Abstract
Background and Aims
The surge in unhealthy alcohol use during the COVID‐19 pandemic may have detrimental effects on the rising burden of alcohol‐associated liver disease (ALD) on liver transplantation (LT) in the USA. We evaluated the effect of the pandemic on temporal trends for LT including ALD.
Approach and Results
Using data from United Network for Organ Sharing, we analyzed wait‐list outcomes in the USA through March 1, 2021. In a short‐period analysis, patients listed or transplanted between June 1, 2019, and February 29, 2020, were defined as the “pre‐COVID” era, and after April 1, 2020, were defined as the “COVID” era. Interrupted time‐series analyses using monthly count data from 2016‐2020 were constructed to evaluate the rate change for listing and LT before and during the COVID‐19 pandemic. Rates for listings (P = 0.19) and LT (P = 0.14) were unchanged during the pandemic despite a significant reduction in the monthly listing rates for HCV (−21.69%, P < 0.001) and NASH (−13.18%; P < 0.001). There was a significant increase in ALD listing (+7.26%; P < 0.001) and LT (10.67%; P < 0.001) during the pandemic. In the COVID era, ALD (40.1%) accounted for more listings than those due to HCV (12.4%) and NASH (23.4%) combined. The greatest increase in ALD occurred in young adults (+33%) and patients with severe alcohol‐associated hepatitis (+50%). Patients with ALD presented with a higher acuity of illness, with 30.8% of listings and 44.8% of LT having a Model for End‐Stage Liver Disease–Sodium score ≥30.
Conclusions
Since the start of COVID‐19 pandemic, ALD has become the most common indication for listing and the fastest increasing cause for LT. Collective efforts are urgently needed to stem the rising tide of ALD on health care resources.
Abbreviations
- ALD
alcohol‐associated liver disease
- AUD
alcohol use disorder
- COVID‐19
coronavirus Disease 2019
- ICU
intensive care unit
- LT
liver transplantation
- MELD‐Na
Model for End‐Stage Liver Disease–Sodium
- SAH
severe alcohol‐associated hepatitis
- UNOS
United Network for Organ Sharing
The exponential increase in the number of reported coronavirus disease 2019 (COVID‐19) cases in the USA has mandated large‐scale health care practice changes across the country. Although organ transplantation is often a medical emergency, the increasing demand and use of hospital resources has adversely affected the ability of transplant centers to perform these life‐saving procedures. Previous analyses have demonstrated that solid organ transplantation within the USA was significantly curtailed by COVID‐19.( 1 ) However, the reduction was driven primarily by a substantial decline in kidney and living donor transplantation volumes during the early phases of the pandemic in March and April 2020.(2,3) Although these analyses appreciated a downtrend in transplant volumes among all organ types, an ongoing follow‐up was warranted to understand how this trend has affected liver transplantation (LT) on both a national and regional level.
In particular, patients with alcohol‐associated liver disease (ALD) are an important subpopulation to consider. Data from early in the pandemic showed a rise in liquor sales during the pandemic.( 2 ) Other recently published studies showed that increased alcohol consumption, which was associated with length of time spent under a shelter‐in‐place order, increased stress, greater alcohol availability, and diagnoses of depression or depressive symptoms.( 3 , 4 , 5 ) ALD is already the leading indication for LT in the USA.( 6 , 7 ) The downstream impact of increased unhealthy alcohol use during the pandemic can have substantial, detrimental effects and may have already affected LT in the USA.
The landscape for LT in the USA has changed dramatically over the past 5 years due to advancements for treating HCV, rising prevalence for NASH, and increased leniency for programs to list those with ALD.(2,6) This has led to declining LT rates for HCV and increasing rates for ALD and NASH. The effect of the COVID‐19 pandemic on LT for these etiologies needs to be explored further. Therefore, we aim to evaluate the effect of the COVID‐19 pandemic on wait‐list outcomes in relation to short‐term and long‐term temporal trends for LT.
Patients and Methods
Study Database and Participants
This study was exempted from IRB approval. We conducted an analysis of prospectively collected data from the United Network for Organ Sharing (UNOS) among all adult (age >18 years) LT wait‐list registrants and recipients. We defined the 9‐month period from June 1, 2019, through February 29, 2020, as the “pre‐COVID era.” Similarly, we defined the 9‐month period from April 1, 2020, through December 31, 2020, as the “COVID era.” To limit confounding from regional variation in the adoption of shelter‐in place statutes during March 2020 and its effect on LT, we excluded data from March 1, 2020, through March 31, 2020, from our comparative analyses. Wait‐list additions or listings were defined as patients initially listed only during the defined era period. Follow‐up data were available until March 1, 2021.
Etiology of liver disease including ALD and severe alcohol‐associated hepatitis (SAH) was determined by distinct primary and secondary diagnostic codes provided by the UNOS registry. Patients listed or transplanted HCV or NASH were also evaluated. Other patient characteristics included sociodemographic characteristics including age, gender, ethnicity/race, and clinical characteristics including laboratory Model for End‐Stage Liver Disease–Sodium score (MELD‐Na), presence of ascites, HE, history of spontaneous bacterial peritonitis, HCC, hemodialysis, mechanical ventilation, and US geographic region.
Temporal Trend Analyses
To compare how pandemic‐associated changes took place within the context of ongoing trends over the last several years, we constructed single‐group interrupted time‐series regression models using monthly cumulative count data from the last 5 years (January 1, 2016, to March 1, 2021) to account for long‐term time trends in the data.( 8 , 9 ) April 1, 2020, represented the start of the COVID‐19 pandemic. Predicted rate during the pandemic was determined using cumulative monthly counts in the pre‐COVID era from January 1, 2016, through February 28, 2020, and compared with actual rates during the pandemic. Using these models, we analyzed changes in rates for overall and etiology‐based (ALD, NASH, and HCV) cumulative listing and transplant trends. In consideration of the idea that consecutive observations in a trend tend to be more similar than observations further apart, our time‐series models were corrected for autocorrelation appropriately to favor the assumption that observations are independent.
We also evaluated trends in wait‐list and LT rates before and during the COVID‐19 pandemic in a short‐term analyses. We split the pre‐COVID and COVID eras into 3‐month periods, resulting in six quarters. The quarterly average number of new wait‐list additions, wait‐list deaths, and LT surgeries performed in the pre‐COVID era (April 1, 2019, to December 31, 2019) were compared to counts during each of the three time periods during the pandemic (April 1, 2020, to June 30, 2020; July 1, 2020, to September 30, 2020; October 1, 2020, to December 31, 2020). We also examined temporal trends in wait‐list and LT in subgroups with ALD, SAH, and by geographic region in the USA.
