1. INTRODUCTION
Approval of the first vaccines against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has dramatically changed the prospects of controlling coronavirus disease 2019 (COVID‐19). According to the first available data, these vaccines appear effective and safe, but no clinical trials have included patients with rheumatic diseases, and no data exists on efficacy and safety in such subjects.
2. CASE REPORT
In this regard, we report the case of a 77‐year‐old male, who presented with necrotic ulcers of the lower limbs in September 2020. In August he had suffered an acute episode of mild‐to‐moderate dyspnea, followed by hematuria and lower limb purpura. Dyspnea and hematuria resolved rapidly after self‐administration of glucocorticoids, whereas purpura worsened, eventually leading to large ulcers. Blood chemistry showed slight elevation of C‐reactive protein (CRP) and serum creatinine, while urinalysis showed proteinuria and hemoglobinuria.
Autoimmunity blood tests were all negative, whereas skin biopsy was compatible with leukocytoclastic vasculitis. A diagnosis of microscopic polyangiitis was eventually made and the patient was treated with prednisone, tapered slowly to 6.25 mg/day, and methotrexate (MTX) 15 mg/week, which led to prompt and complete resolution of skin involvement.
After 4 months of treatment, the patient, by now considered to be in remission, was called for vaccination against COVID‐19 and he decided to temporarily suspend MTX. A few days after the first shot of BioNtech/Pfizer Comirnaty, he experienced severe, rapidly worsening dyspnea and presented at the emergency unit. Arterial blood gas analysis showed severe hypoxemia (pO2 48 mm Hg) and low oxygen saturation, while blood chemistry showed elevation of CRP (3.7 mg/dL) and creatinine (1.55 mg/dL), in addition to hemoglobinuria. Lung function tests showed severe restrictive deficit and severe reduction of diffusing capacity of the lung for carbon monoxide (<35%). High‐resolution computed tomography (HRCT) of the chest showed diffuse “ground‐glass” opacities with superimposed septal thickening and subpleural consolidations (Figure 1).
Figure 1.
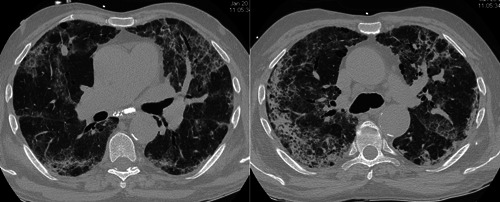
(A) and (B). High‐resolution computed tomography performed on admission to emergency care showed bilateral ground‐glass opacities with superimposed septal thickening (arrows) and subpleural consolidations (arrowheads)
The patient required high‐flow oxygen (12 L/min, FiO2 50%) and was treated with piperacillin/tazobactam and high‐dose intravenous methylprednisolone. After 7 days of hospitalization, the patient was eupneic and no longer required oxygen. Blood chemistry showed normalization of CRP (0.11 mg/dL) and creatinine (1.1 mg/dL) and the patient was discharged in good clinical condition. Nasopharyngeal swab for SARS‐CoV‐2 remained negative, as well as both IgM and IgG performed 1 month after the vaccination. HRCT showed radical rapid improvement of consolidations and ground‐glass opacities, suggesting resolution of vasculitis (Figure 2).
Figure 2.
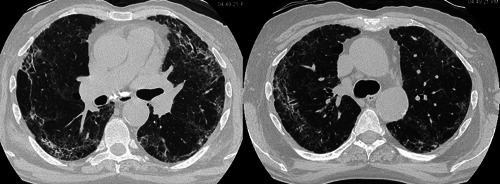
(A) and (B). High‐resolution computed tomography performed 7 days later showed significant improvement in radiological findings (arrows and arrowheads)
3. DISCUSSION
At the present time, four vaccines against COVID‐19 have been authorized by the European Medicines Agency.
Certain scientific societies, 1 , 2 , 3 after an experience with many other nonlive vaccines, do not preclude vaccination of rheumatic patients, nor do they suggest the need to withdraw immunosuppressive treatment, except for rituximab. At the same time, like for any other vaccination, patients to be vaccinated should preferably have low disease activity and a stable dose of immunosuppressants.
It is well known that many autoimmune rheumatic diseases may be triggered by infectious stimuli, particularly viruses. 4 Another major trigger may be vaccines themselves: vaccines may even trigger different subsets of diseases, particularly in immune‐compromised and/or elderly subjects. 5
It is therefore not surprising that SARS‐CoV‐2 may trigger the development and/or relapse of certain autoimmune disorders, including vasculitis. To the best of our knowledge, few papers have reported cases of COVID‐induced vasculitis in adulthood: these have usually concerned IgA‐related, 6 , 7 antineutrophil cytoplasmic antibodies (ANCA)‐associated, 8 , 9 or large‐vessel forms. 10 , 11 , 12 Presentation may be extremely variable and, in cases of lung involvement, the differential diagnosis between idiopathic vasculitis and COVID‐19 may be challenging. 8 , 9 On the other hand, there have not yet been any reports of cases of patients whose rheumatic condition has worsened or even relapsed after vaccination against COVID‐19.
