Abstract
In symptomatic patients with acute Coronavirus disease 2019 (COVID‐19), lymphocytopenia is one of the most prominent laboratory findings. However, to date age and gender have not been considered in assessment of COVID‐19‐related cell count alterations. In this study, the impact of COVID‐19 as well as age and gender on a large variety of lymphocyte subsets was analyzed in 33 COVID‐19 patients and compared with cell counts in 50 healthy humans. We confirm that cell counts of total lymphocytes, B, NK, cytotoxic and helper T cells are reduced in patients with severe COVID‐19, and this tendency was observed in patients with moderate COVID‐19. Decreased cell counts were also found in all subsets of these cell types, except for CD4+ and CD8+ effector memory RA+ (EMRA) and terminal effector CD8+ cells. In multivariate analysis however, we show that in addition to COVID‐19, there is an age‐dependent reduction of total, central memory (CM), and early CD8+ cell subsets, as well as naïve, CM, and regulatory CD4+ cell subsets. Remarkably, reduced naïve CD8+ cell counts could be attributed to age alone, and not to COVID‐19. By contrast, decreases in other subsets could be largely attributed to COVID‐19, and only partly to age. In addition to COVID‐19, male gender was a major factor influencing lower counts of CD3+ and CD4+ lymphocyte numbers. Our study confirms that cell counts of lymphocytes and their subsets are reduced in patients with COVID‐19, but that age and gender must be considered when interpreting the altered cell counts.
Keywords: age‐related, COVID‐19, gender‐related, lymphocytopenia, SARS‐CoV‐2
1. INTRODUCTION
Coronavirus Disease 2019 (COVID‐19) is a high contagious acute respiratory tract infection caused by the severe acute respiratory syndrome coronavirus type 2 (SARS‐CoV‐2) that emerged in China in late 2019. 1 Most people infected with SARS‐CoV‐2 are asymptomatic or have mild to moderate disease with fever, cough, or myalgia, and recover quickly. Especially older humans and patients with comorbidities have an increased risk of developing pneumonia and fatal acute respiratory distress syndrome (ARDS) or sepsis. 2 , 3 , 4 The injury of the respiratory system is thought to be mediated by the virus itself and/or an overstimulated immune system. 5 COVID‐19 is associated with various lab abnormalities of coagulation, inflammatory biomarkers, and biochemical as well as hematologic values dependent on the severity of COVID‐19. 6 The most striking finding in the blood count of patients with COVID‐19 is lymphocytopenia that correlates with the severity of disease. 7 , 8 However, interpretation of virus‐induced alterations of lymphocyte counts should also be considered in the light of age, given that COVID‐19 patients mostly belong to the middle‐aged and old population. A large basic effect of aging is seen on counts of cytotoxic and helper T cells. During the human lifetime, the proportion of naïve T cells within both CD4+ and CD8+ cells decreases dramatically from 45% and 43% to 20% and 10%, respectively. 9 In contrast, the proportion of central memory and effector memory cells within cytotoxic and helper T lymphocytes increases with age. Furthermore, also gender has an impact on lymphocyte counts. In general, counts of total CD3+ T lymphocytes as well as CD8+ cytotoxic T cells and especially CD4+ T helper cells are higher in females compared to males. 10 Thus, the question arises whether the widely reported alterations in counts of lymphocytes and their subsets in patients with COVID‐19 are indeed primarily due to coronavirus infection, or whether gender and age might have a significant impact.
While lymphocytopenia in COVID‐19 patients is well known, the influence of age and gender has hardly been considered so far. The aim of this study was to analyze the influence of age and gender on the total number of lymphocytes, as well as on B‐, T‐ and NK‐cell subsets during COVID‐19.
2. PATIENTS AND METHODS
2.1. Study population and trial design
Study participants with COVID‐19 were recruited for this prospective study at the University Medical Hospital, Augsburg, between April and October 2020. Patients were included immediately after clinical admission and confirmation of a positive qPCR test for SARS‐CoV‐2 from oropharyngeal swab. The time period between onset of COVID‐19 symptoms and study inclusion was restricted to a maximum of 28 days. Exclusion criteria were malignancy, immune deficiency, autoimmune disorder, chronic infection disease, and pregnancy. Every participant was classified according to interim guidance of the World Health Organization (WHO) as mild infection (uncomplicated upper respiratory tract infection), pneumonia (without need for supplemental oxygen), severe pneumonia (with need for supplemental oxygen), acute respiratory distress syndrome (ARDS), sepsis, or septic shock. 11 Patients suffering from severe pneumonia, ARDS, sepsis, or septic shock were categorized as “severe” COVID‐19 in our study, while all other causes were defined as “moderate” COVID‐19.
The study was performed in accordance with the revised declaration of Helsinki and approved by the internal ethics committee (internal review board No. 2020‐12). Signed informed consent was obtained from all participants allowing analysis of all laboratory data presented in this manuscript. Additionally, 50 healthy adult blood donors from the blood bank at University Medical Hospital, Augsburg, served as control group. Control samples were collected, measured, and analyzed during the pre‐COVID‐19 era excluding infection with SARS‐CoV‐2 as described below.
