Abstract
Anti‐severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) immunoglouilin G (IgG) and immunoglouilin M (IgM) antibodies have been widely used to assist clinical diagnosis. Our previous study reported a discrepancy in SARS‐CoV‐2 antibody response between male and female coronavirus disease 2019 (COVID‐19) patients. However, the duration and discrepancy between ages as well as sexes of SARS‐CoV‐2 antibody in convalescent COVID‐19 patients have not been clarified. In this study, a total of 538 health‐examination individuals who were confirmed with SARS‐CoV‐2 infection a year ago were enrolled. Blood samples were collected and detected for IgM and IgG antibodies. Among these convalescent patients, 12.80% were detected positive for IgM antibodies. The positive rates for IgM antibody were close between sexes: for males, this is 9.17% and for females 13.75%. However, the IgG antibody was detected positive in as much as 82.90% convalescent patients and the positive rates were nearly the same between males (82.57%) and females (82.98%). Besides this, the level of IgM and IgG antibodies showed no difference between male and female convalescent patients. The level of IgG antibodies showed a significant difference between ages. The elder patients (over 35 years old) maintained a higher level of IgG antibody than the younger patients (under or equal 35 years old) after recovering for 1 year. In addition, IgG antibody was more vulnerable to disappear in younger patients than in elder patients. Overall, our study identified over 1‐year duration of SARS‐CoV‐2 antibody and age difference of IgG antibody response in convalescent COVID‐19 patients. These findings may provide new insights into long‐term humoral immune response, vaccines efficacy and age‐based personalized vaccination strategies.
Keywords: age difference of SARS‐CoV‐2 antibody response, COVID‐19 convalescent patients, SARS‐CoV‐2 antibody duration
Highlights
Anti‐SARS‐CoV‐2 IgG antibody could maintain over 1‐year in most convalescent COVID‐19 patients.
Anti‐SARS‐CoV‐2 IgG antibody responses may be different between young and elder convalescent COVID‐19 patients.
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is still ongoing and has caused substantial morbidity and mortality. 1 As of May 31, 2021, more than 171,000,000 confirmed cases and 3,500,000 deaths had been reported worldwide. To control the spread of SARS‐CoV‐2 infection, serum rapid detection of SARS‐CoV‐2 specific immunoglobulin M (IgM) and immunoglobulin G (IgG) antibodies have been widely applied in the clinic to assist diagnosis of infection. 2 , 3 , 4 Owing to its high sensitivity, serum SARS‐CoV‐2 antibody detection plays important roles in the identification and isolation of COVID‐19 patients. 5
The levels of IgM and IgG antibodies against SARS‐CoV‐2 nucleoprotein and surface spike protein receptor‐binding domain increase gradually after infection. 6 The IgM antibody is detectable as early as 5 days after symptom onset and the IgG antibody is detectable in one week after symptom onset. 7 It was reported that the IgM antibody level peaked in 2 weeks and then began to decline and disappear, whereas the IgG antibody level continued to go up, reached its peak in 3 weeks, and maintained at a high level beyond 7 weeks. 3 , 8 However, whether the IgM and IgG could persist for a longer time is unclear. Our previous study identified a discrepancy in the SARS‐CoV‐2 IgG antibody response between male and female COVID‐19 patients, 8 while little is known about the SARS‐CoV‐2 antibodies response in convalescent patients between different sexes as well as ages.
In this study, a total of 538 convalescent COVID‐19 patients who came to the Union Hospital for a 1‐year annal health examination were enrolled. Blood samples were collected from all these individuals and detected for SARS‐CoV‐2 specific IgM and IgG antibody. By analyzing SARS‐CoV‐2 antibodies, we identified that 12.80% convalescent patients remained IgM antibody positive and as much as 82.90% convalescent patients still persisted as IgG antibody positive after 1 year of recovery, and no significant difference were observed in antibodies positive rates and average levels between male and female convalescent patients. Noteworthily, we found that the IgG antibody level in younger convalescent patients was significantly lower than elder convalescent patients and the negative rate of IgG antibody in younger patients was higher than elder patients after 1 year of recovery. These findings of our study may be helpful to understand the long‐term duration of humoral immune response, herd immunity and the efficacy of vaccines, which are major concerns in controlling the COVID‐19 pandemic.
