. N = total number of patients in the group, n = number of patients with detectable SARS‐CoV‐2 antibodies in the group. Anti–IL‐6, interleukin 6 inhibitor; JAK‐inh, Janus kinase inhibitor; TNF‐inh, tumor necrosis factor inhibitor.
Among the patients treated with rituximab, the time since the last rituximab infusion did not seem to influence the antibody levels (Figure 3). The median interval between last rituximab treatment and vaccination was 187 days (IQR, 150‐245 days). None of the patients treated with rituximab who developed a detectable antibody response against SARS‐CoV‐2 received combination therapy with methotrexate (Figure 3).
Figure 3.
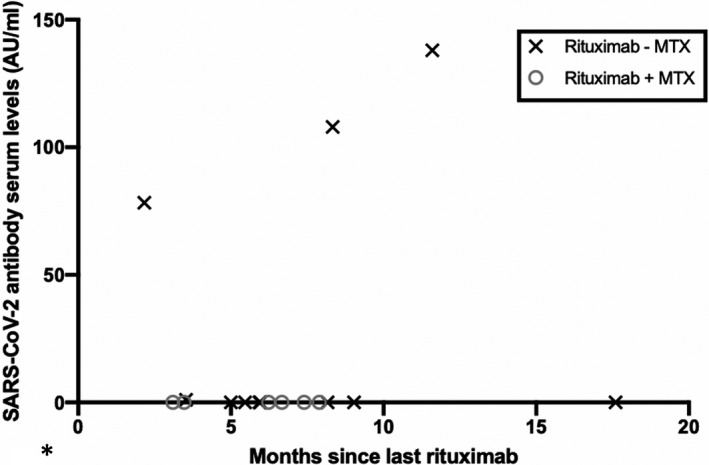
Serum antibody levels against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) after vaccination with Pfizer‐BioNTech BNT162b2 in patients treated with rituximab +/− methotrexate (MTX) and correlation with time since last treatment with rituximab. Anti–IL‐6, interleukin 6 inhibitor; AU, astronomical unit; JAK‐inh, Janus kinase inhibitor; TNF‐inh, tumor necrosis factor inhibitor.
DISCUSSION
Vaccines against SARS‐CoV‐2 are now widely available, and it is recommended that patients with RD be vaccinated (19). In Denmark, the first vaccine to become available was BNT162b2. However, knowledge of the vaccine's efficacy in patients with a compromised immune system and patients treated with immunosuppressive drugs was not available (1).
As a proxy for immunogenicity, we measured the total antibodies against SARS‐CoV‐2 in 134 patients with SLE and RA after a standard two‐dose vaccination with BNT162b2. The assay used had very high sensitivity and specificity, and we observed that 23% of the included patients did not develop detectable antibodies against the vaccine. Treatment, especially BCDT, had a significant impact on the antibody response.
In healthy individuals tested by the research group that developed the BNT162b2 vaccine (3) and in an extensive study of health care workers (4), almost 100% of the individuals who received two doses of BNT162b2 developed antibodies against the vaccine. We observed significantly fewer responders in the current study, in which only 77% developed detectable antibodies. Although immunogenicity is not the same as immunoefficacy for this vaccine, the antibody response correlated well with cellular immunity (3), which is essential for vaccine efficacy against SARS‐CoV‐2. Thus, patients who did not develop antibodies against the vaccine must be suspected of lacking immunity against SARS‐CoV‐2.
Patients in this study were generally older than the average SLE and RA cohorts. This is explained by the Danish vaccine strategy. Both age and diagnosis were taken into account when the first vaccinations became available and were distributed to persons at the highest risk of severe COVID‐19. We did not observe a correlation between antibody levels and age in this cohort, and thus age did not seem to have a significant impact on the vaccine response. However, the influence of treatment choice based on patient age cannot be excluded.
It has previously been demonstrated that age influences the antibody level and, furthermore, that maximum antibody response is seen 4 weeks after the second vaccination (20). It is thus possible that we would have seen a higher antibody response in our patients had we measured the levels 4 weeks after their last vaccination.