2.2. Analysis of lymphocytes and their subsets
EDTA blood samples (EDTA‐blood) were taken from patients and processed within 24 h. Flow cytometry was used to identify and measure total counts of lymphocytes as well as B, T and NK cells and all their subsets. B lymphocytes were identified by the presence of CD19 (CD19‐PC7 IM3628) and were further divided into naïve (IgD+ CD27−; IgD‐FITC B30652, CD27‐ECD B26603), non‐class switched (NCS) memory (IgD+ CD27+), class switched (CS) memory (IgD− CD27+) and transitional (CD24hi CD38hi; CD24‐PE IM1428U, CD38‐PC5 A07780) subsets. T lymphocytes defined by positivity for CD8 or CD4 were subdivided into naïve (CD62L+ CD45RA+), memory T cells (CD4+ CD45RA− CD45RO+/CD8+ CD45RA− CD45RO+), which were further divided into central memory (CD62L+ CD45RA−), effector memory (CD62L− CD45RA−), effector memory RA+ (EMRA) (CD62L− CD45RA+) and activated memory (HLA‐DR+ or CD69+) cells, and FoxP3+ cells. Furthermore, type 1, 2 and 17 CD4+ T helper (Th1/Th2/Th17) cells were identified by using antibodies against CXCR3, CCR4, CCR5 and CCR6. Th1 cells were defined as CD4+ CXCR3+ CCR4− CCR5+ CCR6−, Th2 cells as CD4+ CXCR3− CCR4+ CCR5− CCR6− and Th17 cells as CD4+ CXCR3− CCR4+ CCR5− CCR6+. Within cytotoxic CD8+ T lymphocytes, activated subsets in early (CD28+ CD27+), intermediate (CD28− CD27+) and late (CD28− CD27−) status as well as exhausted (CD279+) and terminal effector (CD279− CD57+) cells were identified. CD56+ T cells were defined as CD56+ CD3+. NK lymphocytes were detected as CD56+ CD3− cells and subdivided into three NK subsets (CD56+ CD16+, CD56dim CD16bright, and CD56bright CD16dim). Detailed information on lymphocyte subset specific immune phenotypes and on fluorochrome‐antibody conjugates are listed in Table S2. The gating strategy is shown in Figure S1 as already described by our research group. 12
2.3. Statistical analysis
Results are given as median values with interquartile ranges for descriptive analysis if not otherwise indicated. Wilcoxon test for univariate analysis was used for all statistical tests (two‐tailed) to detect different numbers of analyzed lymphocyte subsets from patients with moderate or severe COVID‐19 compared with healthy control group as well as analyzing effects of gender. Relative cell count deviations were calculated per 10 years. Impact of age and gender on differences in lymphocyte populations between COVID‐19 patients and healthy subjects was investigated using a fitted multivariate linear regression analysis. p‐values < 0.05 were regarded as statistically significant. Data were analyzed with SPSS 24.0 for Windows (SPSS Inc, Chicago, IL), and R 4.0.2.
3. RESULTS
3.1. Population characteristics
The median age of 33 study participants was 71 years (range: 17–94), and 12 were female and 21 male. One‐third (11/33) of patients had moderate COVID‐19, and two‐thirds of patients (22/33) were categorized as having severe COVID‐19. The time period between onset of COVID‐19 associated symptoms and the measurement of lymphocyte counts and their subsets was 4 days (range: 1–24). A list of patient characteristics including symptoms, and severity of illness are given in Table 1. Healthy controls had comparable characteristics exept for younger age of median 43 years (range: 18–81; p < 0.001) including 17 female and 33 male participants.
TABLE 1.
Patient characteristics
| Age [years]; median (range) | 71 (17–94) |
| Gender | |
| Male; n | 21 (64%) |
| Female; n | 12 (36%) |
| Symptoms type | |
| Cough; n | 18 (55%) |
| Dyspnea; n | 17 (51%) |
| Fever (>38°C); n | 15 (46%) |
| Myalgia; n | 14 (42%) |
| Sore throat; n | 8 (24%) |
| Headache; n | 5 (15%) |
| Diarrhea; n | 3 (9%) |
| Dorsal pain; n | 3 (9%) |
| Nausea; n | 3 (9%) |
| Cardiovascular symptoms; n | 3 (9%) |
| Loss of taste; n | 2 (6%) |
| Emesis; n | 2 (6%) |
| Thoracic pain; n | 2 (6%) |
| Abdominal pain; n | 1 (3%) |
| Dorsal pain; n | 1 (3%) |
| Severity of COVID‐19 (according to WHO) | |
| Mild illness; n | 7 (21%) |
| Pneumonia; n | 4 (12%) |
| Severe pneumonia; n | 10 (30%) |
| ARDS; n | 12 (36%) |
| Treatment in intensive care unit; n | 13 (39%) |
| Need for invasive ventilation; n | 8 (24%) |
3.2. Univariate analyses of lymphocyte populations
COVID‐19 patients with moderate and severe disease had lower total lymphocyte counts (1120/μl and 730/μl, respectively) compared to controls (1884/μl, p = 0.002 and p < 0.001, respectively). Patients with severe COVID‐19 had also reduced cell counts of total CD3+ cells (380/μl vs. 1175/μl, p ≤ 0.001), cytotoxic CD8+ cells (112/μl vs. 292/μl, p ≤ 0.001) and CD4+ helper cells (209/μl vs. 782/μl, p ≤ 0.001) and their subsets except of terminal effector CD8+ cells and EMRA CD4+ and CD8+ cells, respectively. These alterations of cell counts were also seen in the subpopulations of NK (81/μl vs. 226/μl, p ≤ 0.001) and B (71/μl vs. 211/μl, p ≤ 0.001) cells and their subsets. Similar trends were detected in patients with moderate COVID‐19 but without reaching significance in most subtypes of T cells. Cell counts of all lymphocyte subsets are shown in Figure 1 and listed in Table 2.
FIGURE 1.