Sensitivity Analyses
First, we sought to evaluate how state‐to‐state variation in the duration of shelter‐in‐place order statutes for COVID‐19 affected listing and transplants performed for patients with high MELD (MELD‐Na > 30) between eras. Although covariates directly relating to pandemic restrictions or shelter‐in‐place were not available in the UNOS database, to address this point we ascertained the timing of shelter‐in‐place orders for all states, and categorized states based on the implementation and duration of shelter in place during the study time frame using the COVID‐19 US state policies database.( 10 ) We categorized patients into the following groups based on the duration of shelter‐in‐place for the corresponding state of each transplant center at time of listing/transplant: (1) no shelter‐in‐place order (IA, NE, AR, UT, SD, ND, WY, and KY), (2) shelter‐in‐place orders <45 days (AL, AK, FL, GA, ID, KS, MS, MO, MT, NV, OK, RI, SC, TN, TX, and WV), (3) shelter‐in‐place order 45‐80 days (AZ, CO, DE, DC, HI, IL, IN, LA, ME, MD, MA, MI, MN, NC, OH, PA, VT, VA, WA, and WI), and (4) shelter‐in‐place order >80 days (NY, NM, NH, NJ, OR, and CA). We then analyzed the proportions of patients with high MELD at the time of listing and/or transplantation in patients with and without based on whether they were listed/transplanted in states with or without shelter‐in‐place orders.
In February 2020, Organ Procurement and Transplantation Network/UNOS implemented the “acuity circle” policy, which prioritizes allocating liver organs to the sickest candidates within a 150‐250 nautical mile range from a donor service area.( 11 ) In a secondary analysis, we compared median time from listing to transplant for all, patients with ALD, and patients without ALD in high MELD recipients between eras.
Statistical Analyses
We compared clinical and demographic characteristics between wait‐list additions and LT recipients in the pre‐COVID‐19 era with those in the COVID‐19 era. Clinical characteristics among wait‐list additions and LT recipients, including those with ALD, were compared in each era using chi‐square test for categorical variables and Student t test (parametric) and Mann‐Whitney U test for (nonparametric) continuous variables.
Interrupted time‐series analyses were performed using linear regression with Newey–West standard errors to handle autocorrelation in addition to possible heteroskedasticity. Fine and Gray proportional hazard regression models were constructed to evaluate differences in LT rates for ALD and non‐ALD in the pre‐COVID and COVID eras, respectively. The Gray test and Fine‐Gray models allow for the analysis of competing risk events, which, in our study include wait‐list removal due to death, clinical deterioration, or clinical improvement. Follow‐up time for the pre‐COVID era was censored on February 28, 2020, to prevent overlap between the two eras. Patients listed without at least 2 months of follow‐up data in either era were excluded in the regression models. All statistical analyses and data visualizations were performed using STATA Version 13.0 (College Station, TX). Statistical significance was met with a P value < 0.05. This study was approved by the institutional review board at Baylor College of Medicine.
Results
Overall Trends
Cumulative year‐to‐date totals for wait‐list additions, wait‐list dropout (defined as removal from the wait‐list due to death or clinical deterioration), and liver transplants in the USA were similar in 2019 and 2020 (Supporting Fig. S1). Although the number of LT had initially declined during the start of the pandemic, LT volume recovered from May 2020 onward. Aggregate quarterly totals for 2020 (Table 1) demonstrate that the LT recovery was seen across all U.S. regions including the Northeast and Mid‐Atlantic, regions that experienced the largest reduction in transplant volume. However, this recovery was not uniform. Compared with the pre‐COVID era, the Mid‐Atlantic and Southeast regions experienced a reduction in number of transplants performed. In contrast, the Southwest region experienced both an increase in wait‐list additions and transplants in the COVID era. Compared with the pre‐COVID era, wait‐list dropout was lower in the COVID era, which was also observed in nearly all U.S. regions (Table 1).
TABLE 1.
Comparison of Quarterly (3‐Month Period) Wait‐List Additions, Wait‐List Deaths, and LT Surgeries for LT Stratified by US Regions in 2020
| Quarterly Mean in 2019* | April– June 2020 | Percent Change | July–September 2020 | Percent Change | October–December 2020 | Percent Change | Overall 2020 Quarterly Mean † | Percent Change [95% CI] | |
|---|---|---|---|---|---|---|---|---|---|
| Overall | |||||||||
| Additions | 3,223 | 2,819 | −12.1% | 3,279 | +2.2% | 3,161 | −1.47% | 3,086 | −4.0% [−10.9%, 2.9%] |
