To the Editor,
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and the resulting pandemic has led to the rapid production of several vaccines with more than 1.4 billion doses provided to date. 1 Although serious adverse events have remained uncommon, the ChAdOx1 nCov‐19 vaccine, a recombinant chimpanzee adenoviral vector encoding the spike protein of SARS‐CoV‐2, has been associated with thrombotic thrombocytopenia syndrome. 2 We present the first case of hemophagocytic lymphohistiocytosis (HLH) precipitated by the ChAdOx1 nCov‐19 vaccine.
A 68‐year‐old man presented to the hospital on May 8 2021 with 7 days of fevers, rigors, lethargy, and night sweats. He received his first dose of the ChAdOx1 nCov‐19 vaccination on April 20, 2021. On presentation, he had no focal infective symptoms and was commenced on intravenous flucloxacillin and gentamicin to treat fever of unknown origin. There were no clinical features of thrombosis. He had no infectious contacts or contact with animals. His travel history was notable for a trip to a rural area 2 months prior where he reported there were numerous mosquitoes. Physical examination revealed splenomegaly, however, was otherwise unremarkable. His comorbidities included hypertension, gout, and Bowen's disease. His medications included quinapril, diltiazem, prazosin, hydrochlorothiazide, colchicine, and allopurinol. On presentation he had hyponatremia with a sodium level of 125 mmol/L, elevated LDH of 854 U/L (120–250 U/L), low platelet count of 59 × 109/L, elevated ferritin of 8498 μg/L, normal fibrinogen of 2.3 g/L, elevated d‐dimer of more than 10 mg/L ( <0.5 mg/L), elevated aspartate aminotransferase of 223 U/L, and alanine aminotransferase of 121 U/L. Recent blood tests in September 2020 demonstrated a normal platelet count, ferritin level, and liver function tests.
Computed tomography (CT) chest/abdomen/pelvis demonstrated splenomegaly of 16 cm and subcentimetre para‐aortic lymphadenopathy, with no focus of infection. Urine and blood cultures yielded no growth. A nasopharyngeal aspirate for coronavirus disease 2019 (COVID‐19) (real‐time polymerase chain reaction assay) and other respiratory viral infections was negative (Allpex Respiratory Panel, Seegene). Cytomegalovirus and Epstein‐Barr virus serologies (Abbott Architect), monospot, and DNA polymerase chain reactions (Argene) were negative. Hepatitis B, C, and HIV serologies were negative. Q fever (Virion) and rickettsial serologies (immunofluorescence assay) were negative.
A bone marrow biopsy demonstrated evidence of hemophagocytosis (Figure 1). Flow cytometry of bone marrow demonstrated a small population (1% total nucleated cells and 25% of lymphocytes) of CD4−/CD8− TCR gamma‐delta T‐cell population with no aberrant phenotype. Positron emission tomography scan demonstrated fluorodeoxyglucose (FDG) avidity in the spleen and bone marrow, and a 12‐mm FDG‐avid (SUV 3.4) aortocaval lymph node. His fasting triglyceride level was elevated at 2.3 mmol/L. His soluble CD25 was elevated at 733 U/ml (27–118 U/ml), and NK cell function was normal.
Figure 1.
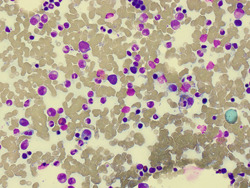
Bone marrow aspirate demonstrating evidence of hemophagocytosis
He had persistent fevers to 39.6°C during the first 4 days of his admission, with a rise in his ferritin to 11 801 μg/L. By the fifth day, his fevers resolved, with improvement in his lethargy and malaise. Four weeks later, his ferritin had reduced to 710 μg/L (Figure 2), platelet count rose to 198 × 109/L, and repeat CT abdomen/pelvis demonstrated resolution of his splenomegaly. Convalescent serologies for influenza, Q fever, rickettsial, Barmah Forest, Ross River, and enterovirus were negative.
Figure 2.
