Abstract
Polymyalgia rheumatica following immunization with Covid‐19 mRNA vaccine: TRL‐7 and TRL‐9 as common link.
Keywords: ASIA syndrome, BNT162b2 vaccine, polymyalgia rheumatica
1. INTRODUCTION
Vaccination against severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2) is a cornerstone in the fight against the coronavirus disease 2019 (COVID‐19) pandemic. Among the currently available vaccines, tozinameran (BNT162b2) is a nucleoside‐modified messenger RNA (mRNA) vaccine encoding the spike (S) protein for the SARS‐CoV‐2.1 Even if its safety has been proved, immune‐mediated diseases flares or new‐onset inflammatory diseases following its administration have recently been reported.2
2. CASE REPORT
In February 2021, the day after the first dose of the BNT182b2 vaccine, a 69‐year‐old woman complained of sudden bilateral pain in the shoulder and pelvic girdles associated with morning stiffness lasting > 2 hours, fever, and general malaise. Until then, she enjoyed excellent health and worked occasionally as a domestic helper. Significant restrictions in her self‐care activities of daily living (ADL) depending on the affected areas were present. She took acetaminophen 1000 mg twice daily for 3 days with resolution of fever, but no improvement on pain. When she was admitted to our outpatient rheumatologic clinic, she had an X‐ray of the chest, shoulders, and pelvic region, revealing no pathologic findings; an abdominal ultrasound (US) showing mild hepatomegaly, and last an 18‐fluorodeoxyglucose positron emission tomography (18‐FDG/PET) associated with total body computed tomography (CT) that excluded pathological findings in other sites (Figure 1). The main laboratory data are listed in Table 1. Polymyalgia rheumatica (PMR) was diagnosed. No clinical manifestation of giant cell arteritis (GCA) was present, and a Doppler US examination of the temporal arteries showed normal findings. Nasal and oropharyngeal swabs were negative for SARS‐CoV‐2 both at the time of diagnosis and 3 days after. When therapy with prednisone 15 mg/day started, her clinical manifestations and ADL quickly improved. The second dose of anti SARS‐CoV‐2 vaccine was not administered. After 5.5 months, she is still taking prednisone 7.5 mg/day and is fine. In Table 1, we listed the main laboratory data after the first dose of vaccine, and after 10 and 30 days of prednisone therapy.
FIGURE 1.
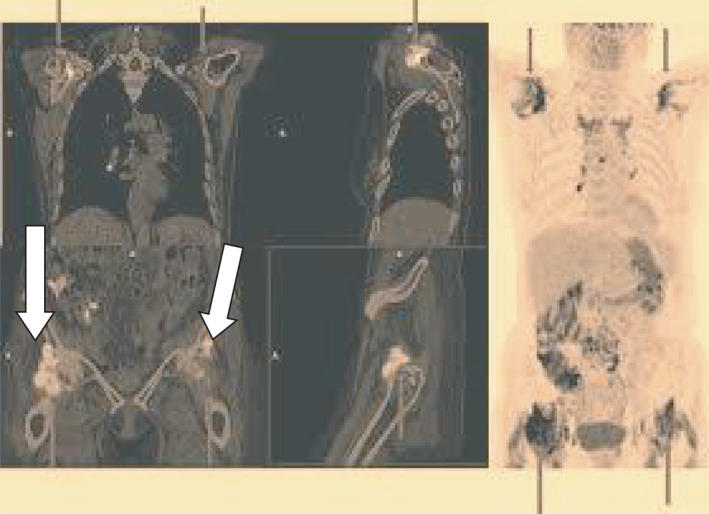
Tracer increased uptake is highlighted in peri‐articular and extra‐articular synovial structures of shoulder (see arrows in the right image) and pelvic girdles (see arrows in the left image)
TABLE 1.
Main laboratory data after the first dose of BNT182b2 vaccine, and after prednisone therapy
| First dose of vaccine | After prednisone | ||
|---|---|---|---|
| (10 days) | (30 days) | ||
| ESR (mm/h) | 105 | 60 | 15 |
| CRP concentrations (n.v. < 5 mg/dL) | 75 | 15 | 2 |
| IL‐6 serum concentrations (n.v. < 50 pg/mL) | 200 | 100 | 20 |
| Hb (n.v. > 12 gr/dL) | 11.0 | 11‐8 | 12.5 |
| Renal, hepatic, and thyroid function tests | n.v. | n.v. | n.v. |
| Blood glucose (mg/dL) | 105 | 230 | 125 |
| RF, ANA, ANCA | n.v. | — | n.v. |
| APCA | n.v. | — | n.v. |
Abbreviations: ANA, antinuclear antibodies; ANCA, anti‐neutrophil cytoplasmic antibodies; APCA, anti protein citrullinated antibodies; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; IL‐6, interleukin‐6; n.v., normal values; RF, rheumatoid factor.