| Deaths | 301 | 274 | −8.4% | 272 | −9.0% | 267 | −10.7% | 271 | −9.4% [−17.8%, −1.0%] |
| Transplants | 2,093 | 1,996 | −4.2% | 2,175 | +4.4% | 2,138 | +2.6% | 2,103 | +1.0% [−3.3%, 5.3%] |
| ALD | |||||||||
| Additions | 1,191 | 1,117 | −5.7% | 1,337 | +12.8% | 1,256 | +6.0% | 1,237 | +4.4% [−4.4%, 13.2%] |
| Transplants | 760 | 758 | +0.3% | 891 | +17.9% | 846 | +11.9% | 832 | +9.1 [−0.6%, 17.6%] |
| SAH | |||||||||
| Additions | 42 | 44 | +4.8% | 81 | +92.9% | 82 | +95.2% | 69 | +64.3% [16.5%, 112.1%] |
| Transplants | 33 | 38 | +15.2% | 65 | +97.0% | 65 | +97.0% | 56 | +69.7% [24.0%, 115.4%] |
| U.S. Region ‡ | |||||||||
| Northeast | |||||||||
| Additions | 385 | 280 | −27.1% | 395 | +2.9% | 373 | −2.9% | 349 | −9.1% |
| Deaths | 54 | 53 | −1.9% | 40 | −25.9% | 33 | −38.9% | 42 | −22.2% |
| Transplants | 193 | 160 | −16.2% | 248 | +29.8% | 233 | +22.0% | 214 | +12.0% |
| Mid‐Atlantic | |||||||||
| Additions | 368 | 309 | −15.6% | 358 | −2.2% | 362 | −1.1% | 343 | −6.3% |
| Deaths | 45 | 47 | +6.8% | 44 | 0% | 38 | −13.6% | 43 | −2.2% |
| Transplants | 245 | 208 | −14.8% | 225 | −7.8% | 233 | −4.5% | 222 | −9.0% |
| Southeast | |||||||||
| Additions | 752 | 655 | −12.7% | 785 | +4.7% | 690 | −8.0% | 710 | −5.3% |
| Deaths | 55 | 54 | 0% | 49 | −9.3% | 44 | −18.5% | 49 | −9.3% |
| Transplants | 536 | 516 | −3.4% | 528 | −1.1% | 530 | −0.7% | 525 | −1.7% |
| North Midwest | |||||||||
| Additions | 691 | 612 | −11.2% | 689 | 0% | 649 | −5.8% | 650 | −6.0% |
| Deaths | 61 | 44 | −27.9% | 40 | −34.4% | 43 | −29.5% | 42 | −45.2% |
| Transplants | 502 | 504 | +0.8% | 533 | +6.6% | 498 | −0.4% | 512 | +2.3% |
| South Midwest | |||||||||
| Additions | 397 | 379 | −3.6% | 404 | +2.8% | 414 | +5.3% | 399 | 1.5% |
| Deaths | 32 | 25 | −21.9% | 38 | +18.8% | 45 | +40.6% | 36 | 11.1% |
| Transplants | 226 | 225 | 0% | 223 | −0.9% | 224 | −0.4% | 224 | 0% |
| Southwest | |||||||||
| Additions | 537 | 510 | −4.7% | 560 | +4.7% | 578 | +8.0% | 549 | +2.6% |
| Deaths | 48 | 46 | −4.2% | 55 | +14.6% | 56 | +16.7% | 52 | +8.3% |
| Transplants | 333 | 334 | +0.6% | 356 | +7.2% | 351 | +5.7% | 347 | +4.3% |
| Northwest | |||||||||
| Additions | 93 | 74 | −19.6% | 88 | −4.3% | 95 | +3.3% | 86 | −7.0% |
| Deaths | 5 | 5 | 0% | 6 | +20.0% | 8 | +60.0% | 6 | +20% |
| Transplants | 58 | 49 | −15.5% | 62 | +6.9% | 69 | +19.0% | 60 | +3.4% |
Quarterly mean number of wait‐list additions, wait‐list deaths, and transplants performed calculated from April 1, 2019, to December 31, 2019.
Quarterly mean number of wait‐list additions, wait‐list deaths, and transplants performed calculated from April 1, 2020, to December 31, 2020.
States included within each UNOS Region:
Northeast: UNOS Region 1 (Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, Eastern Vermont) and UNOS Region 9 (New York, and Western Vermont).
Mid‐Atlantic: UNOS Region 2 (Delaware, District of Columbia, Maryland, New Jersey, Pennsylvania, West Virginia, and Northern Virginia).
Southeast: UNOS Region 3 (Alabama, Arkansas, Florida, Georgia, Louisiana, Mississippi, and Puerto Rico) and UNOS Region 11 (Kentucky, North Carolina, South Carolina, Tennessee, and Virginia).
North Midwest: UNOS Region 7 (Illinois, Minnesota, North Dakota, South Dakota, and Wisconsin), UNOS Region 8 (Colorado, Iowa, Kansas, Missouri, Nebraska, and Wyoming) and UNOS Region 10 (Indiana, Michigan, and Ohio). Great Lakes and Midwest regions combined into North Midwest.
South Midwest: UNOS Region 4 (Oklahoma and Texas).
Southwest: UNOS Region 5 (Arizona, California, Nevada, New Mexico, and Utah).
Northwest: UNOS Region 6 (Alaska, Hawaii, Idaho, Montana, Oregon, and Washington).
Characteristics of Wait‐List Registrants and LT Recipients
Overall
Clinical characteristics of wait‐list additions and LT recipients in the pre‐COVID and COVID eras are given in Table 2. Both wait‐list additions and LT recipients were younger during the COVID era. In addition, there was a higher percentage of listings with Medicaid and private insurance and a lower percentage of Medicare insurance. Gender, ethnicity/race, and geographic region of listing/transplant were not significantly different between eras. During the COVID era, the percentage with ALD significantly increased, accounting for 40% of listings and LTs. The percentage of wait‐list additions with ALD (40.1%) surpassed that for HCV (12.4%) and NASH (23.4%) combined. Median MELD‐Na scores at listing (18 vs. 19, P < 0.001) and at transplant (23 vs. 24, P < 0.001) were also higher in the COVID era. The percentage of patients with a high MELD‐Na scores > 30 at listing (pre‐COVID era: 19.9%. vs. COVID era: 22.1%; P < 0.001) and at transplant (pre‐COVID era: 30.1% vs. COVID era: 33.4%; P < 0.001) increased significantly during the pandemic.
TABLE 2.