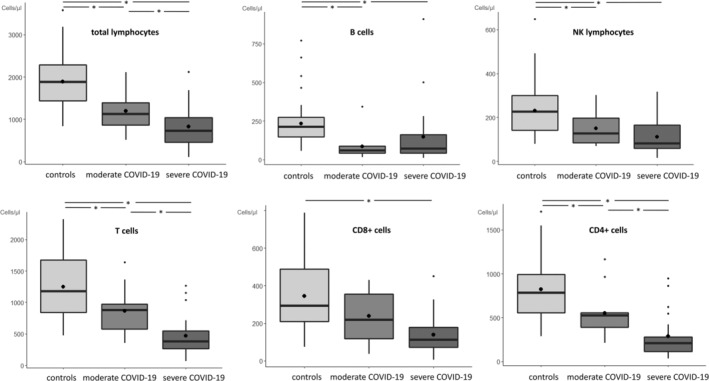
Distribution of counts of total lymphocytes and main subtypes are stratified by healthy controls (light gray), moderate COVID‐19 (medium gray), and severe COVID‐19 (dark gray) patients. Black lines and black points in boxplot represent median and mean values, respectively. *p < 0.05 significant value
TABLE 2.
Lymphocyte and their subset in patients with light or severe COVID‐19 compared to healthy controls. Cell counts are given as median value/μl (interquartile range)
| Healthy controls | Moderate COVID‐19 | p 1 | Severe COVID‐19 | p 2 | p 3 | |
|---|---|---|---|---|---|---|
| Median cell count (interquartile range) (n = 50) | Median cell count (interquartile range) (n = 11) | Median cell count (interquartile range) (n = 22) | ||||
| Total lymphocytes | 1884 (1439–2288) | 1120 (867–1390) | 0.002 | 730 (463–1043) | 0.000 | 0.040 |
| CD3+ cells | 1175 (839–1675) | 879 (576–971) | 0.017 | 380 (263–547) | 0.000 | 0.003 |
| CD8+ cells | 292 (209–488) | 219 (118–354) | 0.058 | 112 (71–179) | 0.000 | 0.053 |
| Naive | 78 (44–138) | 27 (20–124) | 0.298 | 15 (7–35) | 0.000 | 0.032 |
| Memory | 124 (65–164) | 55 (48–86) | 0.017 | 24 (17–66) | 0.000 | 0.123 |
| CM | 35 (14–56) | 16 (8–33) | 0.163 | 8 (6–19) | 0.002 | 0.204 |
| EM | 94 (56–139) | 54 (30–62) | 0.017 | 21 (14–49) | 0.000 | 0.178 |
| EMRA | 51 (23–119) | 24 (17–132) | 0.528 | 45 (19–80) | 0.374 | 0.749 |
| Early | 180 (154–291) | 92 (55–152) | 0.008 | 35 (19–54) | 0.000 | 0.004 |
| Intermediate | 21 (13–34) | 7 (4–26) | 0.050 | 8 (5–14) | 0.000 | 0.778 |
| Late | 51 (28–124) | 27 (16–77) | 0.159 | 58 (23–110) | 0.816 | 0.396 |
| Exhausted | 86 (59–133) | 53 (43–94) | 0.196 | 36 (19–62) | 0.000 | 0.044 |
| Terminal effector | 24 (13–95) | 16 (9–70) | 0.431 | 24 (11–52) | 0.494 | 0.985 |
| Regulatory | 0 (0–1) | 0 (0–1) | 0.616 | 0 (0–0) | 0.022 | 0.336 |
| Il2R+ | 1 (0–1) | 0 (0–0) | 0.019 | 0 (0–0) | 0.004 | 0.462 |
| CD4+ cells | 782 (554–993) | 523 (388–554) | 0.016 | 209 (113–277) | 0.000 | 0.003 |
| Naive | 311 (191–411) | 174 (142–318) | 0.098 | 53 (29–110) | 0.000 | 0.002 |
| Memory | 430 (323–608) | 270 (168–402) | 0.018 | 123 (77–184) | 0.000 | 0.017 |
| CM | 272 (159–406) | 154 (116–304) | 0.149 | 81 (50–135) | 0.000 | 0.040 |
| EM | 147 (94–235) | 91 (53–111) | 0.005 | 39 (21–59) | 0.000 | 0.008 |
| EMRA | 4 (1–31) | 4 (2–19) | 0.688 | 2 (1–5) | 0.107 | 0.105 |
| Th1 | 27 (15–59) | 28 (15–35) | 0.632 | 8 (4–20) | 0.000 | 0.069 |
| Th2 | 45 (30–69) | 34 (27–43) | 0.124 | 14 (10–27) | 0.000 | 0.063 |
| Th17 | 60 (35–81) | 31 (29–37) | 0.013 | 17 (10–36) | 0.000 | 0.029 |
| Regulatory | 43 (29–63) | 24 (20–29) | 0.008 | 12 (9–21) | 0.000 | 0.026 |
| Il2R+ | 17 (9–21) | 10 (7–14) | 0.052 | 6 (3–9) | 0.000 | 0.063 |
| CD3+ CD56+ cells | 45 (18–77) | 47 (15–85) | 0.903 | 29 (8–49) | 0.035 | 0.191 |
| NK cells | 226 (143–300) | 127 (85–197) | 0.017 | 81 (60–166) | 0.000 | 0.097 |
| CD56+ CD16+ | 193 (114–270) | 102 (76–173) | 0.021 | 68 (51–130) | 0.000 | 0.097 |
| CD56dim CD16bright | 15 (11–20) | 10 (5–13) | 0.013 | 4 (3–12) | 0.001 | 0.281 |
| CD56bright CD16dim | 15 (10–19) | 8 (6–12) | 0.009 | 4 (2–7) | 0.000 | 0.003 |
| B cells | 211 (149–274) | 59 (43–87) | 0.000 | 71 (42–161) | 0.000 | 0.418 |
| Naive | 129 (92–171) | 29 (16–50) | 0.000 | 33 (20–71) | 0.000 | 0.585 |
| Non‐class switch memory | 8 (5–13) | 1 (1–2) | 0.000 | 1 (1–2) | 0.000 | 0.985 |
| Class switch memory | 25 (14–43) | 9 (5–11) | 0.000 | 6 (5–11) | 0.000 | 0.985 |
| Transitional | 10 (6–17) | 3 (1–6) | 0.003 | 2 (1–4) | 0.000 | 0.749 |
Note: Significance level are given for comparison between patients with light vs. healthy controls (p 1), patients with severe COVID‐19 vs. healthy controls (p 2), and patients with moderate vs. severe COVID‐19 (p 3).