3. DISCUSSION
PMR is one of the most common inflammatory rheumatic disease in older adults. Bilateral shoulder and hip pain, sometimes accompanied with neck aching, and a morning stiffness lasting > 45 minutes are its typical manifestations. The onset of these manifestations is so sudden that the patient usually remembers the exact day and hour. Additional manifestations, such as fever, general discomfort and fatigue, loss of appetite, and loss of weight, can be present in some patients.3 At present, no specific laboratory tests are available, but inflammatory markers, such as ESR, CRP, and IL‐6 serum concentrations, are usually raised at the time of diagnosis.4 In every day clinical practice, a prompt response to < 20 mg/day prednisolone has commonly been used to confirm the diagnosis.5 Etiology and pathogenesis of PMR are still debated: innate and adaptive immunity are involved in various way,6 and some investigators discussed the possibility that PMR can be an auto‐inflammatory disease.7 Finally, in up to 20% of cases, PMR can be associated to GCA, a chronic granulomatous vasculitis affecting the aorta and its branches, whose most common clinical manifestations are headache, scalp tenderness, and jaw claudication.8, 9
Several diseases can mimic PMR in every day clinical practice. In Table 2, we listed the most common PMR‐mimicking diseases, and the main differential signs and symptoms.10, 11
TABLE 2.
PMR‐mimicking diseases and the signs and symptoms useful for a correct diagnosis
| Disease | Signs and symptoms useful for differential diagnosis |
|---|---|
| Rheumatoid arthritis | Involvement of some joints of the hands (metacarpophalangeal and proximal interphalangeal), positive results of rheumatoid factor (RF) and anti‐cyclic citrullinated peptide antibodies (ACPA), radiographic and ultrasound findings (erosive arthritis, periarticular osteoporosis). |
| Remitting seronegative symmetrical synovitis with pitting edema (RS3PE) | Symmetric multiple synovitis, seronegative for RF and ACPA, causing boxing‐glove swelling with pitting edema of hands and feet. Ultrasound findings: tenosynovitis of extensor tendon sheath. |
| Late‐onset inflammatory spondyloarthropathies, including ankylosing spondylitis and psoriatic arthritis | Inflammatory pain in the lumbar region; radiographic findings of sacroiliitis; psoriasis. |
| Late‐onset systemic lupus erythematosus, scleroderma, Sjogren’s syndrome, vasculitis | Presence of antinuclear antibodies (ANA), presence of anti‐neutrophil cytoplasmic antibodies (ANCA). |
| Idiopathic inflammatory myopathies | Skin rashes, increased serum levels of creatine kinase. |
| Scapulohumeral periarthritis and adhesive capsulitis (“frozen shoulder”) | Restriction of shoulder movements, even in passive; ultrasound and magnetic resonance imaging allow one to diagnose the specific inflammation. Inflammatory markers not raised. |
| Calcium pyrophosphate deposition disease | Monoarthritis; radiographic and ultrasound findings, examination of synovial fluid. |
| Paraneoplastic syndromes | Failure to respond to glucocorticoid therapy or frequent relapses must be considered as elements of suspicion. Furthermore, the presence of atypical clinical manifestations and laboratory findings (among these, macrocytic anemia or bicytopenia), and familiarity for neoplasms should be considered as warning signs. |
Abbreviation: PMR, polymyalgia rheumatic.
PMR may follow vaccination, more frequently influenza vaccines.12 Some investigators hypothesized that this link could be an expression of an autoimmune/auto‐inflammatory syndrome induced by vaccine components called “adjuvants” (ASIA syndrome),13 and other researchers discussed that PMR triggered by vaccination could constitute a subset of milder diseases.14
When we searched the published literature, we found only one case of PMR‐like syndrome following anti SARS‐CoV‐2 vaccination. In short, a 70‐year‐old male patient developed PMR‐like clinical manifestations following 3 days after the first dose of BNT162b2 vaccine and had a fast response to prednisone 40 mg once daily.2 Our patient took a lower dose of prednisone in line with the schedule proposed in 2015 by a European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) collaborative initiative,15 and the most common PMR‐mimicking diseases were assessed both at the time of diagnosis and after the 5 month follow‐up. In particular, laboratory data and a total body CT scan performed after this follow‐up (data not reported) confirmed the first diagnosis.