2. MATERIALS AND METHODS
2.1. Patients
A total of 538 COVID‐19 convalescent patients were enrolled in this study. All the patients were once confirmed as COVID‐19 patients, hospitalized during the epidemic peak period, and discharged from the hospital between late February to March 2020. All these cases enrolled in this study were derived from Wuhan Union Hospital who came to the hospital for a 1‐year annual health examination between March 11 and March 19. The general information of patients was extracted from the electronic medical records system. This study was approved by the Ethics Committee of Wuhan Union Hospital, Tongji Medical College, Huazhong University of Science and Technology.
2.2. Anti‐SARS‐CoV‐2 IgM and IgG antibodies detection
Blood samples from COVID‐19 convalescent patients were centrifuged at room temperature after coagulation. Serum supernatant was then obtained and used for antibody detection. Anti‐SARS‐CoV‐2 IgM and IgG antibodies were detected by chemiluminescent immunoassay using an iFlash 3000 automated chemiluminescent immune analyzer (YHLO Biotechnology Co., Ltd.). The matched regent for detecting SARS‐CoV‐2 IgM and IgG antibodies was developed based on magnetic particle and recombinant protein containing spike protein and nucleocapsid protein of SARS‐CoV‐2. The cat number of SARS‐CoV‐2 IgM and IgG antibodies detecting kit is C86095M and C86095G (YHLO Biotechnology Co., Ltd.). This SARS‐CoV‐2 antibody detecting system has been widely used in clinics and medical research. 9 , 10 , 11 , 12 Briefly, serum and SARS‐CoV‐2 antigen‐coated magnetic particles were incubated for 20 min. After washing, the mouse anti‐human IgM or IgG antibody labeled with acridinium was added and incubated for 4 min. Following another washing cycle, pre‐trigger, and trigger solutions were added to the reaction mixture. The luminescence intensity was then measured and automatically transformed to antibody level based on the standard curve in the chemiluminescent immune analyzer. The cutoff value of IgM and IgG antibodies was 10 AU/ml according to the instruction of the kits. All operations in the detection were in strict accordance with the Standard Operation Procedure.
2.3. Statistics
In this study, GraphPad 6.0 (GraphPad Software Inc.) was applied for mapping and data statistical analysis. The positive rates of IgM and IgG antibodies were analyzed by χ 2 test. The levels of IgM and IgG antibodies were presented as mean ± SEM and analyzed by using one‐way snalysis of variance followed by Dunnett's test or Mann–Whitney U test as indicated in the figure legends. A p value of less than 0.05 was considered statistically significant.
3. RESULT
3.1. Demographic of enrolled COVID‐19 convalescent patients and positive rates of anti‐SARS‐CoV‐2 IgM and IgG antibodies
A total number of 538 convalescent COVID‐19 patients were enrolled in this study. All these patients were once infected with SARS‐CoV‐2 and had recovered for 1 year. Among these patients, males and females were 109 (20.26%) and 429 (79.74%), respectively. The age distribution for male patients and female patients were similar: for male the median age was 37 (interquartiel range [IQR], 33–46) years old, and for female the median age was 36 (IQR, 30–45) years old. For health examination, blood samples were collected from all these convalescent patients and tested for anti‐SARS‐CoV‐2 specific IgM and IgG antibodies. The positive rates of anti‐SARS‐CoV‐2 specific IgM and IgG antibodies are shown in Table 1. The IgM antibody was detected positive in 12.80% of all the convalescent patients. The positive rate of IgM antibody for males was 9.17% and for females was 13.75%. However, IgG antibody was detected positive in as much as 82.90% of these convalescent patients. The positive rates for IgG antibodies were 82.57% for male patients and 82.98% for female patients. The positive rates for both IgM and IgG antibodies in male and female patients were the same as the positive rates of IgG antibody alone. Statistically, no significant difference was observed in IgM and IgG antibodies positive rates between male and female patients. Together, this data indicated that SARS‐CoV‐2 specific IgG or IgM antibody could maintain positivity for over 1 year in most of the convalescent patients, and only a small part of patients with antibodies turned to negative.
Table 1.
