To The Editor,
Increasing detection of reinfections and waning neutralizing antibody (Nab) titers as early as 23 days following initial infection1 raises concerns for herd immunity and the durability of vaccine efficacy.2, 3 Since the first reported reinfection case in August 2020,4 at least 70 confirmed cases have emerged as of April 27, 2021.5 In October 2020, the US Centers for Disease Control and Prevention (CDC) published investigative criteria for suspected SARS‐CoV‐2 reinfections.6 These criteria included any individuals testing positive ≥90 days after their first laboratory‐confirmed SARS‐CoV‐2 infection or symptomatic individuals testing positive 45–89 days after initial infection with paired respiratory specimens.6 Here, we describe a patient infected with two genetically distinct SARS‐CoV‐2 strains detected 19 days apart, indicating that reinfection can occur within a short period.
Ninety‐two SARS‐CoV‐2 positive nasopharyngeal samples (CDC 2019 Novel Coronavirus Real‐Time Reverse Transcriptase‐PCR Diagnostic Panel7) were collected in Columbia, Missouri from March to May 2020. Two samples, collected 19 days apart, were from the same patient. SARS‐CoV‐2 virus isolates were recovered from each of the two samples. The SARS‐CoV‐2 viruses from both clinical swabs were sequenced using Access Array microfluidic (Fluidigm Corporation) and MiSeq systems (Illumina).8 Phylogenetic analyses were performed using BEAST2 (see Supporting Information Appendix for Materials and Methods).
This patient was a female in her 20 s with asthma, obesity, anxiety, and depression, who reported cough, chills, exertional dyspnea, sore throat, dizziness, rhinorrhea, and fever during her initial COVID‐19 diagnosis in March 2020. She tested positive 1 day after symptom onset and was instructed to self‐isolate at home. Nineteen days following her initial positive test, she returned for another COVID‐19 test due to return‐to‐work requirements. Despite her symptoms waning to encompass only productive cough and fatigue, she tested COVID‐19 positive again. She continued to experience persistent cough, fatigue, and dyspnea until 55 days after her initial positive test.
Phylogenetic analyses showed that the two samples contained SARS‐CoV‐2 viruses from two distinct lineages (Figure 1); Sample 1 (GenBank accession No.: MW521480.1; cycle threshold [C t] value = 17.76) belonged to the PANGOLIN A.3 lineage, whereas the Sample 2 (MW521502.1; C t value = 20.36) belonged to the PANGOLIN B.1.1 lineage. Additionally, we compared the sequences between viral isolates and clinical samples. Results showed that sequences from each isolate were identical to the corresponding clinical sample, but those at the first sample and at the second sample were distinct. The virus sequences had 21 nucleotide substitutions relative to each other, encoding 11 nonsynonymous amino acid mutations across five genes (ORF1ab (D75E on nonstructural protein 1 (NSP1), P971L on NSP3, P4715L on NSP12, F6158L on NSP14), ORF8 (V62L, L84S), ORF7a (S81L) ORF10 (I4L), S (D614G) and N (R202K, G203R)). The average sequence depth was 3960 (Day 1 virus) and 3233 (Day 19) reads, and each of those 21‐variation positions had a minimum raw read depth of 1978 reads (Table 1). No diverse polymorphisms were identified among the sequences of the viruses from each clinical sample, suggesting true reinfection rather than a coinfection.
Figure 1.
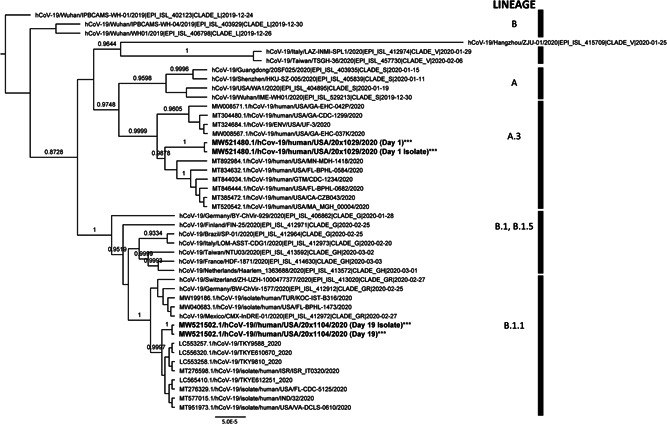
Phylogenetic analyses of SARS‐CoV‐2 viruses from a patient reinfected within 19 days. Bayesian tree of SARS‐CoV‐2 viruses with a reinfection case in our study rooted to hCoV‐19/Wuhan/PBCAMS‐WH‐01/2019 (EPI ISL 402123). The nomenclature of genetic clades was adapted from the PANGOLIN (Phylogenetic Assignment of Named Global Outbreak LINeages) software. Annotated with taxa names and posterior probabilities >0.70. Beast with a HKY substitution model (k = 2.0) with empirical frequencies, strict clock model, and Coalescent Constant Population prior was used. MCMC was used with a chain length of 500,000,000 stored every 50,000 and pre‐burn‐in of 10%. The results were analyzed in Tracer v1.7.1 and convergence was assessed with a cutoff of 200 for the ESS. The consensus tree was generated using TreeAnnotator v2.6.3.0. The trees were visualized with FigTree v1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/). All posterior probabilities of >70%. ESS, effective sample size; HKY, Hasegawa‐Kishino‐Yano; MCMC, Markov chain Monte Carlo
Table 1.