Did our patient have an ASIA syndrome? Three major criteria were present, that is a previous exposure to an external stimulus, and the development of some “typical” clinical manifestations, such as myalgia/myositis, arthralgia/arthritis, and pyrexia.16 The onset of PMR and PMR‐like syndromes after exposure to adjuvant is very uncommon. For instance, only four cases of PMR/PMR‐like syndromes were reported in the ASIA syndrome international registry, which lists > 500 cases.13
Polyethylene glycol (PEG) is present in the lipid film of BNT162b2 vaccine. It has been identified as the culprit of anaphylaxis reactions to anti SARS‐CoV‐2 mRNA vaccines,17 where if it can induce an ASIA syndrome is still debated.2
On the other hand, mRNA vaccine can itself stimulate the innate immunity through endosolic and cytoplasmic nucleic acid receptors, such as Toll‐like receptors (TLRs), especially TLR‐7 and TLR‐9.1 Trafficking of TLR‐7 and TLR‐9 from the endoplasmic reticulum to endosomal compartments is tightly regulated by the chaperone protein UNC93B1.18 It is worthy highlighting that the peripheral mononuclear blood cells of patients with PMR have an increased expression of TLR‐7 and TLR‐9, which resolves with complete disease remission.19 Therefore, TLR‐7 and TRL‐9 could be the common link between PMR and BNT162b2 vaccine, able to favor an over‐production of inflammatory cytokines (IL‐6 among these), at least in genetically predisposed individuals (Figure 2).
FIGURE 2.
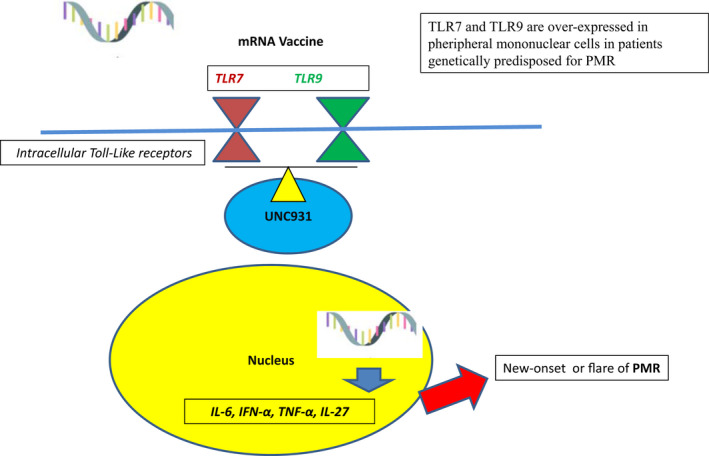
TRL‐7, TRL‐9, mRNA vaccine, and PMR: A working‐hypothesis. PMR, polymyalgia rheumatica
The possibility that COVID‐19 may trigger PMR has recently been reported,20 but no clinical or instrumental manifestation of COVID‐19 was present in our patient, and two consecutive nasal and oropharyngeal swabs were negative for SARS‐CoV‐2.
4. CONCLUSIONS
To date, PMR following anti‐SARS‐CoV‐2 vaccine is exceptional.
We acknowledge that the relationship between the PMR and the SARS‐COV‐2 vaccine cannot assuredly be confirmed by the reported case. Nevertheless, presentation of the symptoms after the vaccine is in favor of this.
Our case report confirms that the interactions among anti SARS‐CoV‐2 mRNA vaccine, its adjuvants, and the human immune system are very complex and not yet fully understood.
CONFLICTS OF INTEREST
Nothing to disclose.
AUTHOR CONTRIBUTIONS
Conceptualization: Manzo. Data curation: Manzo, Natale, and Castagna. Formal analysis: Manzo and Castagna. Writing and original draft preparation: Manzo, Natale, and Castagna. Writing, review, and editing: Castagna and Manzo. Supervision: Manzo. All the authors agreed on the final text.
ETHICAL APPROVAL
The study was approved by the Ethics Committee of Health District no. 59, Sant’Agnello (Naples, Italy) with the following protocol number: SA/06/2021, and conducted in accordance with the requirement of the current version (from 2008) of the Helsinki Declaration of the World Medical Association (WMA) on the ethical principles of conducting medical research involving people.
ACKNOWLEDGEMENT
The authors wish to thank the patient for her collaboration.
INFORMED CONSENT
Written informed consent for publication of clinical details was obtained from the patient.