Demographic of enrolled COVID‐19 convalescent patients and positive rates of SARS‐CoV‐2 IgM and IgG antibodies
| Total | Male | Female | p value | |
|---|---|---|---|---|
| n (%) | 538 | 109 (20.26) | 429 (79.74) | |
| Age, median (IQR) | 36 (31–45) | 37 (33–46) | 36 (30–45) | |
| IgM positive (%) | 69 (12.80) | 10 (9.17) | 59 (13.75) | 0.201 |
| IgG positive (%) | 446 (82.90) | 90 (82.57) | 356 (82.98) | 0.918 |
| Both IgM and IgG positive (%) | 69 (12.80) | 10 (9.17) | 59 (13.75) | 0.201 |
Note: Age is expressed as median (inter‐quartile range [IQR]). The number and percentage of convalescent patients with antibody positive are expressed as n (%). Statistical analyses were performed by χ 2 test.
Abbreviation: IgG, immunoglobulin G; IgM, immunoglobulin M; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
3.2. The levels of SARS‐CoV‐2 IgG and IgM antibodies showed no difference between female and female convalescent COVID‐19 patients
Our previous study reported a different antibody response between male and female COVID‐19 patients, while whether the SARS‐CoV‐2 antibody response works differently in convalescent patients remains unclear. 8 To clarify this, the antibody detecting results of all enrolled convalescent patients were used for analysis. The IgM antibody levels of most convalescent patients were below the cutoff value (10 AU/ml). The average levels of SARS‐CoV‐2 IgM antibody were around 5 AU/ml and showed no difference between male and female convalescent patients (Figure 1A). However, the IgG antibody levels of most convalescent patients were above the cutoff value. The average levels of SARS‐CoV‐2 IgG antibody were about 70 AU/ml and there being no significant difference between sexes as well (Figure 1B). To further determine the potential difference, male and female patients were divided into eight groups (21–25, 26–30, 31–35, 36–40, 41–45, 46–50, 51–55, and 56–66) according to age. The result showed that in all the eight groups, no significant difference was observed for IgM antibody levels, and so too for IgG antibody levels (Figure 1C,D). The above analysis indicated that there was no difference in IgM and IgG antibody levels between male and female convalescent COVID‐19 patients who have recovered for 1 year.
Figure 1.
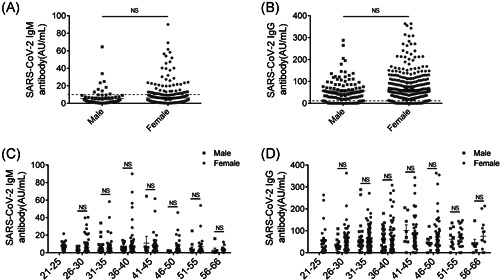
No difference was showed for SARS‐CoV‐2 IgM and IgG antibodies between female and female convalescent COVID‐19 patients. (A) The levels of SARS‐CoV‐2 IgM antibody in male and female convalescent COVID‐19 patients. n (male) = 109, n (female) = 429. Black horizontal dotted line represented cutoff value, 10 AU/ml. (B) The levels of SARS‐CoV‐2 IgG antibody in male and female convalescent COVID‐19 patients. n (male) = 109, n (female) = 429. Black horizontal dotted line represented cutoff value, 10 AU/ml. (C) Comparative analysis of SARS‐CoV‐2 IgM antibody level in different age groups between male and female convalescent COVID‐19 patients. (D) Comparative analysis of SARS‐CoV‐2 IgG antibody level in different age groups between male and female convalescent COVID‐19 patients. All the enrolled convalescent patients were divided into eight groups (21–25, 26–30, 31–35, 36–40, 41–45, 46–50, 51–55, and 56–66) according to age. Data are expressed as mean ± SEM and analyzed by Mann–Whitney U test. NS, No Significance, p > 0.05. COVID‐19, coronavirus disease 2019; IgG, immunoglobulin G; IgM, immunoglobulin M; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
3.3. The SARS‐CoV‐2 IgG antibody presented a lower level and a higher negative rate in the younger convalescent patients than the elder convalescent patients
Next, male and female patients were grouped together to analyze the antibody dynamics going with age. The results showed that the average level of SARS‐CoV‐2 IgM antibody was stable, with slight fluctuations around 5 AU/ml, and no significant differences were observed between any of the age groups (Figure 2A). However, the levels of SARS‐CoV‐2 IgG antibody showed significant differences in patients of different ages. The average level of IgG antibody was relatively low, about 50 AU/ml, in the younger (21–35 years old) convalescent patients and gradually increased to about 80 AU/ml in the elder patients (Figure 2B). Notably, the average levels of IgG antibody for the three younger groups (21–25, 26–30, and 31–35) were all less than 60 AU/ml, while for the elder groups (36–40, 41–45, 46–50, 51–55, and 56–66), the average levels of IgG antibody were all more than 60 AU/ml (Figure 2B). All convalescent patients were then divided into two groups based on the levels of the SARS‐CoV‐2 IgG antibody. Further analysis showed that the average level of SARS‐CoV‐2 IgG antibody in patients under or equal 35 years old was significantly lower than that of patients over 35 years old (Figure 2C). In addition, among patients under or equal 35 years old, the percentage of SARS‐CoV‐2 IgG negative was much higher than that of patients over 35 years old, although no statistical difference was observed (Figure 2D). This data indicated that the SARS‐CoV‐2 IgG antibody in elder convalescent patients was more stable and persisted at a higher level than the younger convalescent patients after recovery for 1 year.