Pairwise comparison of nucleotide and amino acid substitutions
| Gene | 5ʹ‐UTR** | ORF1ab | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NSP1 | NSP3 | NSP4 | NSP12 | NSP14 | |||||||
| Sample | Ct‐Value | Nucleotide Position | 160 | 241 | 313 | 490 | 3037 | 3177 | 8782 | 14,408 | 18,736 |
| Amino Acid Position | NA | NA | 47 | 75 | 924 | 971 | 2839 | 4715 | 6158 | ||
| SARS‐CoV‐2/human/USA/20×1029/2020 (Day 1) | 17.8 | Nucleotide (% reads*) | G (99.82) | C (99.74) | C (99.36) | A (99.09) | C (99.74) | T (99.46) | T (99.38) | C (99.94) | C (99.76) |
| Amino Acid | NA | NA | K | E | F | L | S | P | L | ||
| Sequence Coverage (reads) | 2855 | 4252 | 4892 | 4619 | 5395 | 6310 | 4259 | 3363 | 3034 | ||
| SARS‐CoV‐2/human/USA/20×1104/2020 (Day 19) | 20.4 | Nucleotide (% reads*) | T (99.36) | T (99.12) | T (99.29) | T (99.29) | T (99.70) | C (99.82) | C (99.59) | T (99.75) | T (99.70) |
| Amino Acid | NA | NA | K | D | F | P | S | L | F | ||
| Sequence Coverage (reads*) | 2372 | 3422 | 3972 | 3832 | 4376 | 5187 | 3934 | 2902 | 2396 | ||
| Gene | S | M | ORF7a | ORF8 | N | ORF10 | 3ʹ‐UTR** | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Ct‐Value | Nucleotide Position | 21,658 | 23,403 | 24,034 | 26,729 | 27,635 | 28,077 | 28,144 | 28,881 | 28,882 | 28,883 | 29,567 | 29,700 |
| Amino Acid Position | 32 | 614 | 824 | 69 | 81 | 62 | 84 | 203 | 203 | 204 | 4 | NA | ||
| SARS‐CoV‐2/human/USA/20×1029/2020 (Day 1) | 17.8 | Nucleotide (% reads*) | T (99.80) | A (99.52) | T (99.77) | C (99.74) | T (99.81) | C (99.79) | C (99.30) | G (99.87) | G (99.86) | G (99.38) | C (99.76) | G (99.26) |
| Amino Acid | F | D | N | A | L | L | S | R | R | G | L | NA | ||
| Sequence Coverage (reads) | 7016 | 2538 | 4482 | 4256 | 4852 | 3935 | 5331 | 2316 | 2299 | 2293 | 5025 | 3260 | ||
| SARS‐CoV‐2/human/USA/20×1104/2020 (Day 19) | 20.4 | Nucleotide (% reads*) | C (99.68) | G (99.30) | C (99.52) | T (99.56) | C (99.62) | G (99.59) | T (99.82) | A (99.29) | A (99.34) | C (99.39) | A (99.48) | A (99.73) |
| Amino Acid | F | G | N | A | S | V | L | K | K | R | I | NA | ||
| Sequence Coverage (reads*) | 6328 | 2161 | 3812 | 3665 | 3972 | 3247 | 4446 | 1989 | 1985 | 1978 | 4108 | 2647 | ||
Note: Variants were identified using CLC Workbench and validated using Bowtie 2. Sequence coverage and read counts were calculated using pysamstats. SARS‐CoV‐2/human/USA/20×1029/2020 (GenBank Accession No: MW521480.1). SARS‐CoV‐2/human/USA/20×1104/2020 (GenBank Accession No: MW521502.1).
Abbreviations: M, membrane glycoprotein; NA, not applicable; NSP, nonstructural protein; ORF, open reading frame; S, surface glycoprotein; UTR, untranslated region.
% Depth calculated by (read count)/coverage × 100.
Noncoding region.