Comparison of Sociodemographic and Clinical Characteristics Among Wait‐List Additions and LT Recipients in the USA Before and During the COVID‐19 Pandemic
| Wait‐List Additions* | LT Recipients † | |||||
|---|---|---|---|---|---|---|
| Pre‐COVID Era | COVID Era | P value | Pre‐COVID Era | COVID Era | P Value | |
| June 2019 to February 2020 (n = 9,528), (%) | April 2020 to December 2020 (n = 9,259), (%) | June 2019 to February 2020 (n = 6,325), (%) | April 2020 to December 2020 (n = 6,309), (%) | |||
| Median age [IQR] (years) | 58 [15] | 57 [16] | <0.001 | 58 [15] | 57 [16] | 0.073 |
| Gender | 0.730 | 0.969 | ||||
| Female | 3,593 (37.7) | 3,469 (37.5) | 2,321 (36.7) | 2,313 (36.7) | ||
| Male | 5,935 (62.3) | 5,790 (62.5) | 4,004 (63.3) | 3,996 (63.3) | ||
| Ethnicity/race | 0.239 | 0.085 | ||||
| Caucasian | 6,693 (70.2) | 6,382 (68.9) | 4,512 (71.3) | 4,389 (69.6) | ||
| African American | 666 (7.0) | 646 (7.0) | 447 (7.1) | 458 (7.3) | ||
| Hispanic | 1,636 (17.2) | 1,659 (17.9) | 999 (15.8) | 1,075 (17.0) | ||
| Asian | 363 (3.8) | 395 (4.3) | 237 (3.7) | 273 (4.3) | ||
| Other | 170 (1.8) | 177 (1.9) | 130 (2.1) | 114 (1.8) | ||
| Primary insurance | 0.003 | <0.001 | ||||
| Medicaid | 1,656 (17.4) | 1,692 (18.3) | 924 (14.6) | 1,040 (16.5) | ||
| Medicare | 2,642 (27.7) | 2,345 (25.3) | 1,918 (30.3) | 1,797 (28.5) | ||
| Private | 4,781 (50.2) | 4,771 (51.5) | 3,245 (51.3) | 3,171 (50.3) | ||
| All other | 449 (4.7) | 451 (4.9) | 238 (3.8) | 301 (4.8) | ||
| etiologies of liver disease | ||||||
| Chronic HCV infection | 1,476 (15.5) | 1,148 (12.4) | <0.001 | 1,174 (18.6) | 1,003 (15.9) | <0.001 |
| ALD | 3,546 (37.2) | 3,710 (40.1) | <0.001 | 2,328 (36.8) | 2,495 (39.5) | 0.002 |
| NASH | 2,311 (24.3) | 2,169 (23.4) | 0.183 | 1,491 (23.6) | 1,526 (24.2) | 0.418 |
| Laboratory MELD‐Na score | 18 [14] | 19 [15] | <0.001 | 23 [15] | 24 [15] | <0.001 |
| MELD‐Na score | <0.001 | <0.001 | ||||
| <15 | 3,304 (34.7) | 2,934 (31.7) | 1,360 (21.5) | 1,242 (19.7) | ||
| 15‐19 | 1,906 (20.0) | 1,743 (18.8) | 1,026 (16.2) | 883 (14.0) | ||
| <20 | 5,210 (54.7%) | 4,677 (50.5%) | 2,386 (37.7%) | 2,125 (33.7%) | ||
| 20‐24 | 1,511 (15.9%) | 1,478 (16.0%) | 1,047 (16.6%) | 1,039 (16.5%) | ||
| 25‐29 | 1,005 (10.5%) | 1,063 (11.5%) | 991 (15.7%) | 1,035 (16.4%) | ||
| 30‐34 | 766 (8.0%) | 867 (9.4%) | 770 (12.2%) | 948 (15.0%) | ||
| >35 | 1,036 (10.9%) | 1,174 (12.7%) | 1,131 (17.9%) | 1,162 (18.4%) | ||
| Hepatic decompensation | ||||||
| Moderate‐severe ascites | 2,601 (27.3) | 2,710 (29.3) | 0.003 | 2,049 (32.4) | 2,240 (35.5) | <0.001 |
| Spontaneous bacterial peritonitis | 895 (9.4) | 829 (9.0) | 0.296 | 642 (10.2) | 627 (9.9) | 0.692 |
| Severe HE | 880 (9.2) | 917 (9.9) | 0.120 | 785 (12.4) | 861 (13.6) | 0.039 |
| HCC | 989 (10.4) | 885 (9.6) | 0.060 | 593 (8.9) | 552 (8.7) | 0.220 |
| Hemodialysis | 901 (9.5) | 1,001 (10.8) | 0.002 | 1,047 (16.6) | 1,131 (17.9) | 0.041 |
| Mechanical ventilation/life Support | 424 (4.5) | 398 (4.3) | 0.612 | 566 (8.9) | 484 (7.7) | 0.009 |
| Location at transplant | <0.001 | |||||
| Home | ‐ | ‐ | 4,205 (66.5) | 3,870 (62.2) | ||
| Hospitalized not in ICU | ‐ | ‐ | 1,246 (19.7) | 1,457 (23.4) | ||
| ICU | ‐ | ‐ | 873 (13.8) | 897 (14.4) | ||
| US region | 0.160 | 0.525 | ||||
| Northeast | 1,142 (12.0) | 1,048 (11.3) | 591 (9.3) | 641 (10.2) | ||
| Mid‐Atlantic | 1,104 (11.6) | 1,029 (11.1) | 707 (11.2) | 666 (10.6) | ||
| Southeast | 2,186 (22.9) | 2,130 (23.0) | 1,619 (25.6) | 1,574 (24.9) | ||
| North Midwest | 2,066 (21.7) | 1,950 (21.1) | 1,525 (24.1) | 1,535 (24.3) | ||
| South Midwest | 1,203 (12.6) | 1,197 (12.9) | 679 (10.7) | 672 (10.7) | ||
| Southwest | 1,563 (16.4) | 1,648 (17.8) | 1,008 (15.9) | 1,041 (16.5) | ||
| Northwest | 264 (2.8) | 257 (2.8) | 196 (3.1) | 180 (2.9) | ||
Values (age, MELD‐Na, hepatic decompensation, hemodialysis, and mechanical ventilation/life support) for wait‐list additions reported at time of listing or wait‐list registration.
Values (age, MELD‐Na, hepatic decompensation, hemodialysis and mechanical ventilation/life support) for liver transplant recipient reported at time of transplant.
Abbreviation: IQR, interquartile range.
ALD
Clinical characteristics of patients listed or transplanted with ALD are provided in Table 3. Between eras, young adults (18‐34 years and 35‐50 years) experienced a modest 2.8% absolute increase, but accounted for 35.4% of listings in the COVID era. There was also a corresponding decrease in the number of ALD listed or transplanted aged 65 and above. Similar to the overall comparison, gender, race/ethnicity, and geographic region distributions were similar between the pre‐COVID and COVID eras. Median MELD‐Na at listing (22 vs. 23, P < 0.001) and transplant (27 vs. 28, P < 0.001) increased for ALD during the COVID era. The percentage of patients with ALD listed with MELD‐Na > 30 increased from 26.6% in pre‐COVID to 30.8% in the COVID era, a 15.8% relative increase from prior. In addition, the percentage of ALD transplanted with MELD‐Na > 30 increased from 38.6% to 44.8%, a 16.1% relative increase. A higher proportion of patients with ALD required hemodialysis at listing and transplant in the COVID era. The percentage of patients hospitalized on the general medical floor or intensive care unit (ICU) at the time of transplant also increased from 39.8% to 45.8% between eras (Table 3).