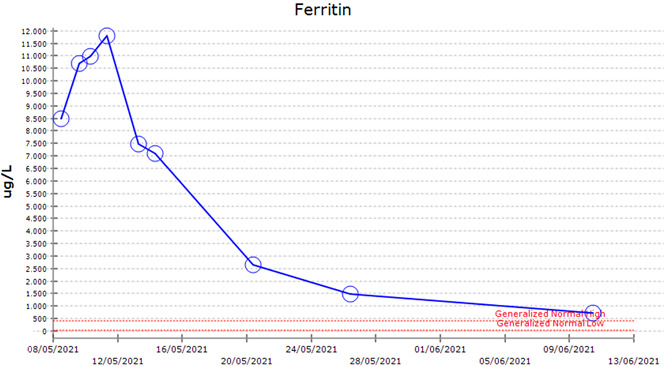
Ferritin level over time
HLH is a disorder of excessive inflammation and tissue destruction characterized by a hyperinflammatory immune state. This condition can be hereditary or precipitated by triggers including infections, malignancy, or medications. 3 The diagnosis of HLH is commonly based on criteria published in the HLH‐2004 study, 4 however, these diagnostic criteria were developed for children and are not validated formally in adults. The H‐score is a diagnostic scoring system for HLH developed for adults based on clinical, biologic, and cytologic parameters. 5 Our patient's H‐score is 250, which is associated with a more than 99% probability of having HLH.
In randomized controlled trials of the ChAdOx1 vaccine, the most common side effects reported were injection‐site pain and tenderness. Fatigue, headache, fevers, and myalgias were the most common systemic adverse reactions. 6 There have been case reports of autoimmune phenomena such as Guillain–Barre syndrome 7 following ChAdOx1 vaccination. There is now a well‐described association between HLH and other systemic inflammatory syndromes in the setting of severe infection with SARS‐CoV‐2. 8 There has been one recently described case report of HLH following a COVID‐19 vaccination, however, this was an inactivated SARS‐CoV‐2 vaccine in a patient with an active EBV infection. 9
In our patient, there was no clear precipitant of HLH other than the ChAdOx1 vaccine. There was no evidence of an infection. The small CD4−/CD8− gamma‐delta T cell population in his bone marrow is commonly seen as a reactive feature in immune dysregulation. 10 The FDG avidity in his spleen and bone marrow is commonly seen in HLH. 11 Due to our patient's clinical stability, resolution of symptoms, and improvement of HLH parameters he did not require HLH specific therapy. It is unclear if he had a pre‐existing genetic predisposition to HLH as genetic testing is pending, however, it is unlikely as he has reached the age of 68 and suffered from previous viral infections without developing HLH.
This is the first reported case of HLH secondary to the ChAdOx1 nCov‐19 vaccine. The syndrome was resolved with conservative management. It is important for clinicians to consider HLH in patients who develop persistent fevers following vaccination.
CONFLICT OF INTERESTS
The authors declare that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
Sylvia Ai, Alexander Awford, and Fernando Roncolato contributed to the draft of the manuscript, management of the patient, and approved the final version for publication.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. World Health Organisation . WHO coronavirus (COVID‐19) dashboard [Internet]. Geneva; May 23, 2021. https://covid19.who.int/?adgroupsurvey={adgroupsurvey}%26gclid=Cj0KCQjw16KFBhCgARIsALB0g8Jw4c4oU7TOhFEAfMcIknUrEuO6gRwmz3_eWIyv89UZqJ4APiWxjfQaAkcaEALw_wcB
- 2. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021;384:2124‐2130. 10.1056/NEJMoa2104882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Filipovich A, McClain K, Grom A. Histiocytic disorders: recent insights into pathophysiology and practical guidelines. Biol Blood Marrow Transplant. 2010;16(suppl 1):S82‐S89. [DOI] [PubMed] [Google Scholar]
- 4. Henter J‐I, Horne A, Aricó M, et al. HLH‐2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124‐131. [DOI] [PubMed] [Google Scholar]
- 5. Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66(9):2613‐2620. [DOI] [PubMed] [Google Scholar]
- 6. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel SU, Khurram R, Lakhani A, Quirk B. Guillain‐Barre syndrome following the first dose of the chimpanzee adenovirus‐vectored COVID‐19 vaccine, ChAdOx1. BMJ Case Rep. 2021;14(4):e242956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Retamozo S, Brito‐Zeron P, Siso‐Almirall A, Flores‐Chavez A, Soto‐Cardenas MJ, Ramos‐Casals M. Haemophagocytic syndrome and COVID‐19. Clin Rheumatol. 2021;40:1233‐1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang LV, Hu Y. Hemophagocytic lymphohistiocytosis after COVID‐19 vaccination. J Hematol Oncol. 2021;87(14):4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roden AC, Morice WG, Hanson CA. Immunophenotypic attributes of benign peripheral blood gammadelta T cells and conditions associated with their increase. Arch Pathol Lab Med. 2008;132(11):1774‐1780. [DOI] [PubMed] [Google Scholar]
- 11. Recht H, Solnes L, Merrill S, Siegelman S. 18‐FDG PET/CT findings in hemophagocytic lymphohistiocytosis: a pictorial review. J Nucl Med. 2020;61(suppl 1):S1140. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


