To the Editor,
1.
On October 2020, Sestili and Fimognari reported that acetaminophen (N‐aetyl‐para‐aminophenol), commonly known as paracetamol, induces or worsens glutathione (GSH) consumption in elderly patients affected by early or mild coronavirus disease 2019 (COVID‐19), thus greatly enhancing the risk of COVID‐19 exacerbation in these patients.1 By early COVID‐19, we mean the typical or commonly acknowledged symptomatology associated with the early phases of COVID‐19, occurring usually when a patient stays at home, that is, fever and dyspnea, besides weakness and pain,2 despite the COVID‐19 symptoms being particularly variable and complex andl only 50% of patients infected with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) developing a forthright symptomatology.2 In any case, fever is one of the most common symptoms during the early stages of COVID‐19, where people use paracetamol quite exclusively.
Reduction of GSH is a condition particularly severe for the individual's antioxidant and anti‐inflammatory response and it is comprehensible that its depletion is crucial for COVID‐19 worsening. Moreover, Zhang and colleagues, recently showed that SARS‐CoV‐2 hijacks folate and one‐carbon metabolism in the infected cell, by remodeling their biochemical turnover at the posttranscriptional level and going ahead with the de novo synthesis of purines.3 Figure 1 shows the fundamental role of GSH in one‐carbon metabolism. SARS‐CoV‐2 uses the cytosolic serine hydroxymethyltransferase‐1 to activate the one‐carbon metabolism for de novo synthesis of purines2 and subtracting serine, and its precursor folic acid hijacks serine from producing cystathionine and therefore GSH (Figure 1). Reduction in plasma and intracellular GSH levels is typical in elderly patients,4 particularly if affected by metabolic syndrome,5 therefore if Sestili and Fimognari are right, elderly patients with prodromic COVID‐19 symptomatology should not be treated with N‐acetyl‐para‐aminophenol.
Figure 1.
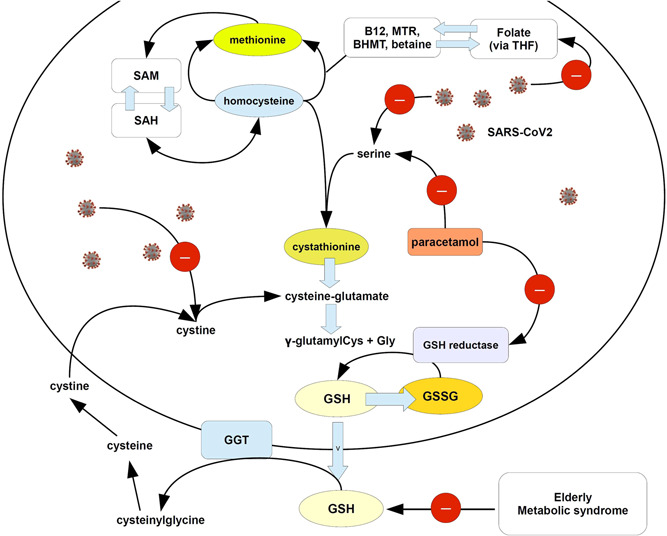
Cartoon showing the possible mechanism of exacerbation of GSH depletion in subjects infected with SARS‐CoV‐2 by paracetamol. As explained in the text, SARS‐CoV‐2 negatively affects the serine pathway leading to cystathionine synthesis and the uptake of folate, dysregulating the one‐carbon metabolism. Folate can be reduced to tetrahydrofolate (THF) and then converted to 5‐methyl THF, whereas a reaction catalyzed by methionine synthetase, transfers the methyl group of 5‐methyl‐THF to homocysteine, generating methionine. SARS‐COV‐2 impairs also other thiolic‐exchanging pathways, such as Cys. On the other hand, paracetamol can inhibit the serine pathway and the sulfur amino acid pathway (see Mast C, Dardevet D, Papet I. Impact of medication on protein and amino acid metabolism in the elderly: the sulfur amino acid and paracetamol case. Nutr Res Rev. 2018 Dec;31(2):179‐192.) and also GSH reductase (see Rousar T, Pařík P, Kucera O, Bartos M, Červinková Z. Glutathione reductase is inhibited by acetaminophen‐glutathione conjugate in vitro. Physiol Res. 2010;59(2):225‐232.). Arrows with red circle and minus sign = inhibition. BHMT, betaine homocysteine methyltransferase; GSH, glutathione; GSSG, oxidized GSH; SAH, S‐adenosyl homocysteine; SAM, S‐adenosylmethionine; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
In addition, Sestili and Fimognari considered the hypothesis that COVID‐19 severity may be caused via a glucose‐6‐phosphate dehydrogenase deficiency, which parallels GSH decrease.6, 7 Actually, in those cases, a warning was forwarded about the use of Tylenol®‐paracetamol, which finally is not recommended.8 Despite some wise recommendations, Linda Geddes spoke about “The fever paradox,” reporting how much paracetamol was abused in the healthcare market to address the symptoms of COVID‐19 in its early development and prevent crowding in hospitalization.9 In Italy, a civil outcry from some physicians, practitioners, and family doctors, is expanding the debate, even in politics, about how best to treat COVID‐19 at home. The civil legacy of these professionals was arranged to prevent the huge concern of elderly people treated with simple paracetamol, counseled to wait under paracetamol therapy for reduced symptoms, yet then often undergoing rapid exacerbation and in many cases even death while being hospitalized.