Figure 2.
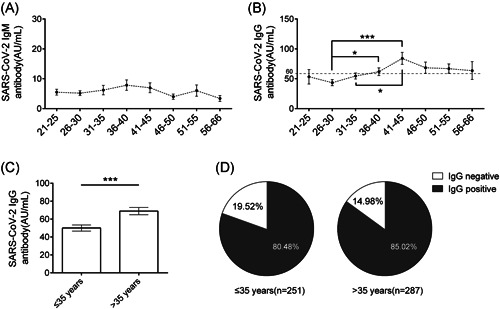
A lower level and a higher negative rate of SARS‐CoV‐2 IgG antibody were observed in the younger convalescent patients than the elder convalescent patients. (A) Dynamics of SARS‐CoV‐2 IgM antibody level in convalescent COVID‐19 patients in different ages. All convalescent patients were divided into eight groups (21–25, 26–30, 31–35, 36–40, 41–45, 46–50, 51–55, and 56–66) according to age. (B) Dynamics of SARS‐CoV‐2 IgG antibody level in convalescent COVID‐19 patients in different ages. Black horizontal dotted line represented 60 AU/ml. (C) The levels of SARS‐CoV‐2 IgG antibody level in younger (under or equal 35 years old, n = 251) and elder (over 35 years old, n = 287) convalescent COVID‐19 patients. (D) The percentage of convalescent patients with SARS‐CoV‐2 IgG antibody negative and IgG antibody positive. Data are expressed as mean ± SEM and analyzed by one‐way ANOVA followed by Dunnett's test (B) or Mann–Whitney U test(C). *p < 0.05, **p < 0.01, ***p < 0.001. ANOVA, analysis of variance; COVID‐19, coronavirus disease 2019; IgG, immunoglobulin G; IgM, immunoglobulin M; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
4. DISCUSSION
Monitoring the humoral immune response and its duration after SARS‐CoV‐2 infection is essential for the assessment of reinfection risk and evaluation of vaccine efficacy. However, the duration of humoral immune response after SARS‐CoV‐2 infection has not been well elucidated. The rapid decline of SARS‐CoV‐2 antibody levels in some patients had heightened public concerns about the long‐term effectiveness of the COVID‐19 vaccine. 13 A previous study reported that the SARS‐CoV‐2 IgG antibody could persist for 6 months in convalescent patients, 14 while the sample size enrolled in this study was a little small. In our study, by detecting serum SARS‐CoV‐2 antibodies of 538 convalescent COVID‐19 patients who once were hospitalized during the epidemic peak period and discharged in Wuhan, we found that although at a low level, 12.80% of convalescent patients still detected positive for SARS‐CoV‐2 IgM antibody. Notably, the SARS‐CoV‐2 IgG antibody detected positive in as much as 82.90% of convalescent patients and maintained relatively high levels. Our study for the first time identified that SARS‐CoV‐2 infection induced specific antibodies responses in convalescent COVID‐19 patients could persist for over 1 year in more than 80% individuals. As the vaccines and natural viral infections have similar immune mechanisms, such a long duration of antibodies in convalescent patients provides insight into evaluating the long‐term efficacy of COVID‐19 vaccines. However, as SARS‐CoV‐2 is a new virus for us, the SARS‐CoV‐2 antibody response still needs further monitoring.
