Abstract
The antiviral remdesivir has been shown to decrease the length of hospital stay in coronavirus disease 2019 (COVID‐19) patients requiring supplemental oxygen. However many patients decompensate despite being treated with remdesivir. To identify potential prognostic factors in remdesivir‐treated patients, we performed a retrospective cohort study of patients hospitalized at NewYork‐Presbyterian Hospital/Weill Cornell Medical Center between March 23, 2020 and May 27, 2020. We identified 55 patients who were treated with remdesivir for COVID‐19 and analyzed inflammatory markers and clinical outcomes. C‐reactive protein (CRP), d‐dimer, and lactate dehydrogenase levels were significantly higher in patients who progressed to intubation or death by 14 days compared to those who remained stable. CRP levels decreased significantly after remdesivir administration in patients who remained nonintubated over the study period. To our knowledge, this is the largest study to date examining inflammatory markers before and after remdesivir administration. Our findings support further investigation into COVID‐19 treatment strategies that modify the inflammatory response.
Keywords: coronavirus disease 2019, inflammatory markers, remdesivir
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) has affected millions of people worldwide and caused a global pandemic. There is evidence that some patients with the most severe form of the disease have an exuberant immune response similar to cytokine release syndrome or sepsis. 1 This phenotype is characterized by persistent fevers, elevated inflammatory markers, and multiorgan failure and is associated with high mortality rates. Retrospective analyses have found that levels of C‐reactive protein (CRP), interleukin‐6 (IL‐6), d‐dimer, ferritin, and lactate dehydrogenase were higher in patients who died compared to survivors. 2 , 3 To date, the only therapies proven to decrease mortality in severe COVID‐19 are immunomodulatory, which suggests the importance of an exuberant immune response in severe COVID‐19. 4 Remdesivir, an antiviral COVID‐19 therapy, has an unknown effect on the inflammatory response in COVID‐19 patients. The aim of this study was to examine the association of inflammatory markers and clinical outcomes in patients treated with remdesivir. This study reflects the clinical experience at a major medical center in New York City at the height of the pandemic.
2. METHODS
To examine the association of inflammatory markers and clinical outcomes in patients treated with remdesivir, we performed a retrospective cohort study of patients hospitalized with severe COVID‐19 pneumonia who were treated with remdesivir at NewYork‐Presbyterian Hospital/Weill Cornell Medical Center between March 23, 2020 and May 27, 2020. These patients were part of Phase 3 randomized, open‐label, multicenter study of remdesivir therapy in patients with severe COVID‐19 (NCT04292899). Severe COVID‐19 was defined as an oxygen saturation of less than or equal to 94% on room air or requirement of supplemental oxygen. For our study, we excluded patients who were mechanically ventilated at the time of remdesivir administration (N = 20), patients who received tocilizumab or eculizumab (N = 4) and patients who received less than three doses of remdesivir (N = 2). Patients who also received hydroxychloroquine before remdesivir were included as this was local standard of care at the time of the study (N = 29). Patients who received corticosteroids were also included (N = 27). Our primary outcome variable was whether patients were on mechanical ventilation or deceased by Day 14 (progressors) or remained alive and nonintubated (nonprogressors). We used the electronic medical record to obtain levels of inflammatory markers including d‐dimer, IL‐6, CRP, and ferritin from 4 days before remdesivir administration to 14 days after. Day 1 was considered the first day of remdesivir administration. We performed descriptive statistics using Fisher's exact test and the Wilcoxon rank‐sum test to compare demographic variables as well as median levels of inflammatory markers in progressors versus nonprogressors. For CRP, we conducted a time‐point analysis by comparing pretreatment (Days 4 through 2), on‐treatment (Days 3 through 9) and posttreatment (Days 10 through 15) levels. A linear mixed‐effects model was used to compare CRP levels for progressors and nonprogressors over time. We fit one model with time‐point and progression status, and a second model that added the interaction between time and progression. Three patients were not included in the mixed‐effects model as they were missing pretreatment CRP data.
3. RESULTS
Of the 55 patients included, 9 were progressors and 46 were nonprogressors. The median age was 66 in progressors and 62 in nonprogressors, and 36% overall were women. The median CRP throughout the study period was significantly higher in progressors compared to nonprogressors (24 vs. 9 mg/L; p < 0.001), as shown in Table 1. The median d‐dimer and lactate dehydrogenase were also higher in progressors than in nonprogressors (871 vs. 576 ng/ml; p = 0.002, 511 vs. 392 U/L; p < 0.001, respectively). Median levels of ferritin and IL‐6 were higher in progressors compared to nonprogressors however the trend did not reach significance (1225 vs. 1033 ng/ml; p = 0.17, 32 vs. 18 pg/ml; p = 0.39, respectively).