Suter and colleagues recently created an algorithm of the best and simplest home therapy for mild symptoms in early COVID‐19, to prevent hospitalization.10 In their retrospective observational study, the control cohort (45 patients on 77; 58.44%) received paracetamol as home therapy, whereas in the cohort of patients following a recommended protocol only 6 of 86 (6.98%) used paracetamol as the leading therapy. The rate of hospitalization was 1.2% for patients undergoing the recommended protocol and 13.1% (p = .007) for patients using predominantly paracetamol, that is, 44 cumulative days of hospitalization (recommended) versus 481 (controls).10 This evidence shows that using paracetamol at home to treat mild COVID‐19 symptoms, particularly in older adults with comorbidity, greatly enhanced the risk of hospitalization for dyspnea from interstitial pneumonia, so increasing the huge concern of crowding the intensive care units. Possible causes of this exacerbation might be the activation of prothrombotic mechanisms, currently reported as the leading pathogenetic cause of COVID‐19, alongside endothelial dysfunction.11 Actually, GSH modulates platelet functions12 and deep venous thrombosis, which may occur in severe COVID‐19, and worsens GSH levels by enhancing glutathione peroxidase.13 Moreover, hospitalization includes also the additional risk to get worse COVID‐19 pneumonia due to hospital‐acquired infections, even increasing the rate of mortality.14
The use of paracetamol to reduce fever should be considered particularly safe, if mild COVID‐19 has not yet been diagnosed, at least in the professional intention of the majority of physicians. On the other hand, fever is one of the early symptoms of a possible SARS‐CoV‐2 infection. Fever is usually associated with inflammation symptomatology (asthenia, muscular pain, cough) and, if more correct therapy information is widespread among healthcare professionals, further therapies, such as nonsteroidal anti‐inflammatory drugs, should have priority in their recommendation.10
In 2019, according to the Italian Agency for Therapic Drugs (AIFA), paracetamol (or acetaminophen, single active principle) represented 11.4% of the total economic burden for therapeutic drugs in Italy and the first drug purchased by the local healthcare units in the country with own expenditures. This rank increased significantly in 2020, reaching an enhancement of about 50 packages/day every 10,000 inhabitants in January‐February 2020, with respect to 16–20 packages purchased in December 2019. These data are easily retrievable from the AIFA website. Certainly, it would be particularly awkward to state that the huge increase in elderly patients entering the intensive care units or the number of deaths for acute and severe respiratory distress from COVID‐19, may have the causative source in the intake of sole paracetamol while staying at home, waiting for a further medical consultancy or hoping for the disappearance of painful symptoms. However, it appears undoubtedly confirmed that patients using paracetamol as the elective home therapy in the early stage of SARS‐CoV‐2 infection, had a higher risk to be hospitalized.10
The Guideline documentation provided by the Ministry of Health on November 30th 2020, then updated on April 26th 2021 by adding nonsteroidal anti‐inflammatory drugs for managing patients with COVID‐19 at home and discouraging them from being hospitalized, suggested: “a watchful waiting attitude” and “paracetamol for treating symptoms” (Note 1). At the indicated date, on the basis of data reported by the Ministry of Health in Italy on November 30th 2020, the relative risk we calculated to be hospitalized following these recommendations should be not far from 1.7981 (CI95 = 1.7234‐1.8760, p < .001), odds ratio = 1.8283 (CI95 = 1.7507‐1.9094, p < .001, considering also the trend reported by others.10 Moreover, the probability to be hospitalized in intensive care units within 10 days of “watchful waiting” may be higher than 65% (65.18%) in a Bayesian calculation. Therefore, resting on this estimation, one possible conclusion must be derived. The pharmacological reasons for this failure have been introduced in this manuscript and should be taken seriously into full consideration to formulate new therapy protocols and approved guidelines.