Age had an impact on total lymphocyte count and most subsets in entire cohort of COVID‐19 patients and healthy controls, as listed in Table 3. Median numbers of total lymphocytes were reduced by 12.5% per 10 years (/10y; Figure 2A). This aging effect could be observed also for CD3+ cells (−14.1%/10y), CD8+ cells (−19.1%/10y; Figure 2B) and some of their subsets (naïve (−38.8%/10y; Figure 2C), memory (−19.6%/10y), CM (−21.1%/10y), EM (−18.3%/10y), early (−29.5%/10y; Figure 2D), intermediate (−14.5%/10y), and exhausted CD8+ cells (−9.6%/10y) as well as for CD4+ cells (−14.8%/10y) and some of their subsets (naïve (−21.5%/10y), memory (−14.2%/10y), CM (−16.0%/10y), EM (−13.0%/10y), regulatory (−12.5%/10y), and Il2R+ CD4+ cells (−18.2%/10y)). Aging had also a negative influence on NK cells (−13.6% /10y) and their subsets (CD56+ CD16+ (−13.4%/10y), CD56dim CD16bright (−17.8%/10y), CD56bright CD16dim (−19.0%/10y)), as well as on B cells (−14.4%/10y) and their subsets (naïve (−23.4%/10y), memory non‐class switch (−24.0%/10y), memory class switch (−10.8%/10y), transitional (−79.3%/10y)).
TABLE 3.
Median cell counts of lymphocytes and their subsets of total cohort including COVID‐19 patients and healthy controls (n = 83), deviation of cell counts dependent on age over lifetime, and p value
| Median cell count/μl (interquartile range) | Relative cell count deviation per 10 years | p value | |
|---|---|---|---|
| Total lymphocytes | 1490 (968–2071) | −12.5% | <0.001 |
| CD3+ cells | 961 (529–1354) | −14.1% | <0.001 |
| CD8+ cells | 231 (138–422) | −19.1% | <0.001 |
| Naive | 49.6 (14–109) | −38.8% | <0.001 |
| Memory | 83 (39–143) | −19.6% | <0.001 |
| CM | 21 (7–44) | −21.1% | <0.001 |
| EM | 65 (29–120) | −18.3% | <0.001 |
| EMRA | 49 (21–108) | +3.5% | 0.541 |
| Early | 153 (54–213) | −29.5% | <0.001 |
| Intermediate | 15 (7–28) | −14.5% | 0.002 |
| Late | 47 (24–121) | −4.0% | 0.49 |
| Exhausted | 65 (40–114) | −9.6% | 0.015 |
| Terminal effector | 23 (11–79) | −7.0% | 0.286 |
| Regulatory | 0.3 (0.1–0.6) | −13.3% | 0.109 |
| Il2R+ | 0.4 (0.2–0.8) | 27.6% | 0.0.956 |
| CD4+ cells | 574 (320–937) | −14.8% | <0.001 |
| Naive | 221 (114–372) | −21.5% | <0.001 |
| Memory | 338 (168–537) | −14.2% | <0.001 |
| CM | 219 (92–344) | −16.0% | <0.001 |
| EM | 98 (53–181) | −13.0% | 0.003 |
| EMRA | 3 (1–19) | −12.9% | 0.215 |
| Th1 | 21 (11–44) | −6.1% | 0.326 |
| Th2 | 34.0 (19–52) | −5.1% | 0.439 |
| Th17 | 38.8 (24–72) | −11.0% | 0.064 |
| Regulatory | 30 (20–52) | −12.5% | <0.001 |
| Il2R+ | 11 (6–19) | −18.2% | <0.001 |
| CD3+ CD56+ cells | 34 (17–67) | −3.5% | 0.549 |
| NK cells | 171 (93–269) | −13.6% | <0.001 |
| CD56+ CD16+ | 137 (80–240) | −13.4% | <0.001 |
| CD56dim CD16bright | 13 (7–19) | −17.8% | <0.001 |
| CD56bright CD16dim | 11 (6–17) | −19.0% | <0.001 |
| B cells | 166 (76–238) | −14.4% | <0.001 |
| Naive | 93 (38–149) | −23.4% | 0.001 |
| Memory non‐class switch | 5 (1–10) | −24.0% | 0.001 |
| Memory class switch | 15 (7–33) | −10.8% | 0.046 |
| Transitional | 6 (3–11) | −79.3% | <0.001 |
FIGURE 2.