Table 1.
Demographics and inflammatory markers in progressors and nonprogressors
| Progressors (n = 9) | Nonprogressors (n = 46) | p Value | |
|---|---|---|---|
| Age | 66 (58, 68) | 62 (56, 69) | 0.82 |
| Sex | 0.46 | ||
| Female | 2 (22%) | 18 (39%) | |
| CRP (mg/dl) | 24 (14, 29) | 9 (5, 15) | <0.001 |
| d‐dimer (ng/ml) | 871 (470, 2656) | 576 (293, 1598) | 0.002 |
| LDH (U/L) | 511 (396, 647) | 392 (315, 494) | <0.001 |
| Ferritin (ng/ml) | 1225 (878, 2085) | 1033 (543, 1657) | 0.17 |
| IL‐6 (pg/ml) | 32 (20, 50) | 18 (9, 120) | 0.39 |
Note: Statistics presented: n (%), median (IQR) throughout the study period.
Abbreviations: CRP, C‐reactive protein; IL‐6, interleukin 6; IQR, interquartile range; LDH, lactate dehydrogenase.
In the mixed‐effect analysis, we found that patients on‐treatment (β = −4.43, p = 0.003) and posttreatment (β = −6.94, p < 0.001) had significantly lower median CRP compared to pretreatment levels. Adjusting for the time period, nonprogressors had significantly lower median CRP compared to progressors (β = −10.01, p < 0.001).
After adding the two‐way interaction for time and progressor status, results showed that median CRP between the two groups on‐treatment compared to pretreatment CRP was significantly different (β = −7.87, p = 0.027). At posttreatment (compared to pretreatment), nonprogressors had significantly decreased CRP compared to progressors (β = −9.76, p = 0.008; Figure 1).
Figure 1.
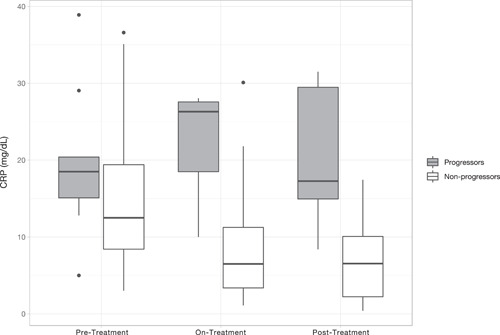
Box‐plot comparing median CRP by time‐point (pretreatment, on‐treatment, posttreatment) and cohort (progressors and nonprogressors). CRP, C‐reactive protein
4. DISCUSSION AND LIMITATIONS
In this observational study, we describe inflammatory markers in patients hospitalized with COVID‐19 and treated with remdesivir. To our knowledge, ours is the largest study to date examining inflammatory markers before and after remdesivir administration. We found that median CRP, d‐dimer, and LDH levels were higher in patients who progressed to intubation or death by 14 days compared to those who did not progress. We found that the median ferritin and IL‐6 levels were also higher in progressors, however, the differences were not significant. It is possible that this is because there were fewer recorded values for ferritin and IL‐6 compared to the other inflammatory markers. Our data are consistent with prior studies and suggest that elevated CRP, d‐dimer, and LDH levels can be used as predictors of poor clinical outcomes including mechanical ventilation and death in patients with severe COVID‐19 pneumonia. Many of these studies however do not disclose which treatment, if any, their patients were receiving. 5 , 6 , 7 , 8 Our study demonstrates that these markers can be reliable reflections of clinical status in patients treated with remdesivir.