TABLE 3.
Comparison of Demographic and Clinical Characteristics Among Wait‐List Additions and LT Recipients With ALD Before and During the COVID‐19 Pandemic
| Wait‐List Additions* | LT Recipients † | |||||
|---|---|---|---|---|---|---|
| Pre‐COVID Era | COVID Era | P Value | Pre‐COVID Era | COVID Era | P Value | |
| June 2019 to February 2020 (n = 3,546), (%) | April 2020 to December 2020 (n = 3,710), (%) | June 2019 to February 2020 (n = 2,328), (%) | April 2020 to December 2020 (n = 2,495), (%) | |||
| Median age [IQR] (years) | 55 [14] | 54 [16] | =0.007 | 54 [15] | 54 [16] | 0.134 |
| Age | 0.010 | 0.088 | ||||
| 18‐34 | 185 (5.2) | 223 (6.0) | 121 (5.2) | 169 (6.8) | ||
| 35‐50 | 976 (27.5) | 1,090 (29.4) | 676 (29.0) | 745 (30.0) | ||
| 50‐64 | 1,846 (52.1) | 1,920 (51.8) | 1,206 (51.8) | 1,256 (50.3) | ||
| >65 | 539 (15.2) | 477 (12.9) | 325 (14.0) | 325 (13.0) | ||
| Gender | 0.835 | 0.695 | ||||
| Female | 1,012 (28.5) | 1,067 (28.8) | 619 (26.6) | 651 (26.1) | ||
| Male | 2,534 (71.5) | 2,643 (71.2) | 1,709 (73.4) | 1,844 (73.9) | ||
| Ethnicity/race | 0.398 | 0.003 | ||||
| Caucasian | 2,635 (74.3) | 2,749 (74.1) | 1,760 (75.6) | 1,836 (73.6) | ||
| African American | 160 (4.5) | 156 (4.2) | 113 (4.9) | 106 (4.2) | ||
| Hispanic | 628 (17.7) | 648 (17.5) | 382 (16.4) | 447 (17.9) | ||
| Asian | 55 (1.6) | 79 (2.1) | 26 (1.1) | 62 (2.5) | ||
| Other | 68 (1.9) | 78 (2.1) | 47 (2.0) | 44 (1.8) | ||
| Primary insurance | ||||||
| Medicaid | 1,656 (17.4) | 1,692 (18.3) | 924 (14.6) | 1,040 (16.5) | ||
| Medicare | 2,642 (27.7) | 2,345 (25.3) | 1,918 (30.3) | 1,797 (28.5) | ||
| Private | 4,781 (50.2) | 4,771 (51.5) | 3,245 (51.3) | 3,171 (50.3) | ||
| All other | 449 (4.7) | 451 (4.9) | 238 (3.8) | 301 (4.8) | ||
| Median laboratory MELD‐Na score [IQR] | 22 [15] | 23 [16] | <0.001 | 27 [15] | 28 [14] | <0.001 |
| MELD‐Na score | 0.001 | <0.001 | ||||
| <15 | 810 (22.8%) | 794 (21.4%) | 290 (12.5%) | 270 (10.8%) | ||
| 15‐24 | 1,326 (37.4%) | 1,263 (34.0%) | 712 (30.6%) | 674 (27.0%) | ||
| 25‐29 | 467 (13.2%) | 510 (13.7%) | 428 (18.4%) | 432 (17.3%) | ||
| 30‐34 | 384 (10.8%) | 475 (12.8%) | 339 (14.6%) | 485 (19.4%) | ||
| >35 | 559 (15.8%) | 668 (18.0%) | 559 (24.0%) | 634 (25.4%) | ||
| Hepatic decompensation | ||||||
| Spontaneous bacterial peritonitis | 468 (13.2) | 460 (12.4) | 0.308 | 345 (14.8) | 332 (13.3) | 0.131 |
| Moderate to severe ascites | 1349 (38.0) | 1409 (38.0) | 0.955 | 972 (41.8) | 1141 (45.7) | 0.005 |
| Severe HE | 414 (11.7) | 449 (12.1) | 0.574 | 364 (15.6) | 410 (16.4) | 0.451 |
| HCC | 194 (5.5) | 190 (5.1) | 0.506 | 125 (5.4) | 106 (4.2) | 0.069 |
| Hemodialysis | 407 (11.5) | 522 (14.1) | 0.001 | 442 (19.0) | 572 (22.9) | 0.001 |
| Mechanical ventilation/life support | 172 (4.9) | 181 (4.9) | 0.956 | 236 (10.1) | 228 (9.1) | 0.240 |
| Location at transplant | <0.001 | |||||
| Home | ‐ | ‐ | 1401 (60.2) | 1329 (54.2) | ||
| Hospitalized not in ICU | ‐ | ‐ | 543 (23.3) | 714 (29.1) | ||
| ICU | ‐ | ‐ | 384 (16.5) | 411 (16.7) | ||
| US region | 0.095 | 0.192 | ||||
| Northeast | 466 (13.1) | 488 (13.2) | 223 (9.6) | 290 (11.6) | ||
| Mid‐Atlantic | 433 (12.2) | 446 (12.0) | 284 (12.2) | 293 (11.7) | ||
| Southeast | 743 (21.0) | 809 (21.8) | 551 (23.7) | 589 (23.6) | ||
| North Midwest | 827 (23.3) | 792 (21.3) | 594 (25.5) | 597 (23.9) | ||
| South Midwest | 435 (12.3) | 438 (11.8) | 258 (11.1) | 253 (10.1) | ||
| Southwest | 528 (14.9) | 634 (17.1) | 342 (14.7) | 398 (16.0) | ||
| Northwest | 114 (3.2) | 103 (2.8) | 76 (3.3) | 75 (3.0) | ||
| SAH, n | 132 | 207 | 110 | 168 | ||
| Median age [IQR] (years) | 41 [12.5] | 40 [14] | 0.363 | 41 [12] | 39.5 [14] | 0.419 |
| Age groups (years) | 0.426 | 0.252 | ||||
| 18‐34 | 25 (18.9) | 48 (23.2) | 20 (18.2) | 38 (22.6) | ||
| 35‐50 | 82 (62.1) | 110 (53.1) | 69 (62.7) | 89 (53.0) | ||
| 50‐64 | 24 (18.2) | 46 (22.2) | 21 (19.1) | 38 (22.6) | ||
| >65 | 1 (0.8) | 3 (1.4) | 0 (0) | 3 (1.8) | ||
| MELD‐Na score | 0.244 | 0.284 | ||||
| 25‐29 | 10 (8.5) | 15 (8.1%) | 9 (9.1) | 18 (11.4) | ||
| 30‐34 | 22 (18.6) | 50 (27.0%) | 14 (14.1) | 33 (20.9) | ||
| >35 | 86 (72.9) | 120 (64.9%) | 76 (76.8) | 107 (67.7) | ||
Values (age, MELD‐Na, hepatic decompensation, hemodialysis, and mechanical ventilation/life support) for wait‐list additions reported at time of listing or wait‐list registration.