Our study may provide new insights into COVID‐19 vaccination strategies for people of different ages. To control this unprecedented pandemic, more than 300 vaccines are now under development and 9 of them have already been approved for emergency use in some countries. 15 , 16 The COVID‐19 vaccine was able to induce humoral responses which neutralizes SARS‐CoV‐2 and thus prevents further morbidity and mortality. 17 , 18 To gain a robust humoral response against SARS‐CoV‐2 infection, an effective and proper vaccination strategy is required. However, the vaccination strategy on different population groups is rarely reported. Presently, the approved vaccine, BNT162b2 messenger RNA vaccine 19 and inactivated vaccine 20 for example, followed a common recommended vaccination dose and interval time for all the people regardless of age and gender. In this study, we identified that the IgG antibody level in elder convalescent patients was much higher than that of in young convalescent patients, which implies that the humoral immune response may be different between younger and elder individuals after SARS‐CoV‐2 infection. Previous studies reported that the duration of SARS‐CoV‐2 clearance was significantly longer in the elderly patients than in the younger patients. 21 , 23 The longer‐term presence of viral antigen in elder patients may be a reason causing such difference in antibody response among patients of different ages. In addition, we found that the IgG antibody in younger individuals was more likely to turn negative than elder individuals after more than 1 year of infection. These findings of our study indicated that the humoral immune response and the protection time in younger individuals may differ with elder individuals after COVID‐19 vaccination. Therefore, different interval times, vaccination doses, and vaccination times should be taken into consideration when vaccinating people of different ages. The humoral immune response after vaccination and specific vaccination strategy for people in different age groups needs further clinical investigation.
A previous study showed that SARS‐CoV‐2 IgM peaked in the 2nd week after the onset of symptoms, and then decreased until disappeared in the 16th week after the onset of symptoms. 24 In this study, we reported that the SARS‐CoV‐2 IgM persisted positive in 12.80% convalescent COVID‐19 patients after recovery for 1 year. Not only in our study, Chuanmiao Liu and colleagues reported that 22.7% of convalescent patients still persisted as IgM antibody positive after 6 months of recovery. 14 Generally, a high level of IgM antibody was considered as an indicator of acute infection or recent reinfection and IgG antibody was regarded as a marker of previous infection. However, in our study, we found that as much as 10 (1.86%) convalescent COVID‐19 patients still detected SARS‐CoV‐2 IgM antibody over 50 AU/ml after 1 year of recovery. Thus, it is not reliable and proper to assist clinical diagnosis of infection only by IgM detecting results. The reason underlying such a long duration of SARS‐CoV‐2 IgM antibody in some convalescent patients is unclear and needs further study.
To our knowledge, this study may have one of the longest durations reported about SARS‐CoV‐2 antibody. Based on a large number of convalescent COVID‐19 patients, we identified that SARS‐CoV‐2 specific IgG antibody was able to persist more than 1 year in most of the convalescent patients, while the IgM antibody disappeared in most of the patients after 1 year of recovery. The IgM and IgG antibodies levels showed no difference between male and female patients. The younger patients maintained a lower level and shorter duration of IgG antibodies than the elder patients. These findings of our study may provide new insights into the long protection of humoral immunity, the risk of reinfection, efficacy of vaccines and vaccination strategy for people in different age groups, which are critical to achieve herd immunity in the current stage of the COVID‐19 pandemic.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Fanfan Zeng, Jinbiao Wang, Jianyu Li, and Guoyun Hu contributed to the detection of serum SARS‐CoV‐2 antibodies. Fanfan Zeng and Mengjun Wu analyzed the data and wrote the manuscript. Lin Wang conceived and supervised the project.
5.
ACKNOWLEDGMENTS
The authors would like to thank all of the doctors and nurses who contributed to blood sample collection and transportation, as well as all the convalescent patients involved in this study. The project was supported by the Department of Clinical Laboratory, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology.
Zeng F, Wu M, Wang J, Li J, Hu G, Wang L. Over 1‐year duration and age difference of SARS‐CoV‐2 antibodies in convalescent COVID‐19 patients. J Med Virol. 2021;93:6506‐6511. 10.1002/jmv.27152
Fanfan Zeng and Mengjun Wu contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the author Fanfan Zeng, upon reasonable request.