Manzo C, Natale M, Castagna A. Polymyalgia rheumatica as uncommon adverse event following immunization with COVID‐19 vaccine: A case report and review of literature. Aging Med. 2021;4:234–238. 10.1002/agm2.12171
REFERENCES
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT182b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383:2603‐2615. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watad A, De Marco G, Mahajna H, et al. Immune‐mediated disease flares or new‐onset disease in 27 subjects following mRNA/DNA SARS‐CoV‐2 vaccination. Vaccines. 2021;9:435. 10.3390/vaccines9050435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.González‐Gay MA, Matteson EL, Castañeda S. Polymyalgia rheumatica. Lancet. 2017;390:1700‐1712. 10.1016/S0140-6736(17)31825-1 [DOI] [PubMed] [Google Scholar]
- 4.Manzo C, Milchert M, Natale M, Brzosko M. Polymyalgia rheumatica with normal values of both erythrocyte sedimentation rate and C‐reactive protein concentration at the time of diagnosis. Rheumatology. 2019;58:921‐923. 10.1093/rheumatology/key431 [DOI] [PubMed] [Google Scholar]
- 5.Manzo C, Camellino D, Manzo C, Camellino D. Polymyalgia rheumatica: diagnostic and therapeutic issues of an apparently straightforward disease. Recenti Prog Med. 2017;108:221‐231. 10.1701/2695.27559 [DOI] [PubMed] [Google Scholar]
- 6.Guggino G, Ferrante A, Macaluso F, Triolo G, Ciccia F. Pathogenesis of polymyalgia rheumatica. Reumatismo. 2018;70:10‐17. 10.4081/reumatismo.2018.1048 [DOI] [PubMed] [Google Scholar]
- 7.Floris A, Piga M, Cauli A, Salvarani C, Mathieu A. Polymyalgia rheumatica: an autoinflammatory disorder ? RMD Open. 2018;4:e000694. 10.1136/rmdopen-2018-000694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villiger PM. Giant cell arteritis: real‐life experience. J Rheumatol. 2021;1:jrheum.210334: 10.3899/jrheum.210334 [DOI] [PubMed] [Google Scholar]
- 9.Manzo C. Widespread headache as the first clinical manifestation of giant cell arteritis in patients affected by polymyalgia rheumatica. Reumatologia. 2016;54:236‐238. 10.5114/reum.2016.63663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ceccato F, Uña C, Regidor M, Rillo O, Babini S, Paira S. Conditions mimicking polymyalgia rheumatica. Reumatol Clin. 2011;7:156‐160. 10.1016/j.reuma.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez‐Gay MA, Garcia‐Porrua C, Salvarani C, Olivieri I, Hunder GG. The spectrum of conditions mimicking polymyalgia rheumatica in Northwestern Spain. J Rheumatol. 2000;27:2179‐2184. [PubMed] [Google Scholar]
- 12.Liozon E, Parreau S, Filloux M, et al. Giant cell arteritis or polymyalgia rheumatica after influenza vaccination: a study of 12 patients and a literature review. Autoimmun Rev. 2021;20:102732. 10.1016/j.autrev.2020.102732 [DOI] [PubMed] [Google Scholar]
- 13.Watad A, Bragazzi NL, McGonagle D, et al. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) demonstrates distinct autoimmune and autoinflammatory disease associations according to the adjuvant subtype: insights from an analysis of 500 cases. Clin Immunol. 2019;203:1‐8. 10.1016/j.clim.2019.03.007 [DOI] [PubMed] [Google Scholar]
- 14.Falsetti P, Conticini E, Acciai C, et al. Polymyalgia rheumatica following infective triggers or vaccinations: a different subset of disease? Reumatologia. 2020;58:76‐80. 10.5114/reum.2020.95360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dejaco C, Singh YP, Perel P, et al. 2015 Recommendations for the management of polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis. 2015;74:1799‐1807. 10.1136/annrheumdis-2015-207492 [DOI] [PubMed] [Google Scholar]
- 16.Borba V, Malkova A, Basantsova N, et al. Classical examples of the concept of the ASIA syndrome. Biomolecules. 2020;10:1436. 10.3390/biom10101436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sellaturay P, Nasser S, Islam S, Gurugama P, Ewan PW. Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID‐19 vaccine. Clin Exp Allergy. 2021;51:861‐863. 10.1111/cea.13874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suthers AN, Sarantopolulos S. TLR7/TLR9‐ and B cell receptor‐signaling crosstalk: promotion of potentially dangerous B cells. Front Immunol. 2017;8:775. 10.3389/fimmu.2017.00775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alvarez‐Rodriguez L, Lopez‐Hoyos M, Beares I, et al. Toll‐like receptor 4 gene polymorphisms in polymyalgia rheumatica and elderly‐onset rheumatoid arthritis. Clin Exp Rheumatol. 2011;29:795‐800. [PubMed] [Google Scholar]
- 20.Manzo C, Castagna A, Ruotolo G. Can SARS‐CoV‐2 trigger relapse of polymyalgia rheumatica? Joint Bone Spine. 2021;88:105150. 10.1016/j.jbspin.2021.105150 [DOI] [PMC free article] [PubMed] [Google Scholar]