A recent study demonstrated that pausing methotrexate 2 weeks prior to vaccination resulted in higher antibody levels in patients with RD after an influenza vaccination (5). In Denmark, endorsed by EULAR, the recommendations have been to continue medication, as the risk of disease flare is regarded as a higher risk than a potential lower vaccine response in a few individuals. From our data, the combination of methotrexate with rituximab and any of the other bDMARDs and Janus kinase inhibitors reduced the number of patients who produced a measurable antibody response; however, the result was not statistically significant. Whether a pause in the DMARD treatment before vaccination could have increased the number of responders remains to be answered by future studies. An alternative to the medication pause could be an additional booster vaccine or an increased interval between vaccines to facilitate an adequate vaccine response. We did not include patients on methotrexate monotherapy and thus cannot conclude anything about this treatment.
BCDT was the treatment associated with the lowest response to the vaccine, with only 24% of patients receiving this treatment having detectable antibodies. This phenomenon is known from studies on both the influenza vaccine and the pneumococcal vaccine (7). Although the EULAR recommends that vaccines in patients receiving BCDT should be administered 1 month before or 6 months after BCDT (8), the time since the last rituximab treatment did not seem to influence who responded to the vaccine in the current study. Only 17 patients in the present study received BCDT, and therefore hard conclusions should not be made. It is, however, remarkable that the patients on BCDT who did elicit an antibody response were not treated in combination with methotrexate. It provokes the thought that, potentially, combination therapy hampers vaccine response more so than monotherapy.
The limitations of this study include a small sample size with only 134 patients and no data concerning disease activity at the time of vaccination. This makes substratifications difficult. All but one of the patients was white; thus the results cannot necessarily be extrapolated to other ethnic groups.
Only antibodies against SARS‐CoV‐2 were measured in this study. The consequence of reduced or unmeasurable antibodies is unknown, as we still lack knowledge of which precise antibody level secures protection. We still lack quantitative assays to measure antibody serum concentrations. Furthermore, the characterization of memory B cells and T cells would also have been advantageous.
Antibodies were measured 1 week after the second vaccination. Because of immunosuppressive treatment, we cannot rule out that patients potentially had a slower response to the vaccine and that antibodies would be detectable in more patients had we measured antibodies at a substantially longer time after the last vaccine dose. Furthermore, it is possible that immunosuppressed patients create lower affinity and/or less effective antibodies, which could have been demonstrated by measuring neutralizing antibodies and comparing with healthy control patients.
In conclusion, patients with RA or SLE have significantly lower immunogenicity compared with the previous studies on healthy individuals, as only 77% were able to mount a detectable serological response to the vaccine. Among patients treated with rituximab, only 23% had detectable anti–SARS‐CoV‐2 antibodies in their serum after vaccination. Our findings warrant particular attention to patients treated with rituximab and call for caution for this patient population concerning COVID‐19 going forward in the pandemic.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Ammitzbøll had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Ammitzbøll, Kragh Thomsen, Erikstrup, Hauge, Troldborg.
Acquisition of data
Ammitzbøll, Bartels, Bøgh Andersen, Risbøl Vils, Dahl Johannsen, From Hermansen, Erikstrup, Troldborg.
Analysis and interpretation of data
Ammitzbøll, Troldborg.
Supporting information
Fig S1
Fig S2
Table S1
ACKNOWLEDGMENTS
The authors would like to acknowledge all the participating patients, and the sparring with The Danish Rheumatish Association in the planning of the project.
Supported by the Danish Rheumatism Association.
No potential conflicts of interest relevant to this article were reported.
Contributor Information
Christian Ammitzbøll, Email: chramm@rm.dk.
Marie‐Louise From Hermansen, Email: maeher@rm.dk.
