INTRODUCTION
The prognostic value of olfactory dysfunction (OD) in coronavirus disease 2019 (COVID‐19) remains controversial, with conflicting reports of the association between OD and COVID‐19 severity. 1 Many of the prognostic studies published so far have important drawbacks that limit the reliability of the results; most are anamnestic studies that do not formally evaluate olfactory function with risk of recall bias, and use “need for hospitalization” alone to determine COVID‐19 severity.
Interstitial pneumonia is an important complication of COVID‐19 and a reliable negative prognostic factor. 2 This study aimed to analyze the correlation between olfactory psychophysical scores and severity of lung involvement detected by chest computed tomography (CT) in COVID‐19 patients suspected of having interstitial pneumonia. We also evaluated whether severity of respiratory disease predicted recovery of OD.
PATIENTS AND METHODS
This prospective study was undertaken at the University Hospital of Sassari (ethical approval PG/2021/5471) and included consecutive patients with polymerase chain reaction (PCR)‐confirmed severe acute respiratory syndrome‐coronavirus‐2 (SARS‐CoV‐2) infection who underwent high‐resolution chest CT scan. Exclusions were as follows: COVID‐19 diagnosis >10 days earlier, psychiatric or neurological diseases, previous trauma, surgery or radiotherapy in the oral and nasal cavities, preexisting OD, allergic rhinitis, chronic rhinosinusitis, and lung disease; non‐consenting patients were also excluded.
Chest CT imaging was performed using a 16‐detector CT scanner (Emotion; Siemens Medical Solutions USA, Inc., Malvern, PA, USA) on admission to the Emergency Department. Images were acquired supine during a single inspiratory breath‐hold, with the following parameters; x‐ray tube 120 kVp, 350 mAs; rotation time 8.10 s; rotation time 0.8 s; pitch 1.5; slice thickness 1 mm; window lung. Lung involvement was staged by the chest CT severity score (CT‐SS) proposed by Yang et al., 3 ranging from 0 (no pneumonia) to 40 points. Each case was independently assessed by three blinded, experienced radiologists. The median CT‐SS was considered.
Psychophysical olfactory evaluation was performed with the Connecticut Chemosensory Clinical Research Center test (CCCRC) within 48 h of the CT and repeated 60 days later by the same researcher, blinded to the CT‐SS results. The test methodology and scoring system have been described in previous studies. 4
The statistical analysis was performed with SPSS 26.0 (IBM, Armonk, NY, USA). The correlation between olfactory scores and CT‐SS was assessed using Pearson's correlation coefficient (statistical significance: p <0.05 with a 95% confidence interval [CI]).
RESULTS
Forty‐six COVID‐19 patients were recruited and completed baseline evaluation. Patient demographics and clinical features are reported in Table 1. At baseline, 35 patients (76.1%) had OD: 12 cases of anosmia (26.1%), severe hyposmia in 10 (21.7%), and moderate hyposmia in 13 cases (28.3%). The mean CT‐SS was 15.2 ± 10.7. No radiological signs of interstitial pneumonia were detected in nine patients. Correlation between CCCRC scores and CT‐SS at baseline was weak and not significant (r s = 0.183; p = 0.222) (Figure 1).
TABLE 1.
General and clinical features of the study population
| Parameter | Value | CI |
|---|---|---|
| Gender, n (%) | ||
| Male | 27 (58.7) | 95% CI, 43.2–73 |
| Female | 19 (41.3) | 95% CI, 27–56.8 |
| Age (years), mean ± SD | 64.5 ± 12 | 95% CI, 61–68 |
| Days from COVID‐19 symptoms onset, mean ± SD | 6.9 ± 2.2 | 95% CI, 6.3–7.5 |
| CT‐SS, mean ± SD | 15.2 ± 10.7 | 95% CI, 12.1–18.3 |