Values (age, MELD‐Na, hepatic decompensation, hemodialysis, and mechanical ventilation/life support) for LT recipient reported at time of transplant.
Abbreviation: IQR, interquartile range.
Listings and transplants for SAH increased from July 2020 onward, and were nearly twice the numbers of listings and transplants in 2019 (Table 1). Overall, SAH listings (pre‐COVID era n = 131 vs. COVID era n = 207) and transplants (pre‐COVID era n = 110 vs. COVID era n = 168) increased by 58.0% and 52.7%, respectively. There were no differences in age or MELD‐Na scores at listing or transplant for SAH in either era.
Figure 1 depicts cumulative incidence rates for undergoing LT among patients with ALD and without ALD listed in the pre‐COVID and COVID eras, adjusted for wait‐list removal due to dropout (death or clinical deterioration) or clinical improvement. Patients with ALD listed in the pre‐COVID era had a 15% overall higher LT rate (subdistribution HR [sHR]: 1.17, 95% CI: 1.09‐1.23; P < 0.001) than patients with non‐ALD etiologies; this disparity increased during the COVID era; patients with ALD had a 50% (sHR: 1.51, 95% CI: 1.41‐1.59; P < 0.001) higher probability of LT than patients with non‐ALD etiologies. Competing risk regression models for wait‐list removal reasons including LT, dropout, and clinical improvement between eras and etiologies are given in Supporting Table S1. In addition, overall rates for wait‐list dropout and clinical improvement decreased by more than 30% (Supporting Table S1). In the pre‐COVID era, patients with ALD had 50% higher removal rate due to clinical improvement (sHR: 1.50, 95% CI: 1.17‐1.92; P = 0.001) than non‐ALD. Conversely, during the COVID era this relationship inverted with ALD experiencing a 50% lower rate for clinical improvement (sHR: 0.49, 95% CI: 0.33‐0.75; P = 0.001) compared with non‐ALD.
FIG. 1.
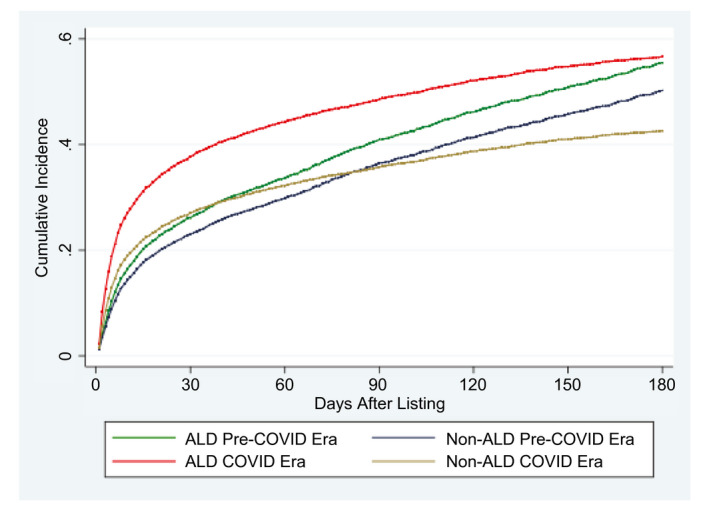
Cumulative incidence rates for LT among patients listed for ALD and non‐ALD in the pre‐COVID and COVID eras.
Temporal Trend Analyses
Table 4 lists the results of the interrupted time‐series models. The monthly rate for overall listings (mean difference −18.27 wait‐list additions per month, −1.74%; P = 0.194) and transplants (+11.04 wait‐list additions per month, +1.57%; P = 0.146) did not significantly change after COVID‐19 (Table 4). For ALD, rate for listings increased significantly in the COVID era by 7.26% (+28.56 wait‐list additions per month; P < 0.001). Similarly, rate for ALD transplants also increased by 10.26% (+27.57 transplants per month; P < 0.001). In contrast, there was a significant decline in the rate of listings in the COVID era for NASH (−13.18 wait‐list additions per month, −5.12%; P < 0.001) and HCV (−35.57 wait‐list additions per month, −21.69%; P < 0.001). NASH transplant rates (+5.01 transplants per month, +3.01%; P < 0.001) increased since the pandemic, whereas HCV transplant rates (−18.47 transplants per month, −14.22%; P < 0.001) declined further in the COVID era.
TABLE 4.
Interrupted Time Series Evaluating Monthly Rate Change for Cumulative Monthly Wait‐List Additions LTs Performed During COVID‐19 Pandemic
| Wait‐List Additions (Number per Month) | LTs (Number per Month) | |||
|---|---|---|---|---|
| Monthly Rate of Increase (95% CI) | P Value | Monthly Rate of Increase (95% CI) | P Value | |
| Overall | 0.194 | 0.146 | ||
| Predicted rate | 1,064.4 (1,057.0,1,071.9) | 701.8 (697.0, 706.7) | ||
| Actual rate | 1,046.2 (1,018.6, 1,073.8) | 712.9 (698.3, 727.5) | ||
| Change in rate | −18.3 (−46.8, 10.3) | +11.0 (−4.3, 26.4) | ||
| % change | −1.74% | +1.57% | ||
| ALD | <0.001 | <0.001 | ||
| Predicted rate | 393.2 (391.3, 395.2) | 258.4 (253.9, 262.9) | ||
| Actual rate | 421.8 (409.0, 434.6) | 286.0 (275.7, 296.2) | ||
| Change in rate | +28.6 (15.6, 41.5) | +27.6 (16.4, 38.7) | ||
| % change | +7.26% | +10.67% | ||
| NASH | 0.001 | <0.001 | ||
| Predicted rate | 257.1 (253.4, 260.9) | 166.6 (165.4, 167.8) | ||
| Actual rate | 244.0 (238.2, 249.7) | 171.6 (169.5, 173.7) | ||
| Change in rate | −13.2 (−20.1, −6.3) | +5.0 (3.1, 6.9) | ||
| % change | −5.12% | +3.01% | ||
| HCV | <0.001 | <0.001 | ||
| Predicted rate | 164.0 (157.7, 170.3) | 129.9 (128.3,131.5) | ||
| Actual rate | 128.5 (126.1, 130.9) | 111.4 (108.9, 114.0) | ||
| Change in rate | −35.6 (−42.4, −28.7) | −18.5 (−21.4, −15.5) | ||
| % change | −21.69% | −14.22% | ||
The predicted rate during the pandemic was determined using cumulative monthly counts in the pre‐COVID era from January 1, 2016, through February 28, 2020.