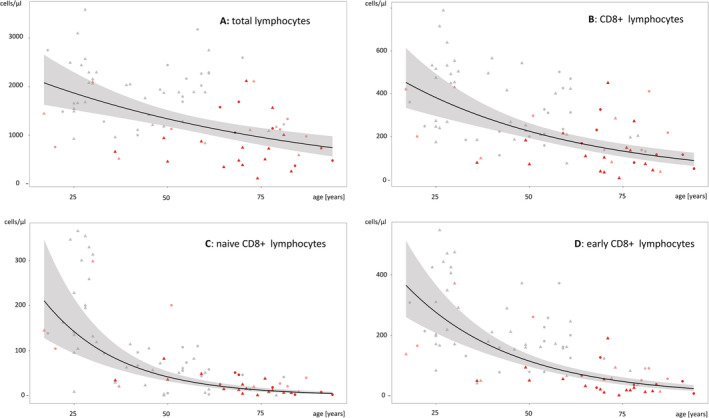
Age dependent distribution of cell counts and median decrease over lifetime (black line, 95% confidence interval in gray) of total lymphocytes (A), CD8+ lymphocytes (B), naïve CD8+ lymphocytes (C), and early CD8+ lymphocytes (D) in patients with moderate COVID‐19 (bright red), severe COVID‐19 (red), and healthy controls (black), respectively. Symbols: circle = female gender, triangle = male gender
Gender influenced the ratio of CD4/CD8 lymphocyte counts in univariate analysis. Female participants had a higher median ratio compared to male in study participants and healthy controls (2.89 (quartile: 1.85–3.94) vs. 2.06 (1.44–3.10); p = 0.024).
3.3. Multivariate analyses of lymphocyte populations
The impact of moderate/severe COVID‐19, gender and increasing age per 10‐year timeframe on total count of lymphocytes and their subsets was estimated using a linear regression model on log‐transformed cell counts, resulting in multiplicative factors (=coefficient [cB]) in a multivariate model (Figure 3, Table S1).
FIGURE 3.
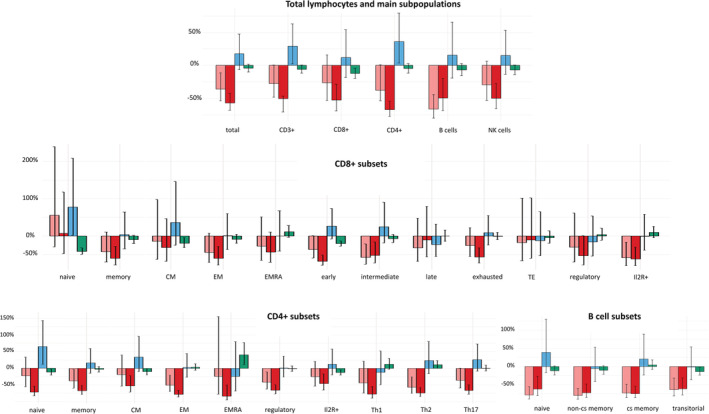
Impact of moderate/severe COVID‐19 (bright red/red) as well as gender (blue) and age (green) on lymphocyte counts were estimated in multivariate analysis. Levels of COVID‐19 columns indicate percentage deviation from normal values of healthy controls. Blue columns indicate mean percentage influence of female gender. Levels of age columns reflect percentage deviation per 10 years. Black bars in columns present 95% confidence interval
Total lymphocyte count was decreased in moderate (cB: 0.640, p = 0.008) and severe (cB: 0.427, p < 0.001) COVID‐19 patients compared to healthy controls, and this decrease was independent of age and gender.
Total T cell populations (CD3+) were also lower in severe COVID‐19 (cB: 0.395, p < 0.001) patients but elevated by femalegender (cB: 1.291, p = 0.033).
Cytotoxic T cells (CD8+) were found with lower levels in patients with severe COVID‐19 (cB: 0.472, p = 0.001), and this effect was even more pronounced with increasing age (cB: 0.876, p = 0.004). Subsets of CD8+ cells showed reduced counts within memory (cB: 0.395, p = 0.003), effector memory (cB: 0.394, p = 0.003), early (cB: 0.320 p < 0.001), intermediate (cB: 0.480, p = 0.010), exhausted (cB: 0429, p < 0.001), and Il2R+ (cB: 0.380, p = 0.002) cell subsets in severe COVID‐19 patients, whereas early CD8+ cells were additionally influenced by increasing age (cB: 0.793, p < 0.001). In patients with moderate COVID‐19, significant reduction of cell counts was also detected for intermediate (cB: 0.427, p = 0.007) and Il2R+ (cB: 0.411, p = 0.014) cytotoxic T cells. Remarkably, numbers of naïve CD8+ cells were not influenced by COVID‐19 but were diminished depending on age (cB: 0.584, p < 0.001) and increased in females (cB: 1.773, p = 0.044). Central memory CD8+ cells were also diminished depending on age (cB: 0.809, p = 0.011) as the only associated factor.
T helper cell (CD4+) counts were lower in patients with severe COVID‐19 (cB: 0.320, p < 0.001), and this cell population was higher in females (cB: 1.363, p = 0.027). All analyzed cell counts of CD4+ subsets were reduced in severe COVID‐19 patients (naïve (cB: 0.278, p < 0.001), memory (cB: 0.326, p < 0.001), CM (cB: 0.477, p = 0.004), EM (cB: 0.222, p < 0.001), EMRA (cB: 0.165, p = 0.002), THC1 (cB: 0.223, p < 0.001), THC2 (cB: 0.260, p < 0.001), THC17 (cB: 0.336, p < 0.001), IlR2+ (cB: 0.540, p = 0.006), regulatory (cB: 0.346, p < 0.001)). Similarly, cell counts of some of these subsets (memory (cB: 0.624, p = 0.036), effector memory (cB: 0.492 p = 0.004), THC2 (cB: 0.433, p = 0.003), and regulatory cells (cB: 0.165, p = 0.002)) were also reduced in moderate COVID‐19 patients. Female gender was associated with higher numbers of naïve helper cells (cB: 1.647, p = 0.012), and age with reduced numbers of naïve (cB: 0.882, p = 0.020), CM (cB: 0.899, p = 0.049), and Il2R+ helper cells (cB: 0.873, p = 0.004). By contrast, the numbers of EMRA cells were increased depending on age (cB: 1.404, p = 0.005) and THC2 (cB: 1.111, p = 0.049).