| Clinical findings, n (%) | ||
| Fever | 38 (82.6) | 95% CI, 68.5–92.2 |
| Asthenia | 41 (89.1) | 95% CI, 76.4–96.4 |
| Cough | 28 (60.9) | 95% CI, 45.3–74.9 |
| Chest pain | 3 (6.5) | 95% CI, 1.4–17.9 |
| Appetite loss | 39 (84.8) | 95% CI, 71.1–93.7 |
| Joint pain | 41 (89.1) | 95% CI, 76.4–96.4 |
| Muscle pain | 40 (87) | 95% CI, 73.7–95.1 |
| Headache | 35 (76.1) | 95% CI, 61.2–87.4 |
| Diarrhoea | 6 (13) | 95% CI, 4.9–26.3 |
| Abdominal pain | 8 (17.4) | 95% CI, 7.8–31.4 |
| Nausea | 8 (17.4) | 95% CI, 7.8–31.4 |
| Conjunctivitis | 1 (2.2) | 95% CI, 0.05–11.5 |
| Urticaria | 0 (0 ) | 97.5% CI, 0–7.7 |
| Sticky throat mucus | 16 (35 ) | 95% CI, 21.3–50.2 |
| Nasal obstruction | 19 (41.3 ) | 95% CI, 27–56.8 |
| Rhinorrhoea | 21 (45.6 ) | 95% CI, 30.9–61 |
| Nasal burning | 17 (37 ) | 95% CI, 23–51 |
| Throat pain | 12 (26.1 ) | 95% CI, 13.4–38.9 |
| Ear pain | 3 (6.5) | 95% CI, 1.4–17.9 |
| Face pain | 1 (2.2) | 95% CI, 0.05–11.5 |
| Swallowing difficulties | 17 (37 ) | 95% CI, 23–51 |
| Voice issues | 3 (6.5) | 95% CI, 1.4–17.9 |
| Mouth burning | 3 (6.5) | 95% CI, 1.4–17.9 |
| Taste loss | 31 (67.4) | 95% CI, 52–80.5 |
| Baseline psychophysical olfactory function assessment, n (%) | ||
| Normal | 11 (23.9) | 95% CI, 12.6–38.8 |
| Mild hyposmia | 0 (0) | 97.5% CI, 0–0.8 |
| Moderate hyposmia | 13 (28.3) | 95% CI, 16–43.5 |
| Severe hyposmia | 10 (21.7) | 95% CI, 10.9–36.4 |
| Anosmia | 12 (26.1) | 95% CI, 14.3–41.1 |
| Baseline self‐reported olfactory function assessment, n (%) | ||
| Normal | 15 (32.6) | 95% CI, 19.5–48 |
| Partial loss | 18 (39.1) | 95% CI, 25.1–54.6 |
| Total loss | 13 (28.3) | 95% CI, 16–43.5 |
| 60‐Day psychophysical olfactory function assessment (n = 42), n (%) | ||
| Normal | 20 (47.6) | 95% CI, 32–63.6 |
| Mild hyposmia | 11 (26.2) | 95% CI, 13.9–42 |
| Moderate hyposmia | 5 (11.9) | 95% CI, 4–25.6 |
| Severe hyposmia | 3 (7.1) | 95% CI, 1.5–19.5 |
| Anosmia | 3 (7.1) | 95% CI, 1.5–19.5 |
| 60‐Day self‐reported olfactory function assessment, n (%) | ||
| Normal | 32 (76.2) | 95% CI, 60.6–87.9 |
| Partial loss | 8 (19) | 95% CI, 8.6–34.1 |
| Total loss | 2 (4.8) | 95% CI, 0.6–16.2 |
Abbreviations: CI, confidence interval; COVID‐19, coronavirus disease 2019; CT‐SS, computed tomography severity score; SD, standard deviation.
FIGURE 1.
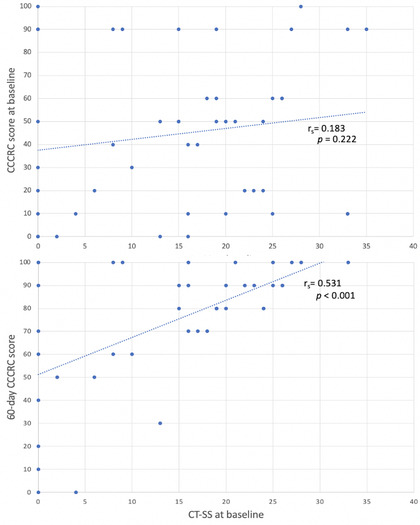
Correlation analysis between CCCRC scores at baseline and 60 days, and CT‐SS at baseline. Abbreviations: CCCRC, Connecticut Chemosensory Clinical Research Center; CT‐SS, computer tomography severity score
Forty‐two subjects completed 60‐day assessment (two patients died and two were lost to follow‐up). At this endpoint, 47.6% of patients had normal olfactory function, mild hyposmia was found in 26.2% whereas moderate or severe dysfunction persisted in 26.1% (Table 1). A significant directly proportional correlation was found between the CCCRC scores at 60 days and the CT‐SS at baseline (r s = 0.531; p < 0.001) (Figure 1).
DISCUSSION
The study of the correlations between olfactory scores and the severity of lung involvement, using a standardized and objective clinical outcome such as the CT‐SS, may help to determine the prognostic value of olfactory disorders in predicting the severity of COVID‐19. The correlation between CCCRC scores at baseline and CT‐SS was weak and not significant. This finding is in contrast with previous studies that found an inverse correlation between severity of COVID‐19 and self‐reported olfactory loss. 1 These may have been influenced by the subjective and retrospective nature of evaluation and by utilization of nonstandardized outcomes such as “need for hospitalization”.