Sensitivity Analyses
Supporting Table S2 lists the differences in the percentage of patients with high MELD wait‐listed or transplanted within the pre‐COVID and COVID eras categorized by the duration of shelter‐in‐place state orders during the pandemic (no shelter‐in‐place order enforced, <40 days, 40‐80 days, and >80 days). The percentage of high MELD overall listings and transplants did not change significantly in the USA without an enforced shelter‐in‐place order. There was a significant increase in overall high MELD listings and transplants in states with longer shelter‐in‐place orders of 40‐80 days. With regard to ALD, both high MELD listings and transplants significantly increased with longer duration of shelter‐in‐place orders of 40‐80 days (pre‐COVID era: 23.1% vs. COVID era: 28.1%; P < 0.001) and >80 days (pre‐COVID era: 19.8% vs. COVID era: 22.2%; P < 0.001) but remained unchanged for non‐ALD. Median time from listing to transplant for patients with high MELD (at time of listing) significantly decreased among all patients, including patients with and without ALD during the COVID era, which also coincided with the start of the acuity circle allocation policy (Supporting Table S1).( 11 )
Discussion
The COVID‐19 pandemic has had a far‐reaching impact on many aspects of health and health care. We found evidence for a substantial and rising burden of ALD since the onset of the pandemic. In our comprehensive analyses of national wait‐list and transplantation data, we found that over 40% of listings were due to ALD. For the first time, ALD accounted for more listings than HCV and NASH combined. In parallel, we found a shift in the severity of liver disease at the time of listing and transplantation, MELD‐Na score at listing and transplant were significantly higher in the COVID era, and much of this trend was due to the higher severity of liver disease seen in patients with ALD. The percentage of patients with ALD listed or transplanted with a MELD‐Na > 30 significantly increased by over 15% during the pandemic. Our data also show that patients with ALD had an advantage over patients with other etiologies, but this disparity grew substantially in the COVID era. Patients with ALD had a 50% higher probability of LT rate than patients with other liver disease. Collectively, these data show that the COVID‐19 pandemic has accelerated the rising burden of ALD with substantial impact on the LT allocation system.
During the COVID era, overall MELD‐Na scores at listing and transplant increased. Over 20% of all wait‐list additions in the COVID era had a MELD‐Na score > 30. This increase in MELD‐Na and severity of hepatic decompensation at presentation and transplant was largely due to the fact that over 30% of listings and 40% of transplants for ALD had a MELD‐Na score > 30—an ominous rising trend that could suggest a larger contribution form alcohol‐associated hepatitis as well. There was also a noticeable shift in the population demographic toward a younger age and Medicaid insurance. In addition, we found that listings and transplants with a diagnosis code for SAH nearly doubled since July 2020—possibly another effect of the pandemic on unhealthy alcohol use, particularly among young adults. Several trials have demonstrated favorable outcomes of early LT for SAH.( 12 , 13 , 14 ) In light of these data, transplant centers have increasingly adopted ALD and SAH as an indication for early LT among carefully selected patients who may not have achieved 6‐month sobriety.( 15 , 16 ) However, our time‐series analysis shows that this change in clinical practice over the past few years for ALD is unlikely to explain the current trends seen during the pandemic. Instead, our data suggest a sharp rise in underlying rate of ALD and SAH during the pandemic and not just an increased consideration for early LT in patients with SAH.
In our sensitivity analyses, we found an association between longer duration of shelter in place and higher severity of disease at the time of listing for ALD. This was not the case for non‐ALD etiologies. These data provide support to the causal effect of the pandemic (and related restrictions) on the sharp rise in ALD and related hepatic decompensation. This is a cause for concern for the lack of appropriate linkage‐of‐care for patients with ALD during the pandemic. Due to “stay at home” regulations, patients with alcohol use disorder (AUD) and ALD who are high‐risk for relapse may no longer have structured non‐alcohol‐related activities and in‐person behavioral counseling programs that were previously readily available. This coupled with the delay in routine health care could have had deleterious effects on patients at risk for unhealthy alcohol use. Although there has been a call for LT to centers to adapt to the pandemic by considering leniencies for ALD LT candidates who may not have appropriate access to care, this strategy will not curtail the anticipated problem.(2) Few patients with ALD receive recommended care for AUD.( 3 ) Access to these services is ever more crucial during the pandemic. Our data call for a well‐coordinated, multidisciplinary effort involving policy stakeholders and health care providers to tackle these alarming trends. It could include use of telehealth and patient outreach programs that address AUD, while simultaneously managing liver disease. Without these efforts, AUD and ALD may have a longstanding negative effect on our health care system, including the liver allocation system, well after the pandemic has subsided.( 17 )
Our study is limited by its retrospective design, inability to evaluate onset of disease presentation, acute‐on‐chronic liver disease, and variation in policies at LT centers in the USA for ALD and SAH as an indication for LT. In February 2020, UNOS implemented a policy limiting a candidate’s maximum exception score to each center’s median MELD‐Na at LT minus 3.( 18 ) This policy was implemented to reduce the inequity in access for LT between patients with and without HCC and would prioritize non‐HCC. This policy may have an increased rate for LT among patients without HCC, including patients with ALD. However, we show no statistical difference in the percentage of patients transplanted with HCC before or during the pandemic. The acuity circle allocation policy was implemented at the start of the pandemic to help prioritize patients with high MELD for LT. In those regards, we found a significant decrease in median time from listing to transplant among patients with high MELD, regardless of etiology. This may suggest that this allocation policy may be contributing to increased LT rates for ALD during the pandemic, as ALD disproportionately had a higher percentage of patients with high MELD compared to other etiologies. Long‐term data are needed to further evaluate the effect of the acuity circle allocation policy on LT outcomes.