CD3+ CD56+ T cells were found to be reduced in patients with severe COVID‐19 (cB: 0.431 p = 0.020) independent of age or gender.
Total B cells were decreased in moderate (cB: 0.335, p < 0.001) and severe (cB: 0.503 p = 0.004) COVID‐19 patients. Numbers of B cells in their defined subsets were also reduced in moderate COVID‐19 patients (naïve (cB: 0.219, p < 0.001), non‐cs‐memory (cB: 0.202, p < 0.001), cs‐memory (cB: 0.272, p < 0.001), transitional (cB: 0.364, p = 0.002)), and such cells were similarly reduced in severely affected patients (naïve (cB: 0.380, p = 0.005), non‐cs‐memory (cB: 0.279, p < 0.001), cs‐memory (cB: 0.256, p < 0.001), transitional (cB: 0.388, p = 0.002)). The reduction of transitional B cells was pronounced by aging (naïve (cB: 0.219, p < 0.001), non‐cs‐memory (cB: 0.202, p < 0.001), cs‐memory (cB: 0.272, p < 0.001), transitional (cB: 0.862, p = 0.020)).
NK cell counts (cB: 0.499, p < 0.001) and counts of their subsets (CD56+ CD16+ (cB: 0.480, p < 0.001), CD56 + bright CD16dim (cB: 0.344, p = 0.014), CD56dim CD16bright were lower in patients with severe COVID‐19 only. An additional negative influence of aging was observed on CD56bright CD16dim (cB: 0.902, p = 0.008) and CD56dim CD16bright (cB: 0.873, p = 0.018) NK cells.
4. DISCUSSION
In this prospective study, we analyzed lymphocyte subsets of 33 elderly patients with different degrees of COVID‐19 severity. In univariate analysis we found lower counts of total lymphocytes and all their subsets, with the exception of CD4+ and CD8+ EMRA cells as well as late and terminal effector T helper cells, in patients with severe COVID‐19. In patients affected by moderate COVID‐19, a similar trend was found for most analyzed cell populations, although this did not always reach statistical significance.
Reduction of lymphocyte numbers in patients with COVID‐19 is well known. 13 , 14 , 15 Usually, the human immune system reacts by inducing proliferation of lymphocytes during the acute phase of viral infections including cytomegalovirus, 16 Epstein Barr virus, 17 and also human immunodeficiency virus. 18 Only few virus infections like avian influenza virus H5N1, 19 respiratory syncytial virus, 20 or swine foot‐and‐mouth disease virus 21 are associated with generalized lymphocytopenia. Different types of coronaviruses belong to this group and likewise present with lymphocytopenia. 22 , 23 The pathophysiology of lymphocytopenia in patients with COVID‐19 has not yet been elucidated. The transient lymphocyte decrease might mainly caused by T cell recruitment to sites of infection, especially to the lungs, leading to extensive lymphocyte infiltration. 24 , 25 After recovery from COVID‐19, normal lymphocyte values can be found again without quantitative restrictions of any lymphocyte subsets, as we have shown recently. 12
In our study, multivariate analysis confirmed the independent negative effect of COVID‐19 on total lymphocyte counts, and on most lymphocytic subsets as seen in univariate analysis. Women had higher CD3+ cells than men, which was mainly due to the higher number of T helper cells. The positive influence of female gender on the level of CD4+ cells in human peripheral blood is well known. 9 , 10 Aging mainly has a negative effect on the number of CD3+ and CD8+ cells, 26 which was also observed in our study, where at least the number of CD8+ cells decreased with age. The used fitted multivariate linear regression analysis was able to identify this significant difference, although the distribution of age between our COVID‐19 cohort and the control group was statistically different.
Looking at detailed T cell subsets in our analysis, naïve CD4+ and CD8+ were significantly influenced by gender as well as age, and COVID‐19 had little or no effect on number of these cell counts. In particular, the number of naïve T cells decreases during life 27 , 28 , 29 which can be explained by an increasing tissue involution of the thymus and the accompanying dysregulation of the thymic epithelial cell architecture. 30 , 31 This aging effect was the only cell‐reducing factor for naïve CD8+ cells in the multivariate analysis of our study, whereas COVID‐19 did not even negatively affect the level of this subset in the severe affected patient cohort as might have been expected from the univariate analysis. Early and CM cytotoxic T lymphocytes also decreased with age in our study, whereas only severe COVID‐19 was found to be a reducing factor for early CD8+ cells. Within CD4+ subsets, numbers of CM, EMRA, THC2, and regulatory CD4+ cells were also reduced by aging, which amplified the reducing effect of severe COVID‐19.
Numbers of total B cells and their subsets were found to be reduced in moderate as well as severe COVID‐19 patients as described before, 8 , 24 with an additional aging effect for transitional B cells. Counts of circulating NK cells and subsets were only reduced in severe COVID‐19 patients in our study as reported in multiple studies probably due to migrations into SARS‐CoV‐2 infected tissues mainly in the lungs. 32 , 33 , 34
Our study has some limitations: the focus of our investigation was centered on adults. Thus, our results are probably not be transferable to children infected by SARS‐CoV‐2 who may have specific age and gender effects on lymphocytes and their subsets. Furthermore, the number of participants is limited especially in the subcohort of moderate COVID‐19.