REFERENCES
- 1.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med 2020;383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tavakol M, Jamee M, Azizi G, Sadri H, Bagheri Y, Zaki‐Dizaji M, et al. Diagnostic approach to the patients with suspected primary immunodeficiency. Endocr Metab Immune Disord Drug Targets 2020;20:157–71. [DOI] [PubMed] [Google Scholar]
- 3.Sahin U, Muik A, Vogler I, Derhovanessian E, Kranz LM, Vormehr M, et al. BNT162b2 induces SARS‐CoV‐2‐neutralising antibodies and T cells in humans. medRxiv. 2020. URL: https://www.medrxiv.org/content/10.1101/2020.12.09.20245175v1. [Google Scholar]
- 4.Ebinger JE, Fert‐Bober J, Printsev I, Wu M, Sun N, Prostko JC, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS‐CoV‐2. Nat Med 2021;27:981–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park JK, Lee YJ, Shin K, Ha Y‐J, Lee EY, Song YW, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2018;77:898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rondaan C, Furer V, Heijstek MW, Agmon‐Levin N, Bijl M, Breedveld FC, et al. Efficacy, immunogenicity and safety of vaccination in adult patients with autoimmune inflammatory rheumatic diseases: a systematic literature review for the 2019 update of EULAR recommendations. RMD Open 2019;5:e001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hua C, Barnetche T, Combe B, Morel J. Effect of methotrexate, anti‐tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta‐analysis. Arthritis Care Res 2014;66:1016–26. [DOI] [PubMed] [Google Scholar]
- 8.Furer V, Rondaan C, Heijstek MW, Agmon‐Levin N, Van Assen S, Bijl M, et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis 2020;79:39–52. [DOI] [PubMed] [Google Scholar]
- 9.Arnold J, Winthrop K, Emery P. COVID‐19 vaccination and antirheumatic therapy. Rheumatology 2021. E‐pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachiller‐Corral J, Boteanu A, Garcia‐Villanueva MJ, de la Puente C , Revenga M, Diaz‐Miguel MC, et al. Risk of severe COVID‐19 infection in patients with inflammatory rheumatic diseases. J Rheumatol 2021. E‐pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Gianfrancesco M, Hyrich KL, Al‐Adely S, Carmona L, Danila MI, Gossec L, et al. Characteristics associated with hospitalisation for COVID‐19 in people with rheumatic disease: data from the COVID‐19 Global Rheumatology Alliance physician‐reported registry. Ann Rheum Dis 2020;79:859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marques CD, Kakehasi AM, Pinheiro MM, Mota LM, Albuquerque CP, Silva CR, et al. High levels of immunosuppression are related to unfavourable outcomes in hospitalised patients with rheumatic diseases and COVID‐19: first results of ReumaCoV Brasil registry. RMD Open 2021;7:e001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasseli R, Mueller‐Ladner U, Hoyer BF, Krause A, Lorenz HM, Pfeil A, et al. Older age, comorbidity, glucocorticoid use and disease activity are risk factors for COVID‐19 hospitalisation in patients with inflammatory rheumatic and musculoskeletal diseases. RMD Open 2021;7:e001464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ammitzbøll C, Andersen JB, Vils SR, Mistegaard CE, Mikkelsen S, Erikstrup C, et al. Isolation, behavioral changes and low seroprevalence of SARS‐CoV‐2 antibodies in patients with systemic lupus erythematosus or rheumatoid arthritis. Arthritis Care Res 2021. E‐pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Listing J, Gerhold K, Zink A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology 2013;52:53–61. [DOI] [PubMed] [Google Scholar]
- 16.Geisen UM, Berner DK, Tran F, Sümbül M, Vullriede L, Ciripoi M, et al. Immunogenicity and safety of anti‐SARS‐CoV‐2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis 2021. E‐pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyarsky BJ, Werbel WA, Avery RK, Tobian AA, Massie AB, Segev DL, et al. Immunogenicity of a single dose of SARS‐CoV‐2 messenger RNA vaccine in solid organ transplant recipients. JAMA 2021;325:1784–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harritshøj LH, Gybel‐Brask M, Afzal S, Kamstrup PR, Jørgensen CS, Thomsen MK, et al. Comparison of sixteen serological SARS‐CoV‐2 immunoassays in sixteen clinical laboratories. J Clin Microbiol 2021;59:e02596–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curtis JR, Johnson SR, Anthony DD, Arasaratnam RJ, Baden LR, Bass AR, et al. American College of Rheumatology guidance for COVID‐19 vaccination in patients with rheumatic and musculoskeletal diseases: version 1. Arthritis Rheumatol 2021. E‐pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh EE, Frenck RW, Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. Safety and immunogenicity of two RNA‐based Covid‐19 vaccine candidates. N Engl J Med 2020;383:2439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Table S1