Due to center variability in the listing diagnosis, our analysis may actually underestimate the number of patients listed and transplanted for SAH. We were unable to evaluate specific patient and donor (offers and acceptances) characteristics, including COVID‐19 status, which are crucial to decision making for transplant programs. In addition, we were unable to evaluate center‐specific factors on decision making for candidates, which was likely heterogenous during this unprecedented pandemic. In our sensitivity analyses, we categorized the duration of shelter‐in‐place orders according to the state for each transplant center. We acknowledge the caveat that some patients may reside in a different state from their transplant center, which may confound our findings regarding the association between duration of shelter‐in‐place orders and high MELD listings.
In summary, there has been an unprecedented rise in rates of listings and transplants for ALD since the beginning of COVID‐19 pandemic. ALD has accounted for more listings than NASH and HCV combined. The higher severity of liver disease at listing and an increasing proportion of young adults among wait‐listed patients are ominous signs and suggest a higher contribution from severe alcohol‐associated hepatitis than indicated by UNOS data. Innovative health care modalities, such as telemedicine and remote health monitoring, could potentially be leveraged to address problems of excessive at‐home drinking that is likely taking place. From a broader perspective, COVID‐19‐related stressors like unemployment, which have been associated with increased alcohol use, should also be carefully considered when caring for patients. Clinicians should ensure that patients with ALD are receiving appropriate and timely linkage‐to‐care, including referral to transplantation for those meeting clinical criteria for LT. Our analysis might only be showing the early effects of increased alcohol consumption that began at the start of the pandemic, and the impact of unhealthy alcohol use may be felt for several years to come.
Author Contributions
K.G., G.C., A.R, and F.K. contributed to the study concept and design, literature review and analysis, drafting and critical revision and editing, and approval of the final version. K.G. analyzed all of the data. P.J, R.H, A.P., B.L.D., S.K.S., D.K., A.A., and J.G. assisted in manuscript preparation and critical appraisal of the manuscript.
Supporting information
Supplementary Material
Acknowledgment
The authors acknowledge UNOS, a non‐profit organization that administrates the Organ Procurement and Transplantation Network, for providing us with a custom database from which their data were collected and analyzed. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the United Network for Organ Sharing/Organ Procurement and Transplantation Network or the U.S. Government.
Potential conflict of interest: Nothing to report.
SEE EDITORIAL ON PAGE 2948
References
Authors names in bold designate shared co‐first authorship.
- 1. Cholankeril G, Podboy A, Alshuwaykh OS, Kim D, Kanwal F, Esquivel CO, et al. Early impact of COVID‐19 on solid organ transplantation in the United States. Transplantation 2020;104:2221‐2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Da BL, Im GY, Schiano TD. Coronavirus disease 2019 hangover: a rising tide of alcohol use disorder and alcohol‐associated liver disease. Hepatology 2020;72:1102‐1108. [DOI] [PubMed] [Google Scholar]
- 3. Barbosa C, Cowell AJ, Dowd WN. Alcohol consumption in response to the COVID‐19 pandemic in the United States. J Addict Med 2020;15:341‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grossman ER, Benjamin‐Neelon SE, Sonnenschein S. Alcohol consumption during the COVID‐19 pandemic: a cross‐sectional survey of US adults. Int J Environ Res Public Health 2020;17:9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weerakoon SM, Jetelina KK, Knell G. Longer time spent at home during COVID‐19 pandemic is associated with binge drinking among US adults. Am J Drug Alcohol Abuse 2021;47:98‐106. [DOI] [PubMed] [Google Scholar]
- 6. Cholankeril G, Ahmed A. Alcoholic liver disease replaces hepatitis C virus infection as the leading indication for liver transplantation in the United States. Clin Gastroenterol Hepatol 2018;16:1356‐1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cholankeril G, Gadiparthi C, Yoo ER, Dennis BB, Li AA, Hu M, et al. Temporal trends associated with the rise in alcoholic liver disease‐related liver transplantation in the United States. Transplantation 2019;103:131‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol 2017;46:348‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McDonald EG, Dendukuri N, Frenette C, Lee TC. Time‐series analysis of health care‐associated infections in a new hospital with all private rooms. JAMA Intern Med 2019;179:1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Raifman JNK, Jones D, Bor J, Lipson S, Jay J, Chan P. COVID‐19 US state policy database. c2021. www.tinyurl.com/statepolicies. Accessed April 26, 2021.
- 11. Chyou D, Karp S, Shah MB, Lynch R, Goldberg DS. A 6‐month report on the impact of the organ procurement and transplantation network/united network for organ sharing acuity circles policy change. Liver Transpl 2021;27:756‐759. [DOI] [PubMed] [Google Scholar]
- 12. Louvet A, Mathurin P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol 2015;12:231‐242. [DOI] [PubMed] [Google Scholar]
- 13. Mathurin P, Moreno C, Samuel D, Dumortier J, Salleron J, Durand F, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med 2011;365:1790‐1800. [DOI] [PubMed] [Google Scholar]
- 14. Lee BP, Samur S, Dalgic OO, Bethea ED, Lucey MR, Weinberg E, et al. Model to calculate harms and benefits of early vs delayed liver transplantation for patients with alcohol‐associated hepatitis. Gastroenterology 2019;157:472 ‐ 480.e475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bangaru S, Pedersen MR, MacConmara MP, Singal AG, Mufti AR. Survey of liver transplantation practices for severe acute alcoholic hepatitis. Liver Transpl 2018;24:1357‐1362. [DOI] [PubMed] [Google Scholar]
- 16. Puri P, Cholankeril G, Myint TY, Goel A, Sarin SK, Harper AM, et al. Early liver transplantation is a viable treatment option in severe acute alcoholic hepatitis. Alcohol Alcohol 2018;53:716‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tapper EB, Asrani SK. The COVID‐19 pandemic will have a long‐lasting impact on the quality of cirrhosis care. J Hepatol 2020;73:441‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Durkin C, Kaplan DE, Bittermann T. T2 hepatocellular carcinoma exception policies that prolong waiting time improve the use of evidence‐based treatment practices. Transplant Direct 2020;6:e597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