In summary, our study confirms the decrease in the total number of lymphocytes, cytotoxic and helper T cells, B and NK cells, and almost all their subsets, especially in COVID‐19 patients with severe course. Since severe COVID‐19 mainly affects elderly patients, it is important to consider the additional effect of aging which leads to reduction in naïve CD4+ and CD8+ cells. Similarly, gender must be taken into account given the diminished decreases of CD3+ and CD4+ lymphocyte counts in female compared to male COVID‐19 patients.
AUTHOR CONTRIBUTIONS
Phillip Löhr: Data curation; formal analysis; methodology; project administration; software; visualization; writing‐original draft. Stefan Schiele: Data curation; formal analysis; software; visualization; writing‐original draft. Tobias Arndt: Data curation; formal analysis; software; visualization; writing‐original draft. Stefanie Grützner: Conceptualization; project administration; supervision. Rainer Claus: Conceptualization; investigation; supervision; writing‐original draft. Christoph Römmele: Project administration. Gernot Müller: Formal analysis; supervision. Christoph Schmid: Conceptualization; supervision; writing‐original draft. Kevin Dennehy: Supervision; writing‐original draft. Andreas Rank: Conceptualization; formal analysis; methodology; project administration; resources; writing‐original draft.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Supporting information
Supplemental Table S1 Impact of moderate/severe COVID‐19, gender and increasing age (per 10 years) on total count of lymphocytes and their subsets estimated as multiplicative factor (= coefficient B) in a multivariate model.
Supplemental Table S2. Identification and gating strategy for detection of main lymphocyte types and their subsets by labeled antibodies using flow cytometry.
Supplemental Figure S1 Gating strategy. (A) Lymphocytes were identified by using forward scatter (FC) and side scatter (SC). (B) Additional CD19 positivity was used to define B cells (Plot 1), which were further subdivided into naïve (IgD+), class switched memory (CD27+), non‐class switched memory (IgD+ CD27+, Plot 2) and transitional B cells (CD24+ CD38+, Plot 3). (C) CD56+ T cells (CD3+ CD56+) were primarily identified (Plot 1). CD3− cells were classified into 3 different subgroups of NK cells (CD56+ CD16+, CD56bright CD56 dim, CD56dim CD16bright, Plot 2). (D) T cells were identified by CD4 or CD8 positivity. Both CD4+ as well as CD8+ cells were subdivided into memory (CD45RA− CD45RO+) (Plots 1 + 6), naïve (CD62L+ CD45RA+), central memory (CD62L+ CD45RA−), effector memory (CD62L− CD45RA−) and effector memory RA+ (“EMRA”) (CD62L− CD45RA+) cells (Plots 3 + 7). To analyze CD8+ cell activity, cells were subdivided into early (CD27+ CD28+), intermediate (CD27+ CD28−), late (CD27− CD28−) (Plot 4), or exhausted (CD279+) and terminal effector (CD279− CD57+) cells (Plot 5). CD4+ T helper cells were classified into Th1 (CXCR3+ CCR4− CCR5+ CCR6−), Th2 (CXCR3− CCR4+ CCR5− CCR6−) and Th17 (CXCR3− CCR4+ CCR5− CCR6+) cells (Plots 8 + 9). (E) CD4+ and CD8+ cells were also subdivided into activated memory cells (HLA‐DR+ or CD69+) (Plots 1–4) and regulatory cells (CD25+ or FoxP3+) (Plots 5–8).
MIFlowCyt MIFlowCyt Item Checklist.
ACKNOWLEDGMENTS
We would like to thank Tatajana Lensjaka and Gabriele Ziegler on behalf of all laboratory staff for the technical support in our laboratory.
Open access funding enabled and organized by Projekt DEAL.
Löhr P, Schiele S, Arndt TT, Grützner S, Claus R, Römmele C, et al. Impact of age and gender on lymphocyte subset counts in patients with COVID‐19. Cytometry. 2021;1–9. 10.1002/cyto.a.24470
REFERENCES
- 1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schaller T, Hirschbuhl K, Burkhardt K, Braun G, Trepel M, Markl B, et al. Postmortem examination of patients with COVID‐19. JAMA. 2020;323:2518–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, et al. Immunology of COVID‐19: current state of the science. Immunity. 2020;52:910–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clin Chem Lab Med. 2020;58:1021–8. [DOI] [PubMed] [Google Scholar]
- 7. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang W, Berube J, McNamara M, Saksena S, Hartman M, Arshad T, et al. Lymphocyte subset counts in COVID‐19 patients: a meta‐analysis. Cytometry A. 2020;97:772–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kverneland AH, Streitz M, Geissler E, Hutchinson J, Vogt K, Boes D, et al. Age and gender leucocytes variances and references values generated using the standardized ONE‐study protocol. Cytometry A. 2016;89:543–64. [DOI] [PubMed] [Google Scholar]
- 10. Andreu‐Ballester JC, Garcia‐Ballesteros C, Benet‐Campos C, Amigo V, Almela‐Quilis A, Mayans J, et al. Values for alphabeta and gammadelta T‐lymphocytes and CD4+, CD8+, and CD56+ subsets in healthy adult subjects: assessment by age and gender. Cytometry B Clin Cytom. 2012;82:238–44. [DOI] [PubMed] [Google Scholar]
- 11. WHO . Clinical management of severe acute respiratory infection when COVID‐19 is suspected. Interim guidance. 13 March 2020. 2020.