The warning must be taken into account, when considering paracetamol in elderly people with a symptomatology presumptive of SARS‐CoV‐2 infection, before being confirmed by a swab. Although as reported in Suter et al.,10 the initial recommendations from AIFA in 2020 included paracetamol as an elective, practical therapy at home to reduce symptoms in COVID‐19 and loosening the vise upon hospitals, the new recommended protocols, proposed by a group of physicians, may cause criticism of the management of pandemic by politicians and academicians in Italy.
Scientific research must always lead the debate towards ameliorating any good proposal and warding off this raw and worrisome emergency.
CONFLICT OF INTERESTS
The authors declare that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
Sergio Pandolfi conceived the rationale, contributed in conceiving the paper, contributed in writing the paper, and revised the paper. Vincenzo Simonetti revised the paper. Giovanni Ricevuti revised the paper, and contributed in the statistics. Salvatore Chirumbolo conceived the paper, performed the study, wrote the manuscript, and submitted the manuscript.
DATA AVAILABILITY STATEMENT
This is not a Research Paper. Anyway, readers may contact the corresponding author any time for elucidations on this paper.
Note 1
REFERENCES
- 1.Sestili P, Fimognari C. Paracetamol‐Induced Glutathione Consumption: Is There a Link With Severe COVID‐19 Illness? Front Pharmacol. 2020;11:579944. 10.3389/fphar.2020.579944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Vito A, Fiore V, Princic E, et al. Predictors of infection, symptoms development, and mortality in people with SARS‐CoV‐2 living in retirement nursing homes. PLOS One. 2021;16(3):e0248009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y, Guo R, Kim SH, et al. SARS‐CoV‐2 hijacks folate and one‐carbon metabolism for viral replication. Nat Commun. 2021;12(1):1676. 10.1038/s41467-021-21903-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sekhar RV, Patel SG, Guthikonda AP, et al. Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation. Am J Clin Nutr. 2011;94(3):847‐853. 10.3945/ajcn.110.003483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sekhar RV, McKay SV, Patel SG, et al. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care. 2011;34(1):162‐167. 10.2337/dc10-1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aydemir D, Ulusu NN. Is glucose‐6‐phosphate dehydrogenase enzyme deficiency a factor in Coronavirus‐19 (COVID‐19) infections and deaths? Pathog Glob Health. 2020;114(3):109‐110. 10.1080/20477724.2020.1751388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdel Hafez SMN. Glucose‐6‐phosphate dehydrogenase deficiency enhances Covid‐19 infection in elderly people. Bratisl Lek Listy. 2020;121(11):786‐788. 10.4149/BLL_2020_128 [DOI] [PubMed] [Google Scholar]
- 8.Richardson SR, O'Malley GF. Glucose 6 Phosphate Dehydrogenase Deficiency. 2020 Jun 25. In: StatPearls [Internet]. StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 9.Geddes L. The fever paradox. New Sci. 2020;246(3277):39‐41. 10.1016/S0262-4079(20)30731-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suter F, Consolaro E, Pedroni S, et al. A Simple, Home‐Therapy Algorithm to Prevent Hospitalization for COVID‐19 Patients. A Retrospective Observational Matched‐Cohort Study medRxiv. 2021. 10.1101/2021.03.25.21254296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonaventura A, Vecchié A, Dagna L, et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID‐19. Nat Rev Immunol. 2021;21(5):319‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuda S, Ikeda Y, Aoki M, Toyama K, Watanabe K, Ando Y. Role of reduced glutathione on platelet functions. Thromb Haemost. 1979;42(4):1324‐1331. [PubMed] [Google Scholar]
- 13.Türker FS, Malbora A, Erisir M. Oxidative status and antioxidant enzyme levels in deep venous thrombosis patients. Am J Cardiovasc Dis. 2021;11(1):176‐183. [PMC free article] [PubMed] [Google Scholar]
- 14.Chirumbolo S, Simonetti V, Franzini M, Valdenassi L, Bertossi D, Pandolfi S. Estimating COVID‐19‐caused deaths in hospitals and healthcare units: did hospital acquired infections play the utmost role? Comments with a proposal. Infect Control Hosp Epidemiol. 2021:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is not a Research Paper. Anyway, readers may contact the corresponding author any time for elucidations on this paper.
Note 1