This report is limited by the unavailability of sera samples to study Nab titers and lack of information regarding the patient's potential contacts with others during the 2‐week isolation period. Nevertheless, this case showed a patient who unknowingly became reinfected with two genetically distinct viruses within 19 days and may have still been infectious after the CDC‐recommended 10 day isolation period.9 Additionally, the CDC has encouraged symptom‐based strategies for ending isolation rather than viral retesting for asymptomatic individuals or for individuals without new symptoms during 90 days after illness onset due to findings that detectable but noninfectious SARS‐CoV‐2 RNA can persist in respiratory samples.9 Larger studies are necessary to test whether the prevalence of reinfection within a short period is high, as shown in this case; if yes, this may pose a challenge of infection control, especially as variants of concern continue to emerge and immune evasion increases despite vaccination efforts.
Reinfections are likely underreported due to lack of multiple sample collections and sequencing from the same individuals. A pressing question remains of whether immunity developed from initial infection protects against other strains. The E484K spike mutation, present in the B.1.351 and P.1 variants of concern, has raised fears over their potential to impact immune escape and reinfection.10 With the mass rollout of COVID‐19 vaccinations, other urgent unknowns include the true occurrence of reinfection, the health impact of subsequent infections, and the duration of immunity generated from infections and vaccinations. Expanding sequencing and surveillance of COVID‐19 reinfections will help address many of these questions.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Xiu‐Feng Wan and Cynthia Y. Tang conceived this study, designed the analysis, and wrote the paper. Tao Li, Yang Wang, and Cynthia Y. Tang collected the data. Jun Hang, Richard Hammer, Detlef Ritter, and Grace M. Lidl contributed data or analysis tools. Cynthia Y. Tang performed the data analyses. Yang Wang, Jane A. McElroy, Richard Hammer, Detlef Ritter, Grace M. Lidl, Richard Webby, and Jun Hang revised the paper.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
We thank Karen Segovia, Simone Camp, and Michelle Beckwith for help with samples. The opinions expressed are the private views of the authors and are not to be conveyed as official or signifying the views of the Department of the Army or the Department of Defense. This study was supported by the National Institutes of Health (5T32LM012410) and the Global Emerging Infections Surveillance Branch of the Armed Forces Health Surveillance Division (ProMIS ID P0140_20_WR_01.Global).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in GenBank: The GenBank Accession Numbers are MW521480.1 (Sample 1) and MW521502.1 (Sample 2).
REFERENCES
- 1.Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS‐CoV‐2 infection in humans. Nat Microbiol. 2020;5(12):1598‐1607. 10.1038/s41564-020-00813-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen JI, Burbelo PD. Reinfection with SARS‐CoV‐2: Implications for Vaccines [Epub ahead of print]. Clin Infect Dis. 2020:ciaa1866. 10.1093/cid/ciaa1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crawford KHD, Dingens AS, Eguia R, et al. Dynamics of neutralizing antibody titers in the months after SARS‐CoV‐2 infection. J Infect Dis. 2020;223:197‐205. 10.1093/infdis/jiaa618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.To KK, Hung IF, Ip JD, et al. COVID‐19 re‐infection by a phylogenetically distinct SARS‐coronavirus‐2 strain confirmed by whole genome sequencing [Epub ahead of print]. Clin Infect Dis. 2020:ciaa1275. 10.1093/cid/ciaa1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.COVID‐19 Reinfection Tracker . 2020 August 28. https://bnonews.com/index.php/2020/08/covid-19-reinfection-tracker/. Accessed April 27, 2021.
- 6.Investigative criteria for suspected cases of SARS‐CoV‐2 reinfection (ICR) . 2020 October 27. https://www.cdc.gov/coronavirus/2019-ncov/php/invest-criteria.html. Accessed March 23, 2021.
- 7.Lu X, Wang L, Sakthivel SK, et al. Panel for detection of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(8):1654‐1665. 10.3201/eid2608.201246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li T, Chung HK, Pireku PK, et al. Rapid high throughput whole genome sequencing of SARS‐CoV‐2 by using one‐step RT‐PCR amplification with integrated microfluidic system and next‐gen sequencing. J Clin Microbiol. 2021;59(5). 10.1128/JCM.02784-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nonaka CKV, Franco MM, Gräf T, et al. Genomic evidence of SARS‐CoV‐2 reinfection involving E484K spike mutation, Brazil. Emerg Infect Dis. 2021;27(5):1522‐1524. 10.3201/eid2705.210191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) . 2021 February 13. Interim guidance on duration of isolation and precautions for adults with COVID‐19. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html. Accessed March 23, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are openly available in GenBank: The GenBank Accession Numbers are MW521480.1 (Sample 1) and MW521502.1 (Sample 2).