An interesting finding is the significant directly proportional correlation between severity of the interstitial pneumonia at baseline and olfactory scores at 60 days. Better olfactory outcomes in more severe cases may be related to treatments prescribed, because most patients with severe respiratory disease were treated with dexamethasone. Preliminary trials of local and systemic corticosteroids suggest benefit in COVID‐19–related anosmia, 5 although further studies are required before these can be widely recommended. 6 However, a post hoc correlation analysis based on whether (25 patients, 59.5%) or not (17 patients, 40.5%) patients were treated with corticosteroids found nonsignificant correlations between CCCRC scores at 60 days and CT‐SS for both subgroups (treated group: r s = 0.318, p = 0.121; not treated group: r s = 0.237, p = 0.361). Potential selection bias should therefore be considered a limitation of the present study.
It could be hypothesized that cytokine storm associated with the severity of pneumonitis might be associated with greater severity and persistence of OD, due to an increase in nasal inflammatory cytokine. A statistically significant directly proportional correlation between the severity of chronic ODs and serum levels of interleukin 6 (IL‐6), released as part of the COVID‐19 cytokine storm has been reported. 7 In contrast, we found that patients with more severe lung disease, in whom levels of serum inflammatory cytokines would expect to be elevated, had better olfactory recovery. The initial injury to the olfactory epithelium is borne largely by sustentacular supporting cells that express highest levels of angiotensin‐converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) receptors. 8 Perhaps, higher circulating pro‐inflammatory cytokines initiate apoptosis of the infected cells and more effective viral clearance, followed by recovery and return of olfactory function. Given the severity of respiratory and other symptoms, transient loss of smell is then neglected by patients in retrospective anamnestic studies. In the absence of severe disease, the virus may persist, 9 allowing ongoing mild inflammation that appears to be associated with downregulation of the expression of olfactory sensory neuron (OSN) receptors 10 or may even allow direct infection of OSNs or indirect secondary damage. This may also represent a mechanism of potential benefit from corticosteroids.
This study recruited relatively small numbers of patients and it is likely that only more severely symptomatic patients underwent radiological investigations. Furthermore, different treatments were given, in part based on severity, which limits interpretation of the long‐term outcomes. Further research should look to include larger numbers of patients receiving standardized medical therapies and a wider range of markers of severity including inflammatory cytokine levels.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
Open Access Funding provided by Universita degli Studi di Sassari within the CRUI‐CARE Agreement.
Amadu AM, Vaira LA, Lechien JR, et al. Analysis of the correlations between the severity of lung involvement and olfactory psychophysical scores in coronavirus disease 2019 (COVID‐19) patients. Int Forum Allergy Rhinol. 2022;12:103–107. 10.1002/alr.22869
Antonio Matteo Amadu and Luigi Angelo Vaira contributed equally to this work and should be regarded as joint first authors.
Claire Hopkins and Giacomo De Riu contributed equally to this work and should be regarded as joint senior authors.
[Correction added on 16 May 2022, after first online publication: CRUI funding statement has been added.]
REFERENCES
- 1. Aziz M, Goyal H, Haghbin H, Lee‐Smith WM, Gajendran M, Perisetti A. The association of “loss of smell” to COVID‐19: a systematic review and meta‐analysis. Am J Med Sci. 2021;361:216‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Francone M, Iafrate F, Masci GM, et al. Chest CT score in COVID‐19 patients: correlation with disease severity and short‐term prognosis. Eur Radiol. 2020;30:6808‐6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang R, Li X, Liu H, et al. Chest CT severity score: an imaging tool for assessing severe COVID‐19. Radiol Cardiothorac Imaging. 2020;2:e200047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vaira LA, Lechien JR, Khalife M, et al. Psychophysical evaluation of the olfactory function: European multi‐center study on 774 COVID‐19 patients. Pathogens. 2021;10:E62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vaira LA, Hopkins C, Petrocelli M, et al. Efficacy of corticosteroid therapy in the treatment of long‐lasting olfactory disorders in COVID‐19 patients. Rhinology. 2021;59:21‐25. [DOI] [PubMed] [Google Scholar]
- 6. Huart C, Philpott CM, Altundag A, et al. Systemic corticosteroids in coronavirus disease 2019 (COVID‐19)‐related smell dysfunction: an international view. Int Forum Allergy Rhinol. 2021;11(7):1041‐1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cazzolla AP, Lovero R, Lo Muzio L, et al. Taste and smell disorders in COVID‐19 patients: role of interleukin‐6. ACS Chem Neurosci. 2020;11(17):2774‐2781. [DOI] [PubMed] [Google Scholar]
- 8. Lechien JR, Radulesco T, Calvo‐Henriquez C, et al. ACE2 & TMPRSS2 expressions in head & neck tissues: a systematic review. Head Neck Pathol. 2021;15:225‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dias De Melo G, Lazarini F, Levallois S, et al. COVID‐19–related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med. 2021;13(596):eabf8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodriguez S, Cao L, Rickenbacher GT, et al. Innate immune signaling in the olfactory epithelium reduces odorant receptor levels: modeling transient smell loss in COVID‐19 patients. medRxiv. Posted June 16, 2020. 10.1101/2020.06.14.20131128. Accessed July 13, 2021. [DOI] [Google Scholar]