- 12. Rank A, Lohr P, Hoffmann R, Ebigbo A, Grutzner S, Schmid C, et al. Sustained cellular immunity in adults recovered from mild COVID‐19. Cytometry A. 2021;99:429–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID‐19). Front Immunol. 2020;11:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID‐19) in Wuhan, China. Clin Infect Dis. 2020;71:762–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He Z, Zhao C, Dong Q, Zhuang H, Song S, Peng G, et al. Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets. Int J Infect Dis. 2005;9:323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gratama JW, Kardol M, Naipal AM, Slats J, Den Ouden A, Stijnen T, et al. The influence of cytomegalovirus carrier status on lymphocyte subsets and natural immunity. Clin Exp Immunol. 1987;69:16–24. [PMC free article] [PubMed] [Google Scholar]
- 17. Niu MT, Stein DS, Schnittman SM. Primary human immunodeficiency virus type 1 infection: review of pathogenesis and early treatment intervention in humans and animal retrovirus infections. J Infect Dis. 1993;168:1490–501. [DOI] [PubMed] [Google Scholar]
- 18. Callan MF. The evolution of antigen‐specific CD8+ T cell responses after natural primary infection of humans with Epstein‐Barr virus. Viral Immunol. 2003;16:3–16. [DOI] [PubMed] [Google Scholar]
- 19. Zitzow LA, Rowe T, Morken T, Shieh WJ, Zaki S, Katz JM. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J Virol. 2002;76:4420–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Donnell DR, Carrington D. Peripheral blood lymphopenia and neutrophilia in children with severe respiratory syncytial virus disease. Pediatr Pulmonol. 2002;34:128–30. [DOI] [PubMed] [Google Scholar]
- 21. Bautista EM, Ferman GS, Golde WT. Induction of lymphopenia and inhibition of T cell function during acute infection of swine with foot and mouth disease virus (FMDV). Vet Immunol Immunopathol. 2003;92:61–73. [DOI] [PubMed] [Google Scholar]
- 22. Li T, Qiu Z, Zhang L, Han Y, He W, Liu Z, et al. Significant changes of peripheral T lymphocyte subsets in patients with severe acute respiratory syndrome. J Infect Dis. 2004;189:648–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang F, Nie J, Wang H, Zhao Q, Xiong Y, Deng L, et al. Characteristics of peripheral lymphocyte subset alteration in COVID‐19 pneumonia. J Infect Dis. 2020;221:1762–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, et al. Single‐cell landscape of bronchoalveolar immune cells in patients with COVID‐19. Nat Med. 2020;26:842–4. [DOI] [PubMed] [Google Scholar]
- 25. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li M, Yao D, Zeng X, Kasakovski D, Zhang Y, Chen S, et al. Age related human T cell subset evolution and senescence. Immun Ageing. 2019;16:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saule P, Trauet J, Dutriez V, Lekeux V, Dessaint JP, Labalette M. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech Ageing Dev. 2006;127:274–81. [DOI] [PubMed] [Google Scholar]
- 28. Goronzy JJ, Fang F, Cavanagh MM, Qi Q, Weyand CM. Naive T cell maintenance and function in human aging. J Immunol. 2015;194:4073–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chinn IK, Blackburn CC, Manley NR, Sempowski GD. Changes in primary lymphoid organs with aging. Semin Immunol. 2012;24:309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol. 2014;14:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Palmer DB. The effect of age on thymic function. Front Immunol. 2013;4:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Song C‐Y, Xu J, He J‐Q, Lu Y‐Q. COVID‐19 early warning score: a multi‐parameter screening tool to identify highly suspected patients. medRxiv. 2020;2020.03.05.20031906. [Google Scholar]
- 33. Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, et al. Functional exhaustion of antiviral lymphocytes in COVID‐19 patients. Cell Mol Immunol. 2020;17:533–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. National Research Project for SARS BG . The involvement of natural killer cells in the pathogenesis of severe acute respiratory syndrome. Am J Clin Pathol. 2004;121:507–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1 Impact of moderate/severe COVID‐19, gender and increasing age (per 10 years) on total count of lymphocytes and their subsets estimated as multiplicative factor (= coefficient B) in a multivariate model.
Supplemental Table S2. Identification and gating strategy for detection of main lymphocyte types and their subsets by labeled antibodies using flow cytometry.
Supplemental Figure S1 Gating strategy. (A) Lymphocytes were identified by using forward scatter (FC) and side scatter (SC). (B) Additional CD19 positivity was used to define B cells (Plot 1), which were further subdivided into naïve (IgD+), class switched memory (CD27+), non‐class switched memory (IgD+ CD27+, Plot 2) and transitional B cells (CD24+ CD38+, Plot 3). (C) CD56+ T cells (CD3+ CD56+) were primarily identified (Plot 1). CD3− cells were classified into 3 different subgroups of NK cells (CD56+ CD16+, CD56bright CD56 dim, CD56dim CD16bright, Plot 2). (D) T cells were identified by CD4 or CD8 positivity. Both CD4+ as well as CD8+ cells were subdivided into memory (CD45RA− CD45RO+) (Plots 1 + 6), naïve (CD62L+ CD45RA+), central memory (CD62L+ CD45RA−), effector memory (CD62L− CD45RA−) and effector memory RA+ (“EMRA”) (CD62L− CD45RA+) cells (Plots 3 + 7). To analyze CD8+ cell activity, cells were subdivided into early (CD27+ CD28+), intermediate (CD27+ CD28−), late (CD27− CD28−) (Plot 4), or exhausted (CD279+) and terminal effector (CD279− CD57+) cells (Plot 5). CD4+ T helper cells were classified into Th1 (CXCR3+ CCR4− CCR5+ CCR6−), Th2 (CXCR3− CCR4+ CCR5− CCR6−) and Th17 (CXCR3− CCR4+ CCR5− CCR6+) cells (Plots 8 + 9). (E) CD4+ and CD8+ cells were also subdivided into activated memory cells (HLA‐DR+ or CD69+) (Plots 1–4) and regulatory cells (CD25+ or FoxP3+) (Plots 5–8).
MIFlowCyt MIFlowCyt Item Checklist.