The present case report describes the first immune‐related adverse event due to Comirnaty in a rheumatic patient considered to be in remission. Relapse presented without skin involvement, but with severe worsening of lung involvement, leading to immediate hospitalization and oxygen therapy. Fortunately, recovery was rapid, and the patient did not require any immunosuppressants other than glucocorticoids.
This case shows that the immune response elicited by vaccination against COVID‐19 may reactivate autoimmune disorders such as vasculitis. The physiopathological mechanisms have not been fully understood; nevertheless, we may hypothesize that vaccination, through the production of antibodies against SARS‐CoV2 spike glycoprotein, eventually cross‐reacting against host peptides, 13 may trigger pre‐existing aberrant immune mechanisms. 14 In the case of ANCA‐associated vasculitis, auto‐antibodies production, and neutrophils and complement activation both lead to vascular damage and vasculitis relapse.
The presentation may be severe and require hospitalization, but symptoms seem to be manageable with conventional treatments. Further data from larger cohorts will provide more robust evidence for the prevention and treatment of immune‐related adverse events of COVID‐19 vaccines.
The withdrawal of MTX may have been a factor in unleashing the vasculitis and an aberrant immune response in our patient. As already suggested by available guidelines, 3 it may therefore be advisable not to suspend immunosuppressants, before vaccination.
CONFLICT OF INTERESTS
The authors declare that there are no conflicts of interest.
ETHICS STATEMENT
The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
CONSENT FOR PUBLICATION
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
AUTHOR CONTRIBUTIONS
Edoardo Conticini, Miriana d'Alessandro, Laura Bergantini, and Elena Bargagli wrote the paper. Francesco Gentili and Maria Antonietta Mazzei performed HRCT and collected radiological images. Edoardo Conticini and Elena Bargagli performed the clinical diagnosis of vasculitis. Elena Bargagli, Luca Cantarini, and Bruno Frediani corrected the draft and supervised the work.
ACKNOWLEDGMENT
No funding was received for this study.
REFERENCES
- 1. Greek Rheumatology Society and Professional Association of Rheumatologists (ERE‐EPERE) . Vaccination against SARS‐CoV‐2 in immunosuppressed patients with rheumatic diseases: position statement of the Greek Rheumatology Society. Mediterr J Rheumatol. 2020;31(4):430‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Specker C, der Ad‐hoc‐Kommission COVID‐19 DGRh, Schulze‐Koops H, der Vorstand DGRh. Impfung gegen SARS‐CoV‐2 bei entzündlich rheumatischen erkrankungen: empfehlungen der DGRh für Ärzte und patienten [Vaccination against SARS‐CoV‐2 in inflammatory rheumatic diseases: recommendations of the German Society for Rheumatology for physicians and patients]. Z Rheumatol. 2021;80(1):43‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bijlsma JW. EULAR December 2020 view points on SARS‐CoV‐2 vaccination in patients with RMDs. Ann Rheum Dis. 2021;80(4):411‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lutalo PM, D'Cruz DP. Diagnosis and classification of granulomatosis with polyangiitis (aka Wegener's granulomatosis). J Autoimmun. 2014;48‐49:94‐98. [DOI] [PubMed] [Google Scholar]
- 5. Falsetti P, Conticini E, Acciai C, et al. Polymyalgia rheumatica following infective triggers or vaccinations: a different subset of disease? Reumatologia. 2020;58(2):76‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. AlGhoozi DA, AlKhayyat HM. A child with Henoch‐Schonlein purpura secondary to a COVID‐19 infection. BMJ Case Rep. 2021;14(1):e239910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Allez M, Denis B, Bouaziz JD, et al. COVID‐19‐related IgA vasculitis. Arthritis Rheumatol. 2020;72(11):1952‐1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bressler MY, Pathak N, Cervellione K, et al. New onset granulomatosis with polyangiitis associated with COVID‐19. Case Rep Dermatol Med. 2021;2021:8877292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luecke E, Jeron A, Kroeger A, et al. Eosinophilic pulmonary vasculitis as a manifestation of the hyperinflammatory phase of COVID‐19. J Allergy Clin Immunol. 2021;147(1):112‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oda R, Inagaki T, Ishikane M, et al. Case of adult large vessel vasculitis after SARS‐CoV‐2 infection. Ann Rheum Dis. 2020. annrheumdis‐2020‐218440. [DOI] [PubMed] [Google Scholar]
- 11. Shergill S, Davies J, Bloomfield J. Florid aortitis following SARS‐CoV‐2 infection. Eur Heart J. 2020;41(44):4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Manenti A, Farinetti A, Manco G, Mattioli A. Vasculitis and aortitis: COVID‐19 challenging complications. J Vasc Surg. 2021;73(1):347‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vojdani A, Kharrazian D. Potential antigenic cross‐reactivity between SARS‐CoV‐2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Akinosoglou K, Tzivaki I, Marangos M. COVID‐19 vaccine and autoimmunity: awakening the sleeping dragon. Clin Immunol. 2021;226:108721. [DOI] [PMC free article] [PubMed] [Google Scholar]