We found that CRP levels decreased significantly after remdesivir administration in nonprogressors compared to progressors. Our results are consistent with the hypothesis that remdesivir attenuates the inflammatory response in a certain subset of patients but not in all. This is in line with several studies showing that COVID‐19 patients treated with a 5 or 10 days course of remdesivir had a shorter time to recovery than those who received placebo. 9 , 10 , 11 Current recommendations from the Infectious Diseases Society of America and the National Institutes of Health advise treatment with remdesivir in hospitalized patients with COVID‐19 who require supplemental oxygen. 12 , 13 Of note, the World Health Organization recommends against remdesivir in light of their Solidarity trial findings that it had no effect on mortality rates or duration of hospital stay in COVID‐19 patients. 14 In addition a recent large cohort study found that remdesivir was associated with longer hospital stays and had no survival benefit. 15 Further work is needed to understand the link between antiviral activity and the subsequent inflammatory response. However because we did not have a control group, it is unclear whether the differences we saw between progressors and nonprogressors were due to remdesivir, the natural course of disease, or another factor, such as corticosteroids, which many of our study patients received (78% of progressors and 38% of nonprogressors). Future studies examining other inflammatory markers including erythrocyte sedimentation rate, fibrinogen, and complement levels may give further insight into prognostic indicators and potential therapeutic targets. 16 , 17
5. CONCLUSION
In sum, we found that inflammatory markers were higher in COVID‐19 patients treated with remdesivir who had poor clinical outcomes compared to patients who remained stable. In addition, CRP levels decreased significantly after remdesivir administration in patients who remained nonintubated over the study period. Our findings support further investigation into COVID‐19 treatment strategies that modify the inflammatory response.
AUTHOR CONTRIBUTIONS
Kate Stoeckle analyzed the data and wrote the manuscript, Britta Witting collected the patient data, Anjile An conducted statistical analyses, Shashi Kapadia and Kristen Marks supervised the findings of this study. All authors discussed the results and contributed to the final manuscript.
CONFLICT OF INTERESTS
Kristen Marks and Shashi Kapadia are investigators on research grants paid to the institution from Gilead Sciences Inc., for the study of hepatitis C unrelated to the current work. Shashi Kapadia receives research funding paid to the institution from Verily Life Sciences for research related to coronavirus disease 2019.
ACKNOWLEDGMENTS
The authors thank the participants and study staff for their dedication to the study. Research grant paid to the institution from Gilead Sciences Inc., for the conduct of Phase 3 remdesivir clinical trial (NCT04292899). Anjile An was partially supported by the Clinical and Translational Science Center at Weill Cornell Medical College (1‐UL1‐TR002384‐01).
Stoeckle K, Witting B, Kapadia S, An A, Marks K. Elevated inflammatory markers are associated with poor outcomes in COVID‐19 patients treated with remdesivir. J Med Virol. 2021;94:384‐387. 10.1002/jmv.27280
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 2020;383(23):2255‐2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. RECOVERY Collaborative Group , Horby P., Lim W. S., et al. Dexamethasone in hospitalized patients with Covid‐19. N Engl J Med. 2020;384(8):693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Herold T, Jurinovic V, Arnreich C, et al. Elevated levels of IL‐6 and CRP predict the need for mechanical ventilation in COVID‐19. J Allergy Clin Immunol. 2020;146(1):128‐136.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poggiali E, Zaino D, Immovilli P, et al. Lactate dehydrogenase and C‐reactive protein as predictors of respiratory failure in CoVID‐19 patients. Clin Chim Acta. 2020;509:135‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu F, Li L, Xu M, et al. Prognostic value of interleukin‐6, C‐reactive protein, and procalcitonin in patients with COVID‐19. J Clin Virol. 2020;127:104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li W, Lin F, Dai M, et al. Early predictors for mechanical ventilation in COVID‐19 patients. Ther Adv Respir Dis. 2020;14:1753466620963017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid‐19—final Report. N Engl J Med. 2020;383(19):1813‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID‐19: a randomized clinical trial. JAMA. 2020;324(11):1048‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garibaldi BT, Wang K, Robinson ML, et al. Comparison of time to clinical improvement with vs without remdesivir treatment in hospitalized patients with COVID‐19. JAMA Netw Open. 2021;4(3):e213071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America Guidelines on the treatment and management of patients with COVID‐19. Clin Infect Dis. 2021. 10.1093/cid/ciaa478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. COVID‐19 Treatment Guidelines Panel . Coronavirus Disease 2019 (COVID‐19) Treatment Guidelines. National Institutes of Health. Accessed July 7, 2021. https://www.covid19treatmentguidelines.nih.gov/ [PubMed]
- 14. WHO Solidarity Trial Consortium C, Pan H, Peto R, et al. Repurposed antiviral drugs for Covid‐19—interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ohl ME, Miller DR, Lund BC, et al. Association of remdesivir treatment with survival and length of hospital stay among US veterans hospitalized with COVID‐19. JAMA Netw Open. 2021;4(7):e2114741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gavriilaki E, Brodsky RA. Severe COVID‐19 infection and thrombotic microangiopathy: success does not come easily. Br J Haematol. 2020;189(6):227. [DOI] [PubMed] [Google Scholar]
- 17. Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with Covid‐19. N Engl J Med. 2021;384(9):795‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