REFERENCES
- 1. Pollard CA, Morran MP, Nestor‐Kalinoski AL. The COVID‐19 pandemic: a global health crisis. Physiol Genomics. 2020;52(11):549‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li Z, Yi Y, Luo X, et al. Development and clinical application of a rapid IgM‐IgG combined antibody test for SARS‐CoV‐2 infection diagnosis. J Med Virol. 2020;92(9):1518‐1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hou H, Wang T, Zhang B, et al. Detection of IgM and IgG antibodies in patients with coronavirus disease 2019. Clin Transl. Immunology, 2020;9(5):e01136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS‐CoV‐2 in patients with COVID‐19. Nat Med. 2020;26(6):845‐848. [DOI] [PubMed] [Google Scholar]
- 5. Zainol Rashid Z, Othman SN, Abdul Samat MN, Ali UK, Wong KK. Diagnostic performance of COVID‐19 serology assays. Malays J Pathol. 2020;42(1):13‐21. [PubMed] [Google Scholar]
- 6. Sun B, Feng Y, Mo X, et al. Kinetics of SARS‐CoV‐2 specific IgM and IgG responses in COVID‐19 patients. Emerg Microbes Infect. 2020;9(1):940‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu X, Wang J, Xu X, Liao G, Chen Y, Hu CH. Patterns of IgG and IgM antibody response in COVID‐19 patients. Emerg Microbes Infect. 2020;9(1):1269‐1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zeng F, Dai C, Cai P, et al. A comparison study of SARS‐CoV‐2 IgG antibody between male and female COVID‐19 patients: A possible reason underlying different outcome between sex. J Med Virol. 2020;92(10):2050‐2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qian C, Zhou M, Cheng F, et al. Development and multicenter performance evaluation of fully automated SARS‐CoV‐2 IgM and IgG immunoassays. Clin Chem Lab Med. 2020;58(9):1601‐1607. [DOI] [PubMed] [Google Scholar]
- 10. Sekine T, Perez‐Potti A, Rivera‐Ballesteros O, et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID‐19. Cell. 2020;183(1):158‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zeng H, Xu C, Fan J, et al. Antibodies in Infants Born to Mothers With COVID‐19 Pneumonia. JAMA. 2020;323(18):1848‐1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu N, Li W, Kang Q, Zeng W, Feng L, Wu J. No SARS‐CoV‐2 detected in amniotic fluid in mid‐pregnancy. Lancet Infect Dis. 2020;20(12):1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ibarrondo FJ, Fulcher JA, Goodman‐Meza D, et al. Rapid Decay of Anti‐SARS‐CoV‐2 Antibodies in Persons with Mild Covid‐19. N Engl J Med. 2020;383(11):1085‐1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu C, Yu X, Gao C, et al. Characterization of antibody responses to SARS‐CoV‐2 in convalescent COVID‐19 patients. J Med Virol. 2021;93(4):2227‐2233. [DOI] [PubMed] [Google Scholar]
- 15. Kumar A, Dowling WE, Román RG, et al. Status Report on COVID‐19 Vaccines Development. Curr Infect Dis Rep. 2021;23(6):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chakraborty C, Sharma AR, Bhattacharya M, Sharma G, Saha RP, Lee SS. Ongoing Clinical Trials of Vaccines to Fight against COVID‐19 Pandemic. Immune Netw. 2021;21(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA Vaccine against SARS‐CoV‐2 — Preliminary Report. N Engl J Med. 2020;383(20):1920‐1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gao Q, Bao L, Mao H, et al. Development of an inactivated vaccine candidate for SARS‐CoV‐2. Science. 2020;369(6499):77‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lamb YN. BNT162b2 mRNA COVID‐19 Vaccine: First Approval. Drugs. 2021;81(4):495‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS‐CoV‐2 vaccine, BBIBP‐CorV: a randomised, double‐blind, placebo‐controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Du X, Yu X, Li Q, et al. Duration for carrying SARS‐CoV‐2 in COVID‐19 patients. J Infect. 2020;81(1):e78‐e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu Y, Mao B, Liang S, et al. Association between age and clinical characteristics and outcomes of COVID‐19. Eur Respir J. 2020;55(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ueno A, Kawasuji H, Miyajima Y, et al. Prolonged viral clearance of severe acute respiratory syndrome coronavirus 2 in the older aged population. J Infect Chemother. 2021;27(7):1119‐1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zheng Y, Larragoite ET, Williams ESCP, et al. Sustainability of SARS‐CoV‐2 Induced Humoral Immune Responses in COVID‐19 Patients from Hospitalization to Convalescence Over Six Months. Virol Sin. 2021;18:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the author Fanfan Zeng, upon reasonable request.


